Abstract
Purpose of Review
The objective of this article is to review the role of intracoronary imaging in the management of patients with spontaneous coronary artery dissection (SCAD). In this review article, we discuss the types of intracoronary imaging including intravascular ultrasound (IVUS) and optical coherence tomography (OCT) and their relative strengths and weaknesses. Additionally, we discuss in detail the findings on IVUS and OCT and how these modalities can be used for optimization of percutaneous coronary intervention (PCI) in SCAD cases.
Recent Findings
While coronary angiography is the first-line investigation for diagnosis of SCAD, it remains inconclusive in a considerable proportion of cases owing to the widely varying appearance of SCAD on angiography. Even though the vast majority of SCAD cases are treated conservatively, PCI may be required in cases with hemodynamic instability. Intracoronary imaging becomes critical in the two aforementioned situations, either for making a conclusive diagnosis of SCAD or for optimization of PCI.
Summary
Intracoronary imaging has a vital role to play in the diagnosis and management of SCAD patients. Interventional cardiologists should be well versed with the findings on IVUS and OCT to diagnose SCAD and utilize it for optimization of PCI. However, it is important to exercise caution in performing intracoronary imaging and follow safe practices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spontaneous coronary artery dissection (SCAD) is defined as a spontaneous dissection of an epicardial coronary artery in the absence of atherosclerosis, trauma, or iatrogenic etiology [1]. It was first described in 1931 on an autopsy report of a 41-year-old female who had no atherosclerotic risk factors but had sudden cardiac death. However the first angiographic report of SCAD described as the presence of extraluminal dye was by Forker et al. in 1973 [2].
The true incidence of SCAD remains largely unknown due to its underdiagnosis [3]. Although its incidence has been reported to be 2–4% of all acute coronary syndrome (ACS) presentations [4, 5], its prevalence is much higher in younger and middle-aged females. For example, in a Canadian series [6], among women < 50 years presenting with acute myocardial infarction (AMI), SCAD accounted for 24% of cases, similar to the 35% reported in a similar Japanese registry [7]. Therefore, there should be high suspicion of SCAD in younger patients lacking traditional risk factors for atherosclerotic coronary artery disease presenting with ACS. SCAD is also the most common cause of myocardial infarction among pregnant women [8]. Although rheumatologic disorders accelerate the atherosclerotic process and are independent risk factors for traditional coronary artery disease, SCAD should be entertained as a plausible cause for ACS in these patients. Similarly, the presence of fibromuscular dysplasia [9], inherited aortopathies, use of exogenous hormonal agents, or a history of a clear precipitant like intense physical or emotional exertion, stimulant/recreational drugs or Valsalva should tip one’s suspicion towards SCAD [10]. Clinically, the vast majority of SCAD patients present as acute coronary syndrome, with a varying proportion of reported ST elevation myocardial infarction (STEMI) (26 to 55%) [11] versus non-ST elevation myocardial infarction (NSTEMI) [12]. Only a minority (2.8 to 10%) of SCAD patients present with ventricular arrhythmia [11]. A percentage of patients classified as MINOCA (myocardial infarction with non-obstructive coronary artery disease), in fact, have SCAD as the primary pathology as indicated in the scientific statement by the American Heart Association [13].
Pathophysiology of SCAD
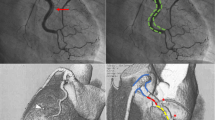
SCAD results from the development of a false lumen in the outer third of the tunica media by one of two proposed mechanisms (Fig. 1). The first is an “inside-out” mechanism where disruption of the intimal layer of the epicardial vessel results in bleeding into the vessel wall or media, forming a false lumen. Progressive bleeding into the false lumen compresses and subsequently obliterates the true lumen causing ACS. The second is an “outside-in” mechanism due to spontaneous bleeding from the vasa vasorum into the media resulting in intramural hematoma with progression and obliteration of lumen of the epicardial vessel resulting in ACS. [14]. Multi-vessel SCAD defined as the occurrence of spontaneous dissections in more than one epicardial artery without continuity has been reported to occur in 5 to 13% of cases [10, 11, 15]. Considering the histopathology, accurate diagnosis of SCAD without optical coherence tomography (OCT), intravascular ultrasound (IVUS), or multi-slice computed tomography (MSCT) can be challenging as the false lumen or the transmural hematoma may not be adequately visualized with routine angiography.
