Abstract
Purpose of Review
Left main coronary artery disease (LMCAD) is a frequently encountered, high-risk presentation of atherosclerosis, traditionally managed with surgical bypass grafting. Percutaneous coronary intervention (PCI) for LMCAD is an increasingly attractive option for patients with low to intermediate complexity disease or patients at extremely high or prohibitive surgical risk. The goal of this review is to outline the current indications and guideline recommendations regarding PCI for LMCAD and the role of intracoronary imaging in optimizing these cases.
Recent Findings
Several recent randomized controlled trials have demonstrated the non-inferiority of PCI in LMCAD compared with CABG. Further, the use of intracoronary imaging techniques (i.e., intravascular ultrasound (IVUS) and optical coherence tomography (OCT)) has an advanced understanding of the features, both pre- and post-intervention, responsible for poor procedural outcomes and the metrics of successful PCI.
Summary
PCI for LMCAD should be considered a viable option for those patients at increased surgical risk with low to intermediate lesion complexity. While not directly evaluated in LMCAD intervention, routine intracoronary imaging use and PCI optimization metrics can help to improve outcomes in LMCAD PCI procedures. Further exploration of intracoronary imaging techniques in LMCAD PCI procedures, as well as the long-term follow-up data comparing patients with LMCAD treated with PCI versus CABG, will more completely define the role of PCI in treating these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Significant left main coronary artery disease (LMCAD) is defined by greater than 50% stenosis seen by angiography. A large observational study of data from the National Cardiovascular Data Registry (NCDR) reviewed over 1.2 million coronary angiograms and demonstrated prevalence of significant LMCAD of 4.2% [1]. Additionally, LMCAD is associated with multi-vessel coronary disease in nearly 70% of cases [2, 3]. For the last several decades, management of significant LMCAD has been via coronary artery bypass graft (CABG) surgery, with several early studies demonstrating clear mortality benefit to surgical bypass compared with optimal medical management [4, 5]. More recently, percutaneous coronary intervention (PCI) has become a viable therapeutic option, especially in patients with disease limited to the LMCA, or with low to intermediate lesion complexity, as well as in those with extremely high or prohibitive surgical risk. Given the established safety and efficacy of PCI, the intention of this review is to address the current indications and practice guidelines for LMCA PCI and the use of intracoronary imaging techniques, namely intravascular ultrasound (IVUS) and optical coherence tomography (OCT), in optimizing PCI outcomes.
LMCAD: Revascularization with PCI Versus CABG
The management of LMCAD has historically been based on data from the Coronary Artery Surgery Study (CASS) [4, 5]. A sub-analysis of LMCAD from the CASS data demonstrated significantly greater 3-year cumulative survival in the surgical group compared with the medically managed group, 91% vs 69% respectively [6]. This effect is present in any stenosis > 50% in the LMCA and is particularly pronounced for those in the highest risk categories, with LMCA stenosis > 75% or reduced left ventricular (LV) function. Overall, increasing severity of LMCAD was associated with decreased survival in both the surgical and medical groups. However, low-risk patients with LM stenosis of 50–59% and normal LV function showed no survival advantage with bypass at 3 years [5]. Together, these trials served as the basis for preferential management of LMCAD with bypass surgery for many years.
With the evolution of percutaneous techniques and devices, PCI has become an increasingly attractive option for patients with CAD. Data from the NCDR demonstrate the considerable expansion of PCI use in patients with LMCAD in the last two decades [1]. The Synergy Between PCI with Taxus vs Cardiac Surgery (SYNTAX) and subsequent SYNTAX-Left Main sub-study demonstrated non-inferiority of PCI for left main stenosis in patients with low to intermediate SYNTAX score (< 33) with regard to all-cause mortality and major adverse cardiac and cerebrovascular events (MACCE) outcomes at 15 months [2, 7, 8]. While a pooled analysis of data from Bypass Surgery Versus Angioplasty Using Sirolimus-Eluting Stent in Patients with Left Main Coronary Artery Disease (PRECOMBAT) and the SYNTAX sub-study showed PCI was associated with significantly higher rates of major adverse events than CABG at 5 years (28.3% vs 23.0% p < 0.045) [9,10], this was driven mainly by the higher rate of repeat revascularization associated with PCI, and the two approaches had otherwise similar outcomes of death, myocardial infarction, and stroke.
