Abstract
Purpose of Review
The scope of this text is to critically review the most important recent advances in the field of optical coherence tomography, both from a clinical, scientific, and technical point of view.
Recent Findings
In recent years, important steps forward have been put in the field of optimization of PCI (with a focus on optimal sizing of stents and optimization of stent expansion after implantation) and the differentiation and its possible applications of various underlying morphologic characteristics of acute coronary syndrome lesions. Several efforts have been made to elucidate underlying mechanical causes of stent thrombosis, based on optical coherence tomography (OCT) imaging. This had led to the recognition of neoatherosclerosis as an important cause for very late stent thrombosis, fueling new research into this area and to the development of intracoronary devices which could be even more safe for patients on the very long term.
Summary
With an ever increasing use for clinical and scientific applications in coronary artery disease, OCT has come to a mature and solid tool in the armamentarium of the coronary artery disease specialist. With new areas deserving more intensified focus and several innovations ahead, it seems that OCT is there to defend its position as the standard intracoronary imaging modality for the next decennium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the course of the last decade, optical coherence tomography (OCT) has been adapted enthusiastically by the interventional community, both for clinical and scientific applications. This is reflected in an exponential rise of the number of peer-reviewed manuscripts in the field over the course of the last years, reaching almost 500 publications in 2016 alone.
The scope of this review is to discuss some of the publications related to OCT which impacted distinctly on the way we practice PCI currently, had a dominant influence on future studies and research work, or offered important new insights into the pathophysiology of coronary artery disease and/or the effect of its different treatment modalities. In a separate section, we focus on important new technological advancements in the field, such as combined imaging methods and keep an eye on future innovations to be expected.
Part A: recent developments in the clinical use of OCT
Important advancements have been seen in the domain of optimization of PCI. Although important steps still need to be taken, the recently published ILUMIEN III trial marks important novelties, especially dealing with the methodology by which OCT is used, for the first time also exploiting the power of OCT in the process preceding actual stent implantation, focusing on the choice of the appropriate size and length of the stent [1••].
Of equal importance is to be considered the EROSION trial [2•], which studied a non-interventional treatment of patients presenting with an acute coronary syndrome in whom plaque erosion (and not plaque rupture) had been identified as an underlying plaque morphology, a concept that had been explored earlier by Prati et al. [3].
A third field which has seen important contributions is the study of the underlying mechanisms of stent failure using OCT. Results from three independent research groups (PESTO, the Bern-Fribourg-Paris-Kopenhagen group, and PRESTIGE) revealed the importance of stent underexpansion and malapposition (fueling arguments for an expanded role of PCI optimization) as underlying mechanisms for stent thrombosis and discovered a high prevalence of neoatherosclerosis (lipid accumulation within stented segments) in cases of very late stent thrombosis [4,5,6].
In-stent neoatherosclerosis was first reported by Nakazawa et al. in a pathology series of stented coronary artery segments [7]. In the subsequent years, OCT has proven extremely useful in delivering new insights into the incidence, predictors for, and pathophysiology of this newly discovered, but very important, entity.
In the field of the development of bioresorbable scaffolds as well, the role of OCT has been substantial, delivering insights into the different stages of the resorption process, enhancing safety of the use of the device through optimization and guidance of the implantation procedure and again, delivering mechanistical insights into the origin of scaffold thrombosis, a currently highly debated issue.
OCT-guided Optimization of PCI
Intuitively, the value of OCT as an adjunctive tool during a PCI procedure, with its unsurpassed detail on the lumen-vessel wall border and stent struts, has been appreciated by many operators. The scientific body of evidence supporting this use is now increasing, step by step.
In the CLI-OPCI study, a non-randomized comparison of 670 patients undergoing PCI with or without OCT guidance, the use of OCT guidance was associated with a significantly lower risk of cardiac death or MI [8]. However, even if in the analysis, adjustments were made for baseline and procedural differences between groups, it was obvious that these results would need confirmation in further investigations.
A second important step was the non-randomized 418-patient ILUMIEN I study, which revealed that physician decision-making was affected by OCT imaging prior to PCI in 57% and post-PCI in 27% of all cases [9]. The information provided by the pre-intervention OCT acquisition led to the selection of a shorter/longer stent length in respectively 25 and 43% of cases and a smaller/larger stent diameter in respectively 31 and 8% of cases.