Schematic representation of the pathophysiology of SCAD. a Accumulation and axial propagation of blood forms a false lumen in the outer third of the tunica media leading to external compression of the true lumen. b Blood may enter through an endothelial-intimal disruption or “tear.” c Or as a result of bleeding from a microvessel within the vessel wall leading to an expanding and compressing false lumen (dotted arrows)
Role of Coronary Angiography in the Diagnosis of SCAD
Coronary angiography despite its limitations remains first-line for the diagnosis of SCAD [14].
The presence of radiolucent lumens and contrast staining in the extraluminal space is descriptive of lesions suggestive of SCAD. However, in a case series described by Saw et al., only 30% of SCAD cases had this classic appearance on coronary angiography [10]. Based on coronary angiography alone, 4 major types of SCAD have been described (Fig. 2) [16].
-
Type 1: “Classic SCAD” with radiolucent lumen and/or extraluminal staining
-
Type 2 A: refers to a segment with diffuse narrowing with “normal” segment proximally and distally
-
Type 2 B: refers to diffuse narrowing extending to the distal end of the vessel
-
Type 3: refers to short segment of stenosis (< 20 mm in length) that may have similar appearance to an atherosclerotic lesion and can only be accurately diagnosed with intracoronary imaging
-
Type 4: refers to complete obliteration of the lumen and typically involves the distal segment of the vessel. This is the most difficult to diagnose and it is usually inferred upon a follow-up angiogram when flow in the vessel is restored.
Angiographic classification of SCAD. Type 1 spontaneous coronary artery dissection (a), type 2A spontaneous coronary artery dissection (b), type 2B spontaneous coronary artery dissection (c), type 3 spontaneous coronary artery dissection (d), type 4 spontaneous coronary artery dissection (e), and intermediate type 1/2 spontaneous coronary artery dissection (f)
Type 2 SCAD appears to be the most common, in occurring in up to two-thirds of all cases, followed by type 1 (29.1%) with type 3 being the rarest (3.5%) [10]. As previously mentioned, multi-vessel involvement has been reported to occur in 5–13% of cases.
It is to be noted that these vessels are inherently fragile and have a higher tendency for iatrogenic dissection from diagnostic catheter engagement at 3.4% as compared to a risk of < 0.2% during routine angiography [17, 18]. Care must be taken to avoid pressure dampening of the catheter and avoid high-pressure injections into coronaries especially in proximal vessel involvement.
Role of Intravascular Imaging in SCAD
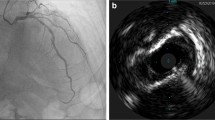
The American Heart Association [1] and European Society of Cardiology [14] in their consensus documents recommend using intravascular imaging only when coronary angiography is inconclusive for diagnosis of SCAD or to aid PCI when indicated for its treatment. Even though awareness about SCAD and its angiographic pattern recognition has improved among cardiologists in recent years, there are still cases when angiography alone may be inconclusive [19]. With greater use of intravascular imaging, SCAD is likely to be recognized as the underlying pathology for a larger subset of patients with MINOCA [13]. In such cases, intracoronary imaging is a valuable tool for diagnosis, since the pathology in SCAD involves the vessel wall rather than the lumen (Fig. 3). Intravascular imaging is particularly useful in type 3 SCAD which closely mimics atherosclerosis on a coronary angiogram. It is to be noted that intravascular imaging poses risk of propagation of coronary dissection due to guidewire entering the false lumen, hydraulic pressure from powerful contrast injections to opacify the vessel for some intravascular imaging modalities, or related to the guiding catheter itself (Fig. 4). Therefore, intracoronary imaging should be performed with great caution and should be reserved for cases where the diagnosis of SCAD is uncertain with routine angiography or to aid percutaneous coronary intervention (PCI) when deemed necessary [14]. Figure 6 shows a schematic representation of the role of intravascular imaging in SCAD and a comparison between the two modalities.