Following these results, the Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease (EXCEL) trial randomized patients with low-intermediate SYNTAX score to PCI versus CABG. It demonstrated non-inferiority of PCI versus CABG for a composite endpoint of all-cause mortality, MI, and stroke at 3 years [11••]. Similarly, the Nordic-Baltic-British Left Main Revascularization Study (NOBLE) trial evaluated PCI versus CABG in all comers at 5 years. While it showed higher rates of major adverse events in the PCI arm (28%) compared with CABG (18%), this was driven by revascularization in the PCI group. Stroke rate and mortality rates were similar between the two groups [12••]. These trial data, further stratified by disease complexity using the SYNTAX score, have made PCI a reasonable alternative to CABG for patients with low and intermediate SYNTAX scores. However, long-term follow-up data from these trials are not yet available.
Intracoronary Imaging during PCI
Given the two-dimensional imaging plane of coronary angiography, the degree of LMCA stenosis can be difficult to assess accurately. Factors such as the absence of a proximal reference point, vessel tortuosity and overlap, or eccentric or bifurcating plaque distribution all increase the challenge. Further, angiography provides no detail regarding the vessel wall characteristics. It is well documented that interobserver grading of angiographic stenoses is inconsistent, and these findings are even more pronounced in the assessment of LMCAD [13]. In the current practice guidelines, revascularization of LMCA stenosis > 50% with PCI has a class IIa LOE B recommendation for patients with low to intermediate stenosis complexity and high surgical risk [14,15,16]. However, given the aforementioned challenges with angiography, decision-making solely on angiographic appearance is inadequate.
Intravascular ultrasound (IVUS), in which an ultrasound-enabled catheter is pulled back across an atherosclerotic lesion, utilizes echocardiographic principles to more directly examine vascular disease and to include plaque characterization and calcification [17]. Several randomized trials have demonstrated the effectiveness of IVUS-guided PCI over angiography alone in improving outcomes in both the era of bare-metal stents (BMS) and drug-eluting stents (DES) [18, 19]. Parise et al. reviewed 7 randomized trials (n = 2193), demonstrating that IVUS guidance of bare-metal stent implantation resulted in a statistically significant increase in post-procedure minimal luminal diameter (MLD) of 0.12 mm (95% CI 0.06–0.18, p < 0.0001) and decreased 6-month angiographic restenosis rates (22% vs 29%, OR 0.64, 95% CI 0.42–0.96, p = 0.02) as well as major adverse cardiac and cerebrovascular events (MACCE) (19% vs. 23%, odds ratio 0.69, 95% CI 0.49 to 0.97, p = 0.03) [18]. More recently, in a meta-analysis of 7 randomized trials (n = 3192) of IVUS-guided vs. angiography-guided DES implantation, IVUS guidance significantly reduced MACCE (6.5% versus 10.3%; odds ratio, 0.60; 95% confidence interval, 0.46–0.77; p < 0.0001). This was largely due to a reduction in the risk of ischemia-driven revascularization (4.1% vs 6.6%; OR 0.60; 95% CI, 0.43–0.84; p = 0.003), although cardiovascular mortality was significantly reduced as well (0.5% versus 1.2%; OR, 0.46; 95% CI, 0.21–1.00; p = 0.05) [19].
In LMCAD, routine IVUS use has been associated with improvement in restenosis rates and stent thrombosis at 3 years [20,21,22]. Mortality and MACCE-free survival rates are also better with IVUS guidance, even possibly out to 10 years. In the subgroup analysis of the EXCEL trial, LMCA stenting was aided by the use of IVUS in 77% of cases and minimal stent area (MSA) < 8.7 mm2 was associated with worse MACE outcomes, cardiovascular death, myocardial infarction, and stent thrombosis compared with MSA > 11 mm2 [11••, 22]. These data suggest that IVUS guidance to optimize LMCAD PCI improves outcomes.