The methodology and protocol of PCI optimization were further elaborated in the ILUMIEN III trial [1••], in which 450 patients were randomly assigned to guidance of PCI by IVUS, OCT or angiography alone. An important focus in ILUMIEN III was on the use of OCT for selection of diameter and length of the stent to be implanted. To accommodate for a problem between lumen vs. vessel sizing in earlier comparative studies between OCT and IVUS, which had systematically led to selection of smaller stent sizes in the OCT group, leading to smaller stent areas, a new concept of stent sizing with OCT, based on EEL (external elastic lamina) to EEL measurements, was introduced. The requirement of the EEL being visible in ≥180° of the vessel circumference in both the proximal and the distal landing zone was met in a substantial number of patients. If this requirement could not be met, stent diameter was decided by OCT automation based on the smallest mean lumen diameter at the reference site. The decision of stent length was based on the selection of appropriate landing zones in longitudinal OCT views. With respect to optimization of the result after stent implantation, an important focus was on correction of stent underexpansion. To accommodate for the problem of vessel tapering in longer stented segments, often leading to comparison of the proximal stent segment with relatively small distal edge reference areas, a separate assessment of the distal and the proximal half of the stented segment was introduced. Furthermore, in contrast to earlier studies using 70 or 80% criteria, ILUMIEN III set the bar higher, with a requirement of at least 90% expansion of the stent compared to the respective reference areas of both of these halves of the stented segment. There were additional criteria for the correction of edge dissections, malapposition, and plaque/thrombus protrusion. Using this methodology, the study met its primary endpoint proving non-inferiority of the specific OCT-based stent optimization strategy compared to IVUS-guided PCI. Furthermore, there were fewer untreated major dissections and areas of stent malapposition. The robust methodology used in this study will form the basis of the upcoming large-scale ILUMIEN IV trial.
Other important contributions come from authors focusing on the choice of the optimal landing zones of stented segments. In an elegant study by Ino et al., evidence was delivered for the importance of avoiding stents landing at the level of a lipid pool. Failing to do so resulted in an elevated risk for periprocedural myocardial infarction and a substantially higher risk for the development of edge restenosis [10].
Assessment of Plaque Morphology in ACS
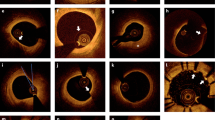
Detailed descriptions of different morphologies of acute coronary syndrome (ACS) lesions had been described in the early years after the clinical introduction of OCT (Fig. 1). A further step forward has been made recently, introducing the concept of a tailored therapy for patients presenting with ACS, based on OCT-derived morphologic characteristics of the culprit lesion. The application of a conservative, only medical therapy for non-severely narrowed culprit lesions with OCT characteristics of plaque erosion (characterized by an intact fibrous cap of the culprit lesion) was first described in a small series of patients by Prati et al. [3]. In the larger-number EROSION study, this concept was elaborated further [2•]. From a pool of patients with ACS (predominantly STEMI) undergoing PCI, 405 had OCT images suitable for culprit lesion evaluation. Consistent with earlier observations, a quarter of these lesions were diagnosed as plaque erosion [11]. Patients with residual diameter stenosis ≥70% on coronary angiogram did not qualify for inclusion in the study. Finally, 60 patients were enrolled and received a medical treatment with dual antiplatelet therapy without stent implantation, of whom 55 completed 1-month follow-up. The majority (more than three quarters) of patients met the primary endpoint (>50% reduction of thrombus volume). All except two patients treated with anti-thrombotic therapy without stent implantation remained free of MACE. This study marks an important proof-of-concept and opens the way towards larger, randomized studies, comparing a medical vs. interventional approach for this type of lesions. It must be acknowledged, however, that it can be difficult (especially in cases with an important amount of residual thrombus during OCT acquisition) to make a firm and definite diagnosis of plaque erosion. In fact, given even the resolution of OCT that is not low enough to discriminate individual endothelial cells, plaque erosion will always be an exclusion diagnosis, made in the absence of cap rupture and provided that there is good image quality without excessive remaining thrombus load.
OCT characteristics of acute coronary syndrome. a Ruptured thin-cap fibroatheroma (TCFA). The lipid pool is indicated with L. There is a thin cap covering the lipid pool, with signs of rupture (indicated with r) and small thrombi in the lumen. b Plaque erosion. Thrombus with irregular contour in the lumen. There are no signs of TCFA or rupture. c Calcified nodule. There is a large calcified burden (indicated with c), of which some part is protruding into the lumen of the vessel. The asterisk is indicating the guidewire artifact
Based on earlier evidence of an increased risk of stent thrombosis in patients treated for ACS compared to stable angina, and more recently, an OCT observation of reduced healing after DES implantation in plaque erosion compared to plaque rupture lesions, there is certainly an important incentive to further investigate this hypothesis [12].