Intracoronary imaging of spontaneous coronary artery dissection by intravascular ultrasound (with outer border of false lumen arrowed, a) and optical coherence tomography showing partial (b) and circumferential (c) false lumens; the site of a fenestration (d) connecting true and false lumens and reduced light penetration through the false lumen (e). Three-dimensional image derived from segmentation of optical coherence tomography image showing how the false lumen tracks around the true lumen and is influenced (and frequently bounded) by side branches (f). Reprinted from Adlam et al. [14] with permission
Guidewire passage into the false lumen demonstrated by optical coherence tomography, with both wire (GW) and optical coherence tomography catheter (C) seen in the false lumen outside the compressed true lumen. Reprinted from Adlam et al. [14] with permission
IVUS
IVUS has an axial resolution of 150 μm and is helpful in delineating intraluminal thrombus, intramural hematoma, intimal tear, and atherosclerotic plaque [14]. It has a greater depth of penetration compared to OCT, therefore allowing visualization of all the three layers of the vessel wall. IVUS is capable of delineating the true lumen from false lumen and is furthermore able to evaluate the extent of the false lumen and intramural hematoma (Fig. 5). The other benefit of this modality is that blood does not attenuate the ultrasound rays, and therefore, it obviates the need for pressurized contrast or saline injection, as is the case with OCT. Additionally, IVUS has a greater penetration depth (10 mm) allowing for visualization of the external elastic lamina and the entire dissected segment even in large caliber arteries or presence of intraluminal red thrombus [20]. The prime limitation of this technology is its relatively poor spatial resolution, as a result of which it is unreliable in identifying the site of communication between the true and false lumen (called the “entry point”) and the intima-medial layer. To mitigate this limitation, some experts recommend a combined use of IVUS and OCT for evaluation and management of SCAD lesions, although the costs of using two imaging modalities may be an impediment [21].
Intracoronary Images of SCAD from different patients using Optical coherence tomography (OCT) images. a Double lumen with a thick intimomedial membrane. b Double lumen with a thin intimal membrane. c Intimal rupture (arrow). d Intracoronary thrombus protruding into the true lumen (arrow). Intravascular ultrasound (IVUS) images. e Elliptical, echogenic, true lumen fully detached from the outer vessel wall. f Double lumen with a side branch (arrow) emerging from the true lumen. g Double lumen with false lumen thrombosis (plus sign). h Intramural hematoma. Notice the 3-layered appearance of the intimomedial membrane and the layered, crescent-shaped, intramural hematoma. Overall, the intimomedial thickness was better measured with OCT due to the better near-field resolution. However, this was challenging in 2 cases in which the dissection trailing edge progressively faded off into an underlying hematoma; IVUS, however, clearly displayed the true thickness of the flap. The true lumen tended to be smaller than the false lumen, and some segments showed an elliptical morphology suggestive of extrinsic compression. Asterisk denotes wire artifact. Reprinted from Paulo et al. [21] with permission
OCT
OCT is a relatively newer catheter-based modality for intracoronary imaging that utilizes near infrared range light with a much higher spatial resolution (axial resolution of 10 to 20 μm). OCT has a penetration depth (defined as the maximum depth that can be imaged) of 1–2 mm. Tissue with high attenuation-like lipid-rich plaque has a lower penetration depth as compared to collagenous material or calcified plaque, which has lower attenuation and therefore greater penetration depth. OCT is a superior tool to coronary angiography or IVUS in the diagnosis and characterization of SCAD as it is able to acquire high-resolution images of intimal and medial layers and the associated pathology [14, 22]. In a case series by Alfonso et al., only 3 out of 11 patients who had confirmed SCAD by OCT had a demonstrable dissection flap on coronary angiography [23]. OCT requires high-pressure injection of contrast to clear the blood in the lumen with potential risk of propagating the dissection plane particularly in proximal vessel SCAD. However, this is more a theoretical risk and numerous reports suggest that with caution and appropriate technique, OCT is safe to perform [19, 24].
OCT allows visualization of the compromise in true lumen, size and extent of the false lumen, presence of associated thrombus, relationship of the false lumen to side branches, and presence of fenestrations, if any (Fig. 3). On OCT, the characteristic double lumen has been reported on all SCAD cases with either evidence of “entry tear” (connection between the true and false lumens) or intramural hematoma (Fig. 5). OCT also allows measurement of the thickness of the intimal-medial dissection membrane [22, 23]. Additionally, OCT also helps in identifying coronary vascular pathology associated with fibromuscular dysplasia (bright echogenic collagen bands interspersed with cellular hyperplasia), which co-exists and predisposes to SCAD. Follow-up OCT has also shed light on the stages of vessel healing with ultimate resolution of dissection or hematoma [25].