Another imaging modality, optical coherence tomography (OCT), utilizes near-infrared light to provide a detailed image of coronary plaque. As light travels faster than sound, OCT provides a more detailed and higher quality image compared with IVUS, enabling better tissue characterization [23].
Two large trials, Optical coherence tomography during percutaneous coronary intervention impacts physician decision-making (ILUMIEN I) and Does optical coherence tomography optimize results of stenting (DOCTORS), indicate that the use of pre- and post-OCT assessment of PCI strategy changes physician management in over 50% of cases and, in the case of DOCTORS, led to a small but significant improvement in post-stenting hemodynamics (FFR 0.94 ± 0.04 vs 0.92 ± 0.05, p = 0.005) [24, 25]. Subsequent studies Comparison of stent expansion guided by optical coherence tomography versus intravascular ultrasound (ILUMIEN II) and Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III) revealed that OCT was non-inferior to IVUS [26, 27]. The ILUMIEN IV trial, currently underway, is a large, randomized controlled trial comparing clinical outcomes between angiography-guided and OCT-guided PCI.
Pre-Stenting Assessment (Table 1)
Catheter-based imaging techniques may be used prior to stent deployment to evaluate vessel size, characterize plaque composition and extent of disease, assess the need for plaque modification with atherectomy, and determine the required stent diameter and length to optimize landing zones. In the PROSPECT study, predictors of MACCE at 3 years were an IVUS MLA < 4.0 mm2 or a total luminal disease burden > 70% [28]. The 2012 Consensus Standards for Acquisition, Measurement, and Reporting of Intravascular Optical Coherence Tomography Studies outline the particular appearance of various disease morphologies including fibrous, lipidic, and calcified diseases.
Given the flaws in angiographic assessment of LMCAD, IVUS assessment of MLA has shown a strong correlation to hemodynamically significant disease by FFR, which was previously validated as a strong predictor of requiring revascularization [29, 30] (Fig. 1). Traditionally, a MLA of 6.0 mm2 was used as a cutoff below which LMCA stenosis is likely significant and warrants revascularization [31]. In the LITRO study, patients with LMCA MLA > 6.0 mm2 had a 2-year cardiac death–free survival > 97%, compared with 95% for those who were revascularized [32]. Only 4% of deferred revascularization patients required intervention in the follow-up period. Recently, however, evidence from Korea indicates that an MLA < 4.8 mm2 is a more reliable predictor of poor outcomes, although this has not been borne out in Western populations [33].
Intracoronary imaging techniques have also proven invaluable in optimizing stent length and size. First, careful IVUS imaging from the left anterior descending (LAD) and left circumflex into the LMCA can provide clear delineation of the extent of disease. For example, Oveido et al. studied 140 patients with angiographic LMCAD and found that continuous plaque from the LMCA into the LAD was seen in 90% of lesions and into the left circumflex in 66% [34]. Plaque localized to the LAD or left circumflex ostia and not involving the LMCA was seen in only 9% and 17%, respectively. This was irrespective of the angiographic Medina classification and clearly impacts stenting strategies. Second, via direct visualization, the proximal and distal stent landing zones can be identified, ideally to a location with the largest luminal area and < 50% stenosis [35••].