TCFA
Another field that will probably benefit from further investigation with OCT is the study of the natural course and potential modulation of vulnerable coronary plaques. Characterized by a thin fibrous layer overlying a lipid core, these plaques are considered prone to rupture and cause acute coronary syndromes. Over the course of the last years, several attempts have been made to study the possible influence of intensive lipid therapy on specific features of plaque vulnerability such as the thickness of the overlying cap [13]. However, some methodologic hurdles such as a considerable variability in the assessment of cap thickness and practical problems such as the large number of patients needed for studies trying to connect these morphologic changes with clinical outcomes of patients [14]. The start of a new era of lipid lowering therapy, with the introduction of PCSK9 inhibitors into clinical practice, marks new opportunities for studying the influence of cholesterol metabolism on plaque morphology with OCT.
OCT in Stent Thrombosis
Three different research groups have assessed the value of OCT in the assessment of mechanistic causes for stent thrombosis [4,5,6]. While they differed slightly in number of patients included and methodology used, there were important parallels in the main findings of the three studies. First, it appeared that, despite the fact these patients usually present with STEMI, it was possible to achieve good quality acquisitions in the great majority of cases. In most of these cases, based on OCT imaging, a dominant and several contributing factors for ST could be attributed (Fig. 2). With malapposition and underexpansion detected in a substantial amount of cases, these studies provide an indirect support for the concept of OCT-guided PCI optimization. Perhaps the most important finding of all three studies was the dominant role of neoatherosclerosis in the origin of very late stent thrombosis (VLST). Neoatherosclerosis could be observed as a mechanism of VLST in BMS, early- and new-generation DES.
OCT mechanisms underlying stent thrombosis. a Severe underexpansion (when compared with proximal and distal reference area) and persistence of uncovered struts in a segment with multiple layers of struts. b Persistence of uncovered struts and struts covered with thrombus. c Neoatherosclerosis with rupture. Lipid infiltration of the neointima is indicated with L, the site of rupture with r. The asterisk is indicating the guidewire artifact
Neoatherosclerosis
In-stent neoatherosclerosis was first reported by Nakazawa et al. in a pathology series of stented coronary artery segments [7]. It was recognized as clusters of lipid-laden foamy macrophages within the neointima with or without necrotic core formation and appeared to occur more frequently and earlier in DES than in BMS.
In the subsequent years, OCT has proven extremely useful in delivering new insights into the incidence, predictors for, and pathophysiology of this newly discovered, but very important phenomenon, associated with different forms of late stent failure, stent thrombosis, and in-stent restenosis (ISR) [15].
While only observed as a cause for VLST in the specific ST OCT registries, a study from Korea suggests that early stages of development of neoatherosclerosis can already be observed before the first year after stent implantation, with hypertension and high pre-stent LDL cholesterol at the time of the index procedure as independent predictors of this phenomenon [16]. Another study confirmed high LDL cholesterol levels as a risk factor for the development of neoatherosclerosis and determined that patients in whom neoatherosclerosis was observed during a routine OCT examination >1 year after stent implantation had a higher incidence of major adverse cardiac events at follow-up [17].
In a substudy of the SIRTAX-LATE OCT study, in-stent neoatherosclerosis appeared to be more common among patients with angiographic and clinical evidence of native atherosclerosis progression, a finding highly suggestive of a similar pathophysiologic background [18].
Recently, several OCT reports have described neoatherosclerosis as a possible mechanism for late bioresorbable scaffold failure [19,20,21]. These are important findings, as these devices were introduced with the aim at eliminating the risks associated with permanent metal caging and the associated long-term healing reactions.
BVS
Being clinically available from the early times of first in-human implantations of current generation bioresorbable scaffolds, OCT has played a pivotal role in our understanding of the behavior of these devices at the time of initial deployment in the vessel wall, the specific characteristics of resorption of the device over time, and more recently, the reasons why these devices can fail in certain cases, leading to scaffold dismantling, thrombosis, or restenosis [19, 22•] (Fig. 3).
Independent series by Karanasos et al. and Sotomi et al. reported a high incidence of malapposition, underexpansion, and incomplete lesion coverage in their series of cases of scaffold thrombosis studied with OCT, providing again a strong argument for PCI optimization using OCT when implanting these devices and providing hope for a reduced incidence of thrombosis with future thinner strut alterations of the currently used devices [23•, 24].
