Abstract
Purpose of Review
Our objective is to review the current status of OCT-guided treatment of calcified coronary artery disease.
Recent Findings
New treatment modalities provide multiple options for approaching interventions involving calcified lesions.
Summary
Coronary artery calcification is associated with stent underexpansion and worse procedural outcomes. Optimizing stent expansion is essential to reduce restenosis and the need for revascularization. Optical coherence tomography (OCT) allows for accurate diagnosis and detailed characterization of calcified lesions. The features of coronary artery calcification are determinant of the optimal lesion preparation and treatment strategy. We recommend an OCT-guided treatment approach for calcified coronary lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Severely calcified coronary artery disease represents a challenging lesion subset that is often encountered in patients treated with percutaneous coronary intervention (PCI). The presence of calcification is often underappreciated by angiography alone [1•]. Accurate recognition and classification of coronary artery calcification permits appropriate decision-making to determine the optimal treatment approach. We herein review the role of optical coherence tomography (OCT) in guiding the treatment of calcified lesions with PCI.
Impact of Calcification
Coronary artery calcification is associated with decreased procedural success and worse clinical outcomes [2,3,4]. Specifically, underexpansion which is one of the strongest predictors of future stent thrombosis and in-stent restenosis is closely related to the degree of lesion calcification [5]. Atherectomy, the term coined by John Simpson in the 1980s, refers to device-based plaque modification [6]. The value of intravascular imaging with the use of atherectomy has been recognized since the initial reports of atherectomy use [7]. Historically, atherectomy devices were reserved for uncrossable or undilatable lesions. In recent years, there has been a paradigm shift to an upfront lesion preparation approach to modify calcified plaque to maximize stent expansion. This comes with an important distinction; with lesion preparation, the goal is not simply to facilitate stent delivery, but rather to optimize stent expansion.
Role of OCT
Intravascular imaging assessment is essential to accurately assess the burden of calcified plaque [8,9,10]. Two independent studies conducted decades apart both corroborated the significant inaccuracies of angiography alone for the detection and diagnosis of coronary artery calcifications [11, 12]. Mintz et al. initially reported that angiography detected calcium in just 38% lesions, whereas intravascular ultrasound (IVUS) detected lesion calcium in 73% of lesions [13]. The more contemporary study, which compared OCT in addition to IVUS and angiography, found similar conclusions [12].
Intravascular imaging should be performed on all lesions with suspected calcification undergoing PCI to guide selection of appropriate therapies and ensure adequate stent expansion is achieved. Occasionally, angiographically severe calcification can be found to be only mild-moderate in severity following intravascular imaging assessment and does not require lesion preparation beyond balloon-based therapies. Conversely, and more commonly, severe calcification is significantly underappreciated by angiography alone. This is associated with greater morbidity [14]. Unrecognized severe calcification in patients undergoing PCI increases the likelihood for stent underexpansion.
Intravascular imaging allows for more than mere quantification and diagnosis of coronary artery calcification. Beyond assessing mild, moderate, or severe calcification, there are important morphologic characteristics that influence the optimal treatment strategy. An algorithmic approach to integrate OCT into PCI is recommended to ensure comprehensive assessment and procedure planning [15•]. OCT can safely and quickly provide information influencing treatment decisions in real time [16, 17].
Coronary Calcification Classification
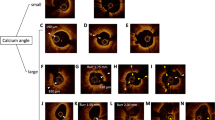
Angiographic calcification is classified as mild, moderate, or severe. The use of intravascular imaging allows for more specific characterization and quantification of calcification. Beyond determining severity of calcification, it is important to determine if the calcification is superficial or deep (Fig. 1) and eccentric or concentric (Fig. 2). These factors are often determinant of the optimal treatment technique.
It is important to recognize the presence of calcified nodule, which is often underappreciated by angiography alone. OCT studies have demonstrated calcified nodules are present in greater than 6% of patients with angiographically nonobstructive lesions and in roughly one third of severely calcified culprit lesions in patients with ACS [18•, 19].