Calcification remains an additional significant predictor of poor outcomes following PCI and the utilization of intracoronary imaging can aid procedural planning for calcified lesions [36]. Calcification may impair stent delivery and expansion. High-pressure balloon inflation used to dilate severe calcification can also lead to coronary perforation. Angiography has been shown to routinely underestimate the degree of calcification within lesions [36]. IVUS and OCT are particularly effective in the detection of plaque calcification and location, whether subintimal or deep (Fig. 2). And, while there is still no consensus regarding techniques to modify calcific disease prior to stent deployment, accurate knowledge of the relative burden may alter decision-making [37]. Early data on the safety and efficacy of calcium-modifying strategies presented in the ORBIT II trial, and subsequent sub-analysis out to 3 years of clinical follow-up, showed that in patients with severely calcified disease with an arc of calcium > 270° and calcification length > 15 mm by IVUS, orbital atherectomy was safe and 3-year target vessel revascularization rates were low at 7.8% [38]. Based on these data, lesion preparation with calcium-modifying strategies is generally recommended at this degree of calcification.
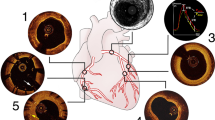
a Diagnostic angiogram of a patient with distal left main lesion declined for CABG and referred for PCI. b Wide arc of calcium (> 270°) noted on intravascular ultrasound (IVUS). c Final angiogram following PCI with rotational atherectomy and IVUS-guided stent implantation. d IVUS images post-stenting and post-dilatation demonstrating well-expanded stent
Post-Stenting Assessment (Table 2)
The benefit of intracoronary imaging in assessing the success of stent deployment in non-left main interventions has been clearly established and is related to optimizing stent expansion, residual landing zone disease or dissection, and treating malapposition. The degree to which stent underexpansion impacts clinical outcomes is clear for non-LMCA intervention in both the BMS and DES eras [39, 40]. Regarding LMCAD PCI, Kang et al. investigated the relationship between MSA and clinical outcomes with 403 patients receiving immediate post-stent deployment IVUS assessment of minimum stent area (MSA) and a subsequent 9-month follow-up angiogram to assess for significant in-stent restenosis (ISR) [39]. Using MSA cutoffs of 8.2 mm2 in the proximal left main, 7.2 mm2 in the polygon of confluence, and 6.3 mm2 in the ostial LAD, the authors demonstrated that ISR rates were considerably higher in the group in which post-dilation MSA fell below these cutoffs. Further, when utilizing these cutoffs, major cardiac event–free survival was improved (98.1 ± 0.9% versus 90.2 ± 2.6%, p ≤ 0.001). In another trial of 670 patients, 550 of whom underwent DES placement to a non-LM stenosis, multivariate analysis demonstrated that smaller MSA, as determined by IVUS, was a strong predictor of in-stent restenosis over 6 months of follow-up [40] (Fig. 3).
Several studies have also revealed the predictive nature of inflow/outflow disease (residual plaque burden at stent edges or geographic miss) for restenosis [41,42,43]. In the DES era, several studies have demonstrated the degree of post-intervention edge disease predictive of ISR, ranging from > 47 to > 52% disease [42, 43]. Most recently, Kang et al. published a prospective cohort analysis of 820 patients (987 total lesions) in which reference segment disease burden was collected for 1668 segments (987 distal, 681 proximal) [41]. Post-PCI, 37% of the angiographically normal proximal and 21% of the distal reference segments had a disease burden characterized as > 50% by IVUS. Using an adjusted cutoff of > 55%, edge restenosis rates (2.1% to 3.4% risk of ISR depending on the type of DES) were predicted with a sensitivity of 81% and specificity of 80%. Importantly, the negative predictive value of this cutoff was > 99%, suggesting that optimizing PCI to an edge disease burden < 55% post-PCI decreased ISR at 9 months [42, 43]. Figure 4 demonstrates a case of stent edge dissection and ISR visualized by OCT.
Stent malapposition, in which the expanded stent does not completely appose to the vessel wall, has also been studied and shown to contribute to very late stent thrombosis, although it does not cause ISR as long as the stent is fully expanded [44,45,46] (Fig. 5).