Comparison of IVUS Versus OCT for Diagnosis of SCAD
As discussed above, both imaging modalities have their relative advantages and disadvantages for the diagnosis of SCAD (Fig. 6). While OCT has better spatial resolution and reveals more intraluminal pathology, there is a risk of propagation of dissection especially when proximal vessels are involved due to contrast injections during OCT. An expert consensus document on intravascular imaging by the European society of cardiovascular interventions recommends using IVUS as the preferred imaging modality when there is evidence of false lumen (type 1) and in small caliber and tortuous vasculature [26]. IVUS with its better penetration depth may also be better in large caliber proximal vessels whose external elastic lamina has been further stretched by the presence of a false lumen. However, OCT provides better diagnostic clarity in type 3 and 4 SCAD, which cannot be conclusively diagnosed on angiography. As previously mentioned, OCT provides better visualization of details such as intramural hematoma, thrombus, entry tear, fenestrations, and relationship of false lumen to the side branches [27].
Role of Intravascular Imaging in Guiding PCI in SCAD
Observational data has revealed that PCI in SCAD is associated with increased risk of intraprocedural complications, poor angiographic outcomes, and increased mortality [10,11,12, 15]. There are potential catastrophic complications reported with PCI such as guide catheter induced dissection, wiring the false lumen, propagation of false lumen/intramural hematoma proximally or distally, resultant side-branch closure, persistent distal dissection, and acute vessel closure [4, 11, 28]. Therefore, PCI is typically reserved only for cases with hemodynamic instability or ongoing ischemia with chest pain associated with compromise in flow in a major epicardial artery [1]. When PCI is deemed necessary, utilizing intravascular imaging becomes critically important in order to guide intervention and optimize procedural outcomes [23]. Intracoronary imaging provides anatomic details such as the location of entry tear, extent of false lumen, presence of intramural hematoma, and involvement of side braches. Intracoronary imaging should be used to confirm the position of guidewire (Fig. 4) and plan PCI strategy including the length and diameter of stent. If intramural hematoma is visualized, it is important to also determine its extent because the goal of PCI is to cover the hematoma with a long drug-eluting stent extending 5 to 10 mm proximally and distally, so as to allow propagation of the intramural hematoma when compressed by the stent [1]. Intracoronary imaging also helps confirm adequate expansion and apposition of the stent thereby decreasing the chances of stent thrombosis. Image-guided optimization of PCI is particularly important to mitigate the risk of late stent thrombosis that occurs due to resorption of the intramural hematoma as the artery heals [22]. Additionally, intracoronary imaging has also been reported to aid in the utilization of cutting balloon angioplasty to create fenestrations in the intramural hematoma and decompress the false lumen into the true lumen [29]. Cutting balloon angioplasty has been reported to be successful in restoration of flow as a stand-alone therapy [29] or prior to stenting [30].
Conclusion
The majority of SCAD cases do not have the classic angiographic appearance of radiolucent lumen and/or extra-luminal staining. In cases where coronary angiography is not diagnostic or when PCI is indicated for treatment of SCAD, intracoronary imaging should be performed albeit with caution. While OCT has emerged as the imaging modality of choice owing to its high spatial resolution, there may be a role for combined assessment with IVUS and OCT in certain cases. When PCI is indicated for the treatment of SCAD, intracoronary imaging becomes crucial to help delineate details such as the extent of dissection, identification of entry tear, presence of intramural hematoma, and involvement of side branches. Ensuring adequate stent expansion and apposition using intravascular imaging is important in preventing late stent thrombosis that may occur due to acquired malapposition following resorption of intramural hematoma.
References
Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137(19):e523–57.
Forker AD, Rosenlof RC, Weaver WF, Carveth SW, Reese HE. Primary dissecting aneurysm of the right coronary artery with survival. Chest. 1973;64(5):656–8.
Tweet MS, Gulati R, Hayes SN. Spontaneous coronary artery dissection. Curr Cardiol Rep. 2016;18(7):60.
Mortensen KH, Thuesen L, Kristensen IB, Christiansen EH. Spontaneous coronary artery dissection: a Western Denmark Heart Registry study. Catheter Cardiovasc Interv. 2009;74(5):710–7.
Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H, et al. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5(3):263–70.