OCT-Based Calcium Scoring System
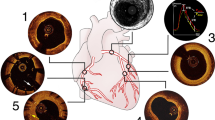
OCT allows unique in vivo characterization of coronary artery calcification. Recognition of the features of calcified plaque that can predict stent underexpansion is important for selecting appropriate lesion preparation strategies. An OCT-based calcium scoring system can be used to quickly calculate the risk of a calcified lesion for stent underexpansion [20••]. If pre-stent OCT demonstrates maximum calcium angle > 180°, continuous length > 5 mm, and calcium thickness > 0.5 mm, the lesion is at risk for stent underexpansion with PCI (Fig. 3). Consequently, these lesions should be treated with an upfront lesion preparation strategy to facilitate plaque modification and calcium fracture prior to stent implantation. This can be achieved with lesion preparation with either intravascular lithotripsy, orbital atherectomy, or rotational atherectomy.
Rotational Atherectomy
Rotational atherectomy was first introduced by David Auth and colleagues in the 1980s and has been the predominant atherectomy modality in use for calcified coronary artery disease [21, 22]. Rotational atherectomy uses a diamond-coated elliptical burr to rotate concentrically while advancing in a forward direction. The Rotablator system (Boston Scientific Corporation, Marlborough, MA) historically was controlled using a console, with activation by a foot pedal; however, more recent iterations include the RotaPro rotational atherectomy system (Boston Scientific Corporation, Marlborough, MA) which integrates the controls into the console. Rotational atherectomy burr sizes vary from 1.25 to 2.5 mm.
Initial trials comparing rotational atherectomy with conventional approaches failed to demonstrate a major difference in outcomes [23,24,25]. The PREPARE-CALC trial randomized patients with severely calcified lesions undergoing treatment with PCI to rotational atherectomy vs. lesion preparation with scoring or cutting balloons. The investigators found that with contemporary PCI, rotational atherectomy was associated with greater strategy success (98% vs. 81%, p < 0.001) [26]. Overall revascularization at 9-month follow-up was lower in those treated with rotational atherectomy (8% vs. 21%, p = 0.01), with no significant difference in other clinical outcomes at 9 months in the 200 randomized patients [26]. OCT intravascular imaging studies suggest that use of a cutting balloon following lesion preparation with rotational atherectomy is associated with calcium fracture and greater stent expansion compared with conventional balloon following rotational atherectomy [27].
Orbital Atherectomy
Orbital atherectomy was approved for the treatment of severely calcified coronary lesions prior to stent implantation in 2013. The Diamondback 360 coronary orbital atherectomy system (Cardiovascular Systems, Inc., St. Paul, MN) uses a diamond-coated, eccentrically mounted 1.25-mm burr that orbits bi-directionally at 80,000 rpm on low speed and 120,000 rpm on high speed [28]. The ORBIT II trial demonstrated the safety and efficacy of orbital atherectomy in patients with severe calcified coronary artery disease [29]. At 3-year follow-up, patients treated with orbital atherectomy had a 10.2% rate of target vessel revascularization [30]. In a real-world, multicenter registry that included high-risk patients excluded in the ORBIT II trial, orbital atherectomy was associated with low complication rates, including a perforation rate of 0.7%, and favorable results at 1-year follow-up [31, 32].
The mechanism of action of orbital atherectomy includes polishing of the surface of calcified plaque, with a characteristic smooth, concave ablation and calcium fracture as a result of pulsatile force, which can be appreciated on OCT assessment following treatment (Fig. 4) [33]. Calcium fracture as a result of plaque modification contributes to greater stent expansion [34]. OCT-based imaging studies have found that compared with rotational atherectomy, orbital atherectomy is associated with greater calcium modification in lesions with larger lumen area, whereas the effect in lesions with smaller lumen area is similar between devices [35••].