In 2019, Choi et al. published a large prospective registry study of 6005 patients undergoing PCI of complex lesions with DES. Complex lesions were defined as a bifurcation lesion, chronic total occlusion, left main disease, long lesion, multivessel PCI, multiple stent implantation, in-stent restenosis, or heavily calcified lesion. IVUS was used in 27% of cases and was associated with a decreased risk of cardiac death (10.2% vs 16.9%, HR 0.573; 0.46–0.71, p < 0.001) vs angiography-guided PCI, strongly suggesting utilizing IVUS for complex intervention [47••].
Criteria for Optimal PCI
Criteria from several trials have been used to optimize PCI using intracoronary image guidance. The MUSIC trial defined successful PCI based on criteria including stent expansion > 90% of the average reference cross-sectional area (CSA) or > 100% of a smaller reference CSA with complete apposition and symmetric expansion [48]. The 2018 ULTIMATE trial was a large, randomized trial of all comers undergoing PCI using angiography or IVUS guidance, with criteria for success including minimal cross-sectional area > 5.0 mm [2] (or 90% of distal reference lumen cross-sectional area), plaque burden at proximal and distal stent edges < 50%, and no edge dissection involving media with a length > 3 mm [35••]. No specific coronary imaging criteria have been defined for optimizing LMCAD PCI. In the NOBLE trial, IVUS assessment prior to stenting was performed in less than half of the procedures. Approximately 75% of patients underwent post-PCI stent assessment via IVUS, though values necessitating post-stent deployment optimization were not stated [12]. Criteria for successful PCI outlined in the ongoing ILUMIEN IV trial include MSA > 90% of the proximal and distal reference vessel lumen, any disease protrusion into the stent lumen > 0.2 mm, untreated reference segment disease (MLA < 4.5 mm2 within 4 mm of the stent edge), edge dissection > 60% of the vessel circumference > 3 mm in length, and stent malapposition.
Current Recommendations and Society Guidelines
As discussed, stable coronary disease involving the LMCA jeopardizes a large territory of myocardium and is potentially life-threatening if progressive or unstable. Recommendations for the management of these complex lesions are offered in both the 2012 ACC/AHA Guideline for the Diagnosis and Management of Patients with Stable Ischemic Heart Disease (and 2014 Focused Updated) and the recently released 2018 ESC/EACTS Guidelines on myocardial revascularization [14,15,16].
Notably, both guideline documents emphasize the importance of complex case review via an interdisciplinary “Heart Team” comprising non-interventional or clinical cardiologists, interventionalists, and cardiac surgeons. The general purpose of the discussion is to most appropriately select a revascularization plan based on an individual patient’s coronary anatomic complexity, clinical presentation and comorbid disease, surgical risk, and personal preferences after a full informed consent process. Several reports from various centers have demonstrated the reproducibility of these discussions and that Heart Team complex case review can reduce self-referral biases and specialty practice variation.
The 2012 ACC/AHA guidelines for management of stable ischemic heart disease gave a class I recommendation for bypass surgery in patients with LMCAD. Given the well-established prognostic benefit of surgery, largely due to long-term patency of left internal mammary artery (LIMA) grafts, surgery has been the treatment of choice for many years. Based on the SYNTAX trial data and several small randomized trials, PCI was considered a class IIa recommendation in patients with low to intermediate coronary anatomic complexity (SYNTAX score ≤ 33) and elevated surgical risk. However, PCI was not recommended for those with highly complex disease (SYNTAX score ≥ 33). These guidelines, however, do not reflect the data from the EXCEL and NOBLE trials.
The 2018 ESC guideline document retains the class I indication for bypass surgery in all patients without prohibitive surgical risk. However, PCI is recommended as an alternative to surgery for those patients deemed poor candidates for surgery in whom anatomic complexity is low (class I) and considered reasonable (class IIa) in those with intermediate SYNTAX [21,22,23,24,25,26,27,28,29,30], but is not recommended for those with the most complex disease with SYNTAX ≥ 33 (class III). If PCI is undertaken, it is recommended that it be performed by trained operators that perform > 25 LM PCI cases yearly at high-volume centers. Importantly, intracoronary imaging is recommended to optimize PCI in the management of LMCAD (class IIa).