Saw J, Aymong E, Mancini GBJ, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30(7):814–9.
Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, Nakao K, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol. 2016;207:341–8.
Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, et al. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation. 2014;129(16):1695–702.
Saw J, Poulter R, Fung A, Wood D, Hamburger J, Buller CE. Spontaneous coronary artery dissection in patients with fibromuscular dysplasia: a case series. Circ Cardiovasc Interv. 2012;5(1):134–7.
Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, et al. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7(5):645–55.
Tweet MS, Eleid MF, Best PJM, Lennon RJ, Lerman A, Rihal CS, et al. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7(6):777–86.
Mahmoud AN, Taduru SS, Mentias A, Mahtta D, Barakat AF, Saad M, et al. Trends of incidence, clinical presentation, and in-hospital mortality among women with acute myocardial infarction with or without spontaneous coronary artery dissection: a population-based analysis. JACC Cardiovasc Interv. 2018;11(1):80–90.
Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139(18):e891–908.
Adlam D, Alfonso F, Maas A, Vrints C, Writing Committee, al-Hussaini A, et al. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39(36):3353–68.
Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, et al. Management and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 2015;116(1):66–73.
Saw J. Coronary angiogram classification of spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2014;84(7):1115–22.
Prakash R, Starovoytov A, Heydari M, Mancini GBJ, Saw J. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2016;9(17):1851–3.
Maehara A, Mintz GS, Castagna MT, Pichard AD, Satler LF, Waksman R, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol. 2002;89(4):466–8.
Saw J, Mancini GBJ, Humphries K, Fung A, Boone R, Starovoytov A, et al. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87(2):E54–61.
Alfonso F, Paulo M, Dutary J. Endovascular imaging of angiographically invisible spontaneous coronary artery dissection. JACC Cardiovasc Interv. 2012;5(4):452–3.
Paulo M, Sandoval J, Lennie V, Dutary J, Medina M, Gonzalo N, et al. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2013;6(7):830–2.
Franco C, Eng L, Saw J. Optical coherence tomography in the diagnosis and management of spontaneous coronary artery dissection. Interv Cardiol Clin. 2015;4(3):309–20.
Alfonso F, Paulo M, Gonzalo N, Dutary J, Jimenez-Quevedo P, Lennie V, et al. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59(12):1073–9.
Motreff P, Malcles G, Combaret N, Barber-Chamoux N, Bouajila S, Pereira B, et al. How and when to suspect spontaneous coronary artery dissection: novel insights from a single-centre series on prevalence and angiographic appearance. EuroIntervention. 2017;12(18):e2236–43.
Cade J, Mintz GS, Silva Filho RM, Caixeta A. Spontaneous coronary artery dissection and healing documented by optical coherence tomography. Einstein (Sao Paulo). 2016;14(3):435–6.
Johnson TW, Räber L, di Mario C, Bourantas C, Jia H, Mattesini A, et al. Clinical use of intracoronary imaging. Part 2: acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: an expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2019;40(31):2566–84.
Macaya F, Salazar CH, Pérez-Vizcayno MJ, Salinas P, Jiménez-Quevedo P, Nombela-Franco L, et al. Feasibility and safety of intracoronary imaging for diagnosing spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2019;12(4):763–4.
Conraads VM, Vorlat A, Colpaert CG, Rodrigus IE, de Paep RJ, Moulijn AC, et al. Spontaneous dissection of three major coronary arteries subsequent to cystic medial necrosis. Chest. 1999;116(5):1473–5.
Yumoto K, Sasaki H, Aoki H, Kato K. Successful treatment of spontaneous coronary artery dissection with cutting balloon angioplasty as evaluated with optical coherence tomography. JACC Cardiovasc Interv. 2014;7(7):817–9.
Alkhouli M, Cole M, Ling FS. Coronary artery fenestration prior to stenting in spontaneous coronary artery dissection. Catheter Cardiovasc Interv. 2016;88(1):E23–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Swamy, P.M., Parwani, P., Mamas, M.A. et al. Role of Intravascular Imaging in the Diagnosis and Treatment of Spontaneous Coronary Artery Dissection. Curr Cardiovasc Imaging Rep 13, 27 (2020). https://doi.org/10.1007/s12410-020-09547-x
Published:
DOI: https://doi.org/10.1007/s12410-020-09547-x