Intravascular Lithotripsy
Intravascular lithotripsy (IVL) is a novel catheter-based device that allows for disruption of calcified plaque with fracturing of calcium due to sonic pressure waves that are released from emitters during balloon inflation at 4 atm [36, 37]. IVL for the treatment of calcified coronary artery disease is commercially available in Europe; however, it is currently limited to clinical investigation in the USA. Due to its ease of use, IVL represents promising technology for both de novo coronary calcification and in-stent restenosis with underexpanded stents due to severe calcification [38,39,40]. Data from the DISRUPT CAD III study will inform the long-term results following lesion preparation with IVL.
OCT-Guided Treatment of Calcified Lesions
The goal of lesion preparation with severely calcified lesions is calcium fracture to facilitate stent expansion [41]. We recommend an algorithmic approach to treating calcified lesions (Fig. 5). Our approach is grounded in OCT and its ability to efficiently characterize the degree of calcification and systematically inform optimal device selection as well as assess treatment efficacy. OCT is essential prior to stent implantation in calcified lesions to ensure appropriate stent sizing and adequate lesion preparation. When the OCT imaging catheter cannot cross the lesion due to severe stenosis, pre-dilatation with small, non-compliant balloons can facilitate pre-stent intravascular imaging. The treatment approach for lesion preparation should be guided by the OCT calcium score (Fig. 3). Lesions with a calcium score of 4 should be approached with adjunctive therapies to include intravascular lithotripsy, orbital atherectomy, or rotational atherectomy. OCT following lesion preparation allows for recognition of calcium fracture and determination if additional lesion modification is necessary prior to stent implantation.
OCT-guided approach to calcified lesions. An algorithmic, OCT-based approach should be incorporated to optimize treatment of calcified lesions undergoing PCI to ensure adequate lesion preparation and optimal stent expansion. IVL intravascular lithotripsy, NC non-compliant, OCT optical coherence tomography, OA orbital atherectomy, RA rotational atherectomy
Following stent implantation, OCT should be performed to ensure adequate endpoints have been reached, with further stent optimization employed as needed. Liberal use of balloon pre- and post-dilatation are employed as needed before and after each step to ensure optimal results.
Future Outlooks
While comparisons have been reported between orbital and rotational atherectomy, these have been based on observational registries and there are no randomized trial comparing these modalities [42,43,44]. While there are advantages for each device in certain lesion subtypes and clinical scenarios, either may be used in most cases of severe calcification. The key is to ensure adequate lesion preparation is achieved, and for an operator to use the device with which they are most proficient.
There are several ongoing pivotal clinical trials that will provide important insight into the role of OCT and lesion preparation strategies in PCI. The ILUMIEN IV trial (NCT03507777) is a randomized, multicenter global clinical trial that will be the largest intravascular imaging trial to date and is comparing OCT-guided PCI with angiographic-guided PCI. The ECLIPSE randomized trial (NCT03108456) is also ongoing and will be the largest randomized PCI trial of patients with severely calcified coronary artery disease. The ECLIPSE trial (NCT03108456) is comparing a lesion preparation strategy with orbital atherectomy prior to stent implantation vs. conventional balloon angioplasty prior to stent implantation. The DISRUPT CAD III (NCT03595176) study is assessing the safety and efficacy of the novel intravascular lithotripsy device. Both the ECLIPSE trial and DISRUPT CAD III studies have pre-specified OCT sub-studies that will also provide additional important insight into the treatment of calcified coronary artery disease. Table 1 summarizes the ongoing pivotal OCT studies.
Conclusions
Successful PCI and reduction in future revascularization is closely tied to final stent expansion. Coronary artery calcification is often underappreciated by angiography alone. OCT permits recognition of calcified plaque with accurate anatomic and morphologic characterization. Lesion preparation based on the presence, severity, and morphology of coronary calcium can facilitate optimal stent expansion. We recommend an OCT-based algorithmic approach to the diagnosis and treatment of calcified plaque in all patients treated with PCI.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Barbato E, Shlofmitz E, Milkas A, Shlofmitz R, Azzalini L, Colombo A. State of the art: evolving concepts in the treatment of heavily calcified and undilatable coronary stenoses - from debulking to plaque modification, a 40-year-long journey. EuroIntervention. 2017;13(6):696–705 A review of severe coronary calcification and contemporary treatment approaches.