Conclusions
With growing data, PCI is being increasingly used in the management of selected patients with LMCAD. PCI optimization with intracoronary imaging techniques prior to and following stent deployment has established benefits despite potential challenges such as procedural time, operator education, and cost. Given the large territory at risk, we believe that optimization of PCI with intracoronary imaging should be considered standard of care in LMCAD PCI and be performed routinely.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Huang HW, Brent BN, Shaw RE. Trends in percutaneous versus surgical revascularization of unprotected left main coronary stenosis in the drug-eluting stent era—a report from the American College of Cardiology-National Cardiovascular data registry (ACC-NCDR). Catheter Cardiovasc Interv. 68:867–72.
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72.
Taggart DP, Kaul S, Boden WE, Ferguson BT. Revascularization for unprotected left main stem coronary artery stenosis: stenting or surgery. J Am Coll Cardiol. 2008;51(9):885–92.
Takaro T, Hultgren HN, Lipton MJ, Detre KM. Survival in subgroups of patients with left main coronary artery disease. Veterans Administration Cooperative Study of Surgery for Coronary Arterial Occlusive Disease. Circulation. 1982;66(1):14–22.
Chaitman B, et al. Effect of coronary bypass surgery on survival patterns in subsets of patients with left main coronary artery disease: report of the collaborative study in coronary artery surgery (CASS). Am J Cardiol. 1981;48:765–77.
Takaro T, et al. The VA cooperative randomized study of surgery for coronary arterial occlusive disease II. Subgroup with significant left main lesions. Circulation. 1976;54(6):107–17.
Mohr FW, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet. 2013;381(9867):629–38.
Morice M-C, Serruys PW, Kappetein AP, Feldman TE, Ståhle E, Colombo A, et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the synergy between percutaneous coronary intervention with Taxus and Cardiac Surgery trial. Circulation. 2014;129:2388–94.
Park S-J, Kim Y-H, Park D-W, Yun SC, Ahn JM, Song HG, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364:1718–27.
Ahn JM, Roh JH, Kim YH, Park DW, Yun SC, Lee PH, et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease: 5-year outcomes of the PRECOMBAT study. J Am Coll Cardiol. 2015;65:2198–206.
•• Stone GW, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease (EXCEL). N Engl J Med. 2016;375:2223–35 XCEL and NOBLE trial demonstrated the non-inferiority of PCI for LMCAD in patients at high surgical risk with low to intermediate complexity disease.
•• Makikallio T, et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in treatment of unprotected left main stenosis (NOBLE): a prospective, randomised, open-label, non-inferiority trial. Lancet. 2016;388(10061):2743–52 XCEL and NOBLE trial demonstrated the non-inferiority of PCI for LMCAD in patients at high surgical risk with low to intermediate complexity disease.
Fisher LD, Judkins MP, Lesperance J, Cameron A, Swaye P, Ryan T, et al. Reproducibility of coronary arteriographic reading in the Coronary Artery Surgery Study (CASS). Catheter Cardiovasc Diagn. 1982;8:565–75.
Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2012;126:354–471.
Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2014;64:1929–49.
Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165.
Garcìa-Garcìa HM, Gogas BD, Serruys PW, Bruining N. IVUS-based imaging modalities for tissue characterization: similarities and differences. Int J Card Imaging. 2011;27(2):215–24.
Parise H, Maehara A, Stone GW, Leon MB, Mintz GS. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am J Cardiol. 2011;107(3):374–82.
Elgendy, IY et al. Outcomes with intravascular ultrasound-guided stent implantation: a meta-analysis of randomized trials in the era of drug-eluting stents. Circ Cardiovasc Interv. 2016; 9 (4).