OCT and DAPT
The optimal duration of duration of dual antiplatelet therapy (DAPT) after stent implantation has been a heavily debated clinical issue over the course of the last years. Although hoped for by some, it is unlikely that a strategy in which the assessment of stent strut coverage by OCT would guide further duration of DAPT. Not only is OCT an invasive imaging modality, associated with the inherent risks and costs of an interventional procedure, but it would also take a very high number of patients and a considerable duration of follow-up for a trial investigating the correlation of the burden of uncovered struts with a relatively rare potential adverse outcome such as stent thrombosis. Moreover, recent studies have shown that the healing process of the vessel wall after stent implantation is a dynamic process, with regions with uncovered struts getting covered later in follow-up, well-apposed struts turning malapposed and zones with a healthy-appearing neointimal coverage sometimes degrading into neoatherosclerotic lesions. This makes it even hard to make long-term assumptions on long-term DAPT therapy based on an intermediate snap-shot image [25].
However, the registries on OCT-derived mechanisms of stent thrombosis described above have delivered adequate explanations for some phenomena observed in the DAPT trial, such as the apparently inevitable catch-up in cases of stent thrombosis after discontinuation of DAPT, whether after 12 or 30 months (most probably explained by one or more mechanical or healing problems at the level of the stented segment, such as underexpansion, malapposition, stent deformation, or the persistence of a burden of uncovered struts, triggering sudden thrombotic occlusion of the stent immediately following withdrawal of the antiplatelet drug, irrespective of the time since implantation of the device) [26]. The observation of neoatherosclerosis being the dominant mechanism of stent thrombosis in a substantial amount of cases of VLST is a plausible explanation for the fact that stent thrombosis can occur at long term after implantation, even with the patient still being on DAPT.
On the other hand of the spectrum, OCT studies indicating clearly incomplete healing of stent struts in the short term after stent implantation and a considerable catch-cup in strut coverage in currently used second-generation DES, all along the first months after implantation [27], help balancing the discussion with proponents of ever-shortening DAPT, to as low as even 1 month, based on studies definitely underpowered for rare thrombotic events.
Part B: technological developments in the field of OCT
Combination of OCT and IVUS
OCT and IVUS are commercially available intravascular imaging modalities for the visualization of coronary arteries. OCT utilizes a high-speed, near-infrared (i.e. central wavelength of approximately 1.3 μm) laser and conventional IVUS, an ultrasound transducer at a frequency of approximately 40 MHz [28, 29]. The main differences between IVUS and OCT are the mode of acquisition, resolution, and tissue penetration.
IVUS data are acquired using a slow speed pullback (typically a few millimeters per second), while OCT dataset acquisition requires blood displacement through a short contrast injection and data are acquired in 2 to 3 s at higher pullback speeds (up to 40 mm/s).
The axial resolution of OCT is approximately 15 μm in tissue, conventional 40 MHz IVUS has a resolution of 100 μm, and HD-IVUS (i.e. a high resolution 60 MHz IVUS technique recently introduced to the market) approximately 40 μm at a price of reduced tissue penetration. The higher resolution of OCT allows to visualize fine features that are not visible to IVUS, including an improved visualization of stent strut apposition and coverage, plaque fibrotic cap, small dissections, and thrombi [28]. The higher tissue penetration of IVUS has been used in the clinic to estimate the lipid/necrotic plaque burden for a given coronary segment. However, it is important to remark that OCT offers a better tissue penetration in calcium, and it is capable of visualizing the inner and outer border of the plaque where IVUS cannot. This has been demonstrated to be a clinically relevant feature that provides guidance for the intervention of calcified arteries [30].
The combination of both modalities in a single catheter allows for the acquisition of high-resolution data and the quantification of calcified plaque extension and plaque burden at the same time. Several research groups demonstrated the feasibility of a dual-modality catheter for the simultaneous acquisition of OCT and IVUS [31, 32]. Although OCT imaging can achieve a very fast frame rate (the feasibility of OCT imaging during diastolic phase at more than 4000 images per second has been demonstrated [33]), IVUS limits the maximum frame rate of a hybrid catheter to approximately 100 frames/s, to maintain ultrasound tissue penetration.