Genereux P, Madhavan MV, Mintz GS, Maehara A, Palmerini T, Lasalle L, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) trials. J Am Coll Cardiol. 2014;63(18):1845–54.
Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. 2015;8(4):461–71.
Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Genereux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63(17):1703–14.
Maehara A, Ben-Yehuda O, Ali Z, Wijns W, Bezerra HG, Shite J, et al. Comparison of stent expansion guided by optical coherence tomography versus intravascular ultrasound: the ILUMIEN II study (observational study of optical coherence tomography [OCT] in patients undergoing fractional flow reserve [FFR] and percutaneous coronary intervention). JACC Cardiovasc Interv. 2015;8(13):1704–14.
Stack RS. New interventional technologies in cardiology. Mayo Clin Proc. 1989;64(7):867–70.
Yock PG, Linker DT, White NW, Rowe MH, Selmon MR, Robertson GC, et al. Clinical applications of intravascular ultrasound imaging in atherectomy. Int J Card Imaging. 1989;4(2–4):117–25.
Shlofmitz E, Kuku KO, Waksman R, Garcia-Garcia HM. Intravascular ultrasound-guided drug-eluting stent implantation. Minerva Cardioangiol. 2019.
Maehara A, Matsumura M, Ali ZA, Mintz GS, Stone GW. IVUS-guided versus OCT-guided coronary stent implantation: a critical appraisal. JACC Cardiovasc Imaging. 2017;10(12):1487–503.
Parviz Y, Shlofmitz E, Fall KN, Konigstein M, Maehara A, Jeremias A, et al. Utility of intracoronary imaging in the cardiac catheterization laboratory: comprehensive evaluation with intravascular ultrasound and optical coherence tomography. Br Med Bull. 2018;125(1):79–90.
Mintz GS, Douek P, Pichard AD, Kent KM, Satler LF, Popma JJ, et al. Target lesion calcification in coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol. 1992;20(5):1149–55.
Wang X, Matsumura M, Mintz GS, Lee T, Zhang W, Cao Y, et al. In vivo calcium detection by comparing optical coherence tomography, intravascular ultrasound, and angiography. JACC Cardiovasc Imaging. 2017;10(8):869–79.
Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation. 1995;91(7):1959–65.
Hong MK, Mintz GS, Lee CW, Park DW, Choi BR, Park KH, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27(11):1305–10.
• Shlofmitz E, Shlofmitz RA, Galougahi KK, Rahim HM, Virmani R, Hill JM, et al. Algorithmic Approach for optical coherence tomography-guided stent implantation during percutaneous coronary intervention. Interv Cardiol Clin. 2018;7(3):329–44 Review of an algorithmic approach to incorporating optical coherence tomography for routine use with percutaneous coronary interventions.
Shlofmitz E, Garcia-Garcia HM, Rogers T, Khalid N, Chen Y, Kajita AH, et al. Techniques to optimize the use of optical coherence tomography: insights from the Manufacturer and User Facility Device Experience (MAUDE) database. Cardiovasc Revasc Med. 2019;20:507–12.
Prati F, Cera M, Ramazzotti V, Imola F, Giudice R, Albertucci M. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervention. 2007;3(3):365–70.
• Yamamoto MH, Maehara A, Song L, Matsumura M, Chin CY, Losquadro M, et al. Optical coherence tomography assessment of morphological characteristics in suspected coronary artery disease, but angiographically nonobstructive lesions. Cardiovasc Revasc Med. 2018; The authors highlight the importance of intravascular imaging when coronary disease is suspected but not appreciated by angiography alone.
Lee T, Mintz GS, Matsumura M, Zhang W, Cao Y, Usui E, et al. Prevalence, predictors, and clinical presentation of a calcified nodule as assessed by optical coherence tomography. JACC Cardiovasc Imaging. 2017;10(8):883–91.
•• Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention. 2018;13(18):e2182–e9 The authors introduce a new classification system to predict the risk of stent underexpansion based on the severity of calcification on optical coherence tomography.
Tomey MI, Kini AS, Sharma SK. Current status of rotational atherectomy. JACC Cardiovasc Interv. 2014;7(4):345–53.
Lee MS, Gordin JS, Stone GW, Sharma SK, Saito S, Mahmud E, et al. Orbital and rotational atherectomy during percutaneous coronary intervention for coronary artery calcification. Catheter Cardiovasc Interv. 2018;92(1):61–7.
Abdel-Wahab M, Richardt G, Joachim Buttner H, Toelg R, Geist V, Meinertz T, et al. High-speed rotational atherectomy before paclitaxel-eluting stent implantation in complex calcified coronary lesions: the randomized ROTAXUS (Rotational Atherectomy Prior to Taxus Stent Treatment for Complex Native Coronary Artery Disease) trial. JACC Cardiovasc Interv. 2013;6(1):10–9.
Reifart N, Vandormael M, Krajcar M, Gohring S, Preusler W, Schwarz F, et al. Randomized comparison of angioplasty of complex coronary lesions at a single center. Excimer Laser, Rotational Atherectomy, and Balloon Angioplasty Comparison (ERBAC) study. Circulation. 1997;96(1):91–8.
Dill T, Dietz U, Hamm CW, Kuchler R, Rupprecht HJ, Haude M, et al. A randomized comparison of balloon angioplasty versus rotational atherectomy in complex coronary lesions (COBRA study). Eur Heart J. 2000;21(21):1759–66.
Abdel-Wahab M, Toelg R, Byrne RA, Geist V, El-Mawardy M, Allali A, et al. High-speed rotational atherectomy versus modified balloons prior to drug-eluting stent implantation in severely calcified coronary lesions. Circ Cardiovasc Interv. 2018;11(10):e007415.
Amemiya K, Yamamoto MH, Maehara A, Oyama Y, Igawa W, Ono M, et al. Effect of cutting balloon after rotational atherectomy in severely calcified coronary artery lesions as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 2019.
Shlofmitz E, Martinsen BJ, Lee M, Rao SV, Genereux P, Higgins J, et al. Orbital atherectomy for the treatment of severely calcified coronary lesions: evidence, technique, and best practices. Expert Rev Med Devices. 2017;14(11):867–79.
Chambers JW, Feldman RL, Himmelstein SI, Bhatheja R, Villa AE, Strickman NE, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7(5):510–8.
Lee M, Genereux P, Shlofmitz R, Phillipson D, Anose BM, Martinsen BJ, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18(4):261–4.
Lee MS, Shlofmitz E, Goldberg A, Shlofmitz R. Multicenter registry of real-world patients with severely calcified coronary lesions undergoing orbital atherectomy: 1-year outcomes. J Invasive Cardiol. 2018;30(4):121–4.
Lee MS, Shlofmitz E, Kaplan B, Alexandru D, Meraj P, Shlofmitz R. Real-world multicenter registry of patients with severe coronary artery calcification undergoing orbital atherectomy. J Interv Cardiol. 2016;29(4):357–62.
Shlofmitz E, Shlofmitz R, Lee MS. Orbital atherectomy: a comprehensive review. Interv Cardiol Clin. 2019;8(2):161–71.
Yamamoto MH, Maehara A, Kim SS, Koyama K, Kim SY, Ishida M, et al. Effect of orbital atherectomy in calcified coronary artery lesions as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 2018.
•• Yamamoto MH, Maehara A, Karimi Galougahi K, Mintz GS, Parviz Y, Kim SS, et al. Mechanisms of orbital versus rotational atherectomy plaque modification in severely calcified lesions assessed by optical coherence tomography. JACC Cardiovasc Interv. 2017;10(24):2584–6 A comparative assessment of the impact and mechanism of orbital atherectomy vs. rotational atherectomy.