Andell P, Karlsson S, Mohammad MA, Götberg M, James S, Jensen J, Fröbert O, Angerås O, Nilsson J, Omerovic E, Lagerqvist B, Persson J, Koul S, Erlinge D. Intravascular ultrasound guidance is associated with better outcomes in patients undergoing unprotected left main coronary artery stenting compared with angiography guidance alone. Circ Cardiovasc Interv 2017; 10.
de la Torre Hernandez JM, Baz Alonso JA, Gómez Hospital JA, Alfonso Manterola F, Garcia Camarero T, Gimeno de Carlos F, et al. IVUS-TRONCO-ICP Spanish Study. Clinical impact of intravascular ultrasound guidance in drug-eluting stent implantation for unprotected left main coronary disease: pooled analysis at the patient-level of 4 registries. JACC Cardiovasc Interv. 2014;7:244–54.
Park SJ, Kim YH, Park DW, Lee SW, Kim WJ, Suh J, et al. MAIN-COMPARE investigators. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;2:167–77.
Roleder T, Jąkała J, Kałuża GL, Partyka Ł, Proniewska K, Pociask E, et al. The basics of intravascular optical coherence tomography. Postepy Kardiol Interwencyjnej. 2015;11(2):74–83.
Wijns W, Shite J, Jones MR, Lee S, Price M, Fabiocchi F, et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J. 2015;36(47):3346–55.
Meneveau N, Souteyrand G, Motreff P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non–ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS (does optical coherence tomography optimize results of stenting) study. Circulation. 2016;134:906–17.
Maehara A, Ben-Yehuda O, Ali Z, Wijns W, Bezerra H, Shite J, et al. Comparison of stent expansion guided by optical coherence tomography versus intravascular ultrasound: the ILUMIEN II study (observational study of optical coherence tomography [OCT] in patients undergoing fractional flow reserve [FFR] and percutaneous coronary intervention). J Am Coll Cardiol Intv. 2015;8:1704–14.
Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomized controlled trial. Lancet. 2016;388:2618–28.
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35.
Hamilos M, Muller O, Cuisset T, Ntalianis A, Chlouverakis G, Sarno G, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505–12.
Motreff P, Rioufol G, Gilard M, Caussin C, Ouchchane L, Souteyrand G, et al. Diffuse atherosclerotic left main coronary artery disease unmasked by fractal geometric law applied to quantitative coronary angiography: an angiographic and intravascular ultrasound study. EuroIntervention. 2010;5(6):709–15.
Jasti V, Ivan E, Yalamanchili V, Wongpraparut N, Leesar MA. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;110:2831–6.
de la Torre Hernandez JM, Hernández Hernandez F, Alfonso F, Rumoroso JR, Lopez-Palop R, Sadaba M, et al. Prospective application of pre-defined intravascular ultrasound criteria for assessment of intermediate left main coronary artery lesions results from the multicenter LITRO study. J Am Coll Cardiol. 2011;58:351–8.
Park S-J, Ahn J-M, Kang S-J, Yoon S-H, Koo B-K, Lee J-Y, et al. Intravascular ultrasound-derived minimal lumen area criteria for functionally significant left Main coronary artery stenosis. JACC Cardiovasc Interv. 2014;7(8):868–74.
Oviedo C, Maehara A, Mintz GS, Araki H, Choi SY, Tsujita K, et al. Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations: where is the plaque really located? Circ Cardiovasc Interv. 2010;3:105–12.
•• Junjie Zhang, Xiaofei Gao, Jing Kan, Zhen Ge, Leng Han, Shu Lu, NailiangTian, Song Lin, Qinghua Lu, Xueming Wu, Qihua Li, Zhizhong Liu, YanChen, Xuesong Qian, Juan Wang, Dayang Chai, Chonghao Chen, Xiaolong Li, Bill, D. Gogas, Tao Pan, Shoujie Shan, Fei Ye, Shao-Liang Chen. Intravascular ultrasound-guided versus angiography-guided implantation of drug-eluting stent in all-comers: the ULTIMATE trial. J Am Coll Cardiol. 2018, 25553. The ULTIMATE trial demonstrated that in all-comer PCI cases, compared to angiography guidance alone, IVUS use was associated with a lower rate of target vessel failure at 12 months.
Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovasc Imaging. 2015;8:461–71.
Koyama T, Okura H, Kume T, Fukuhara K, Neishi Y, Hayashida A, et al. Calcifed plaque ablated by rotational atherectomy visualised by optical coherence tomography. EuroIntervention. 2015;11:e1.
Chambers JW, Feldman RL, Himmelstein SI, Bhatheja R, Villa AE, Strickman NE, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7:510–8.
Kang SJ, Ahn JM, Song H, Kim WJ, Lee JY, Park DW, et al. Comprehensive intravascular ultrasound assessment of stent area and its impact on restenosis and adverse cardiac events in 403 patients with unprotected left main disease. Circ Cardiovasc Interv. 2011;4:562–9.
Hong MK, Mintz GS, Lee CW, Park DW, Choi BR, Park KH, et al. Intravascular ultrasound predictors of angiographic restenosis after sirolimus-eluting stent implantation. Eur Heart J. 2006;27:1305–10.
Kang S-J, et al. Intravascular ultrasound predictors for edge restenosis after newer generation drug-eluting stent implantation. Am J Cardiol. 2013;111:1408–14.
Liu J, Maehara A, Mintz GS, Weissman NJ, Yu A, Wang H, et al. An integrated TAXUS IV, V, and VI intravascular ultrasound analysis of the predictors of edge restenosis after bare metal or paclitaxel-eluting stents. Am J Cardiol. 2009;101:501–6.
Morino Y, Tamiya S, Masuda N, Kawamura Y, Nagaoka M, Matsukage T, et al. Intravascular ultrasound criteria for determination of optimal longitudinal positioning of sirolimus-eluting stents. Circ J. 2010;74:1609–16.
Kimura M, Mintz GS, Carlier S, Takebayashi H, Fujii K, Sano K, et al. Outcome after acute incomplete sirolimus-eluting stent apposition as assessed by serial intravascular ultrasound. Am J Cardiol. 2006;98:436–42.
Steinberg DH, Mintz GS, Mandinov L, Yu A, Ellis SG, Grube E, et al. Long-term impact of routinely detected early and late incomplete stent apposition: an integrated intravascular ultrasound analysis of the TAXUS IV, V, and VI and TAXUS ATLAS workhorse, long lesion, and direct stent studies. J Am Coll Cardiol Intv. 2010;3:486–94.
Guo N, Maehara A, Mintz GS, He Y, Xu K, Wu X, et al. Incidence, mechanisms, predictors, and clinical impact of acute and late stent malapposition after primary intervention in patients with acute myocardial infarction: an intravascular ultrasound substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Circulation. 2010;122:1077–84.
•• Choi KH, Song YB, Lee JM, Lee SY, KyuPark T, Yang JH, et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in patients undergoing complex procedures. JACC Cardiovasc Interv. 2019;12:607–20 In a prospective review, Choi et al. demonstrated that, in patients undergoing predefined “complex” PCI procedures (including PCI for LMCAD), IVUS reduced the risk of all-cause death, myocardial infarction, stent thrombosis, and target vessel revascularization over angiography guidance alone at 64 months of follow-up.
de Jaegere P, Mudra H, Figulla H, et al. Intravascular ultrasound-guided optimized stent deployment: immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting In Coronaries Study (MUSIC Study). Eur Heart J. 1998;19:1214–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David Elison has nothing to disclose.
Antoniette Birs has nothing to disclose.
Jie Zhao has nothing to disclose.
Ravi Hira receives personal fees from Abbott Vascular.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Elison, D., Birs, A., Zhao, J. et al. Intravascular Ultrasound and Optical Coherence Tomography in the Procedural Planning and Execution of Left Main Coronary Artery Percutaneous Coronary Intervention. Curr Cardiovasc Imaging Rep 12, 25 (2019). https://doi.org/10.1007/s12410-019-9506-4
Published:
DOI: https://doi.org/10.1007/s12410-019-9506-4