Dini CS, Tomberli B, Mattesini A, Ristalli F, Valente S, Stolcova M, et al. Intravascular lithotripsy for calcific coronary and peripheral stenoses. EuroIntervention. 2019.
Brinton TJ, Ali ZA, Hill JM, Meredith IT, Maehara A, Illindala U, et al. Feasibility of shockwave coronary intravascular lithotripsy for the treatment of calcified coronary stenoses. Circulation. 2019;139(6):834–6.
Ali ZA, McEntegart M, Hill JM, Spratt JC. Intravascular lithotripsy for treatment of stent underexpansion secondary to severe coronary calcification. Eur Heart J. 2018.
Watkins S, Good R, Hill J, Brinton TJ, Oldroyd KG. Intravascular lithotripsy to treat a severely under-expanded coronary stent. EuroIntervention. 2018.
Alfonso F, Bastante T, Antuna P, de la Cuerda F, Cuesta J, Garcia-Guimaraes M, et al. Coronary lithoplasty for the treatment of undilatable calcified de novo and in-stent restenosis lesions. JACC Cardiovasc Interv. 2019;12(5):497–9.
Fujino A, Mintz GS, Lee T, Hoshino M, Usui E, Kanaji Y, et al. Predictors of calcium fracture derived from balloon angioplasty and its effect on stent expansion assessed by optical coherence tomography. JACC Cardiovasc Interv. 2018;11(10):1015–7.
Meraj PM, Shlofmitz E, Kaplan B, Jauhar R, Doshi R. Clinical outcomes of atherectomy prior to percutaneous coronary intervention: a comparison of outcomes following rotational versus orbital atherectomy (COAP-PCI study). J Interv Cardiol. 2018;31(4):478–85.
Koifman E, Garcia-Garcia HM, Kuku KO, Kajita AH, Buchanan KD, Steinvil A, et al. Comparison of the efficacy and safety of orbital and rotational atherectomy in calcified narrowings in patients who underwent percutaneous coronary intervention. Am J Cardiol. 2018;121(8):934–9.
Lee MS, Park KW, Shlofmitz E, Shlofmitz RA. Comparison of rotational atherectomy versus orbital atherectomy for the treatment of heavily calcified coronary plaques. Am J Cardiol. 2017;119(9):1320–3.
Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, et al. Comparison between Optical COherence tomography guidance and Angiography guidance in percutaneous coronary intervention (COCOA): study protocol for a randomized controlled trial. J Cardiol. 2018;72(2):170–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
F. Sosa is an employee of Abbott Vascular.
Z. Ali has served as a consultant to Abbott Vascular, Boston Scientific, Opsens Medical, Cardinal Health, and Canon; has equity/options in Shockwave Medical; and has received research grants from Abbott Vascular, the National Heart, Lung, and Blood Institute, and Cardiovascular Systems Inc.
R. Waksman has served on the advisory boards of Abbott Vascular, Amgen, Boston Scientific, Cardioset, Cardiovascular Systems Inc., Medtronic, Philips, and Pi-Cardia Ltd.; as a consultant for Abbott Vascular, Amgen, Biosensors, Biotronik, Boston Scientific, Cardioset, Cardiovascular Systems Inc., Medtronic, Philips, and Pi-Cardia Ltd.; has received grant support from Abbott Vascular, AstraZeneca, Biosensors, Biotronik, Boston Scientific, and Chiesi; has served on the speakers bureaus of AstraZeneca and Chiesi; and has invested in MedAlliance.
A. Jeremias has received educational grants from and served as a consultant for Abbott Vascular and Philips; and has served as a consultant for Opsens.
All other authors have no conflict of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Shlofmitz, E., Sosa, F.A., Ali, Z.A. et al. OCT-Guided Treatment of Calcified Coronary Artery Disease: Breaking the Barrier to Stent Expansion. Curr Cardiovasc Imaging Rep 12, 32 (2019). https://doi.org/10.1007/s12410-019-9509-1
Published:
DOI: https://doi.org/10.1007/s12410-019-9509-1