Figure 4 shows an example of an IVUS-OCT system reproduced from Li et al. [34]. A dual-modality system is obtained by combining a conventional OCT system with an ultrasonic pulser/receiver. The hybrid catheter is composed by a torque cable, hosting both the OCT optical fiber and the IVUS electrical wires. The torque cable is encapsulated within a transparent outer sheath (as normally done in standalone OCT and IVUS solutions), to protect the vessel during torque cable rotation and pulling back. The OCT optics and the IVUS transducer are sitting next to each other, enabling co-registered, simultaneous data acquisition. The feasibility of a 2.7F catheter potentially suitable for clinical imaging has been demonstrated, enabling simultaneous in vivo imaging at 72 frames/s at a pullback speed of 18 mm/s [34]. Examples of OCT-IVUS images acquired in ex vivo cadaver arteries are shown in Fig. 5, illustrating potential benefits of IVUS and OCT complementary imaging. The translation to the clinic of hybrid OCT-IVUS solutions can be expected in a near-future.
a Example of a combined OCT and IVUS system. Conventional OCT and IVUS systems are combined at the level of the pullback console. Data from the two different systems are collected simultaneously by a single digitizer and subsequently processed into an image. b, c An example of simultaneous OCT and IVUS imaging, respectively. d An example of a hybrid 2.7F catheter design, combining the OCT optics and the IVUS transducer at the end of a torque-cable. This image is reproduced from a paper from Li et al., published in the journal Scientific Reports [34]
Examples of simultaneous OCT and IVUS imaging of an ex vivo human coronary plaque. a, b Movat’s pentachrome and Hematoxylin-Eosin histology sections, respectively. A calcified plaque is visible between 12 and 9 o’clock and a lipid-rich plaque between 3 and 10 o’clock. OCT imaging (d) shows high-resolution details of the plaque fibrotic cap as well as the calcium extension between 3 and 6 o’clock. The IVUS image (c) complements OCT, visualizing lipid-rich plaque burden. This image is reproduced from an article by Li et al., from the journal Catheterization and Cardiovascular interventions [32]
Combination of OCT with NIRS
OCT has been extensively used for the assessment of lipid-rich plaques and thin-cap fibroatheromas (TCFA) [35]. Lipid-rich plaques appear to OCT having diffused borders and a rapid signal decay [28, 36]. This is due to lipid optical properties in the near-infrared, showing both elevated optical attenuation and backscattering coefficients [10]. The characterization of lipid-rich plaques is typically based on image interpretation, which is intrinsically a subjective assessment. Automated methods for the analysis of lipids have also been proposed [37, 38], and the accuracy of OCT to detect lipid has been the matter of several studies [36, 39]. Despite the fact that a good reproducibility and accuracy has been shown, recent research suggested that other vessel components, including macrophage accumulations, calcified nodules, smooth muscle cells, rich fibrous tissue, proteoglycans, and loose connective tissue, may present similar optical features [40]. Additionally, OCT lipid assessment can be further confounded by the presence of image artifacts [41].
The combination of OCT with near-infrared spectroscopy (NIRS) has been proposed to address some of the limitations illustrated above and the feasibility of an OCT-NIRS catheter demonstrated [42]. NIRS typically uses a broad-bandwidth laser source, exploiting the fact that lipids show a peak of absorption approximately at 1200 nm and a second peak around 1400 nm. Near-infrared light is sent to the vessel wall, it propagates through the tissue, and it is collected at a second location (i.e. illumination and collection are separated). The spectrum of the collected light is analyzed, and the presence of lipid-rich tissue is determined. Commercial IVUS-NIRS technology typically displays NIRS as a ring around the IVUS cross-sectional image and as a 2D map (the so called chemogram), illustrating the probability of lipid distribution for an entire coronary segment. Similar solutions have been adopted for the visualization of OCT-NIRS imaging (Fig. 6). The use of NIRS imaging modality has been investigated in several studies and clinical trials [43,44,45], and it is commercially available for use in the coronary arteries in combination with IVUS. NIRS holds potential to complement OCT imaging for an improvement assessment of lipid-rich plaques.
An example of OCT-NIRS imaging, suggesting the capability of near-infrared spectroscopy to complement OCT for a better identification of lipid-rich plaques. Image (a) shows an example of fibrotic plaque and (b) an example of lipid plaque between 4 and 7 o’clock. OCT identification of lipid is based on image interpretation, which may be affected by inter- and intra-operator variability. This image is reproduced with permission from an article by Fard et al., published in the journal Optics Express [42]
Combination of OCT with Molecular Imaging (NIRF)
As discussed in the previous sections, OCT has the capability of visualizing vessel features in high-resolution depicting in details lumen morphology and tissue microstructure. OCT can provide information about tissue type, discriminating the main plaque components including fibrotic, calcific, and lipid-rich plaques. Despite its high resolution, OCT cannot provide details on the functional state of the artery wall nor provide details about molecular and biological processes of atherosclerosis. It has been recognized that the underlying mechanisms of coronary plaque development involve a complex interaction between mechanical, structural, compositional, cellular, and molecular processes in the vessel wall [46]. Near-infrared fluorescence molecular imaging (NIRF) has the capability to detect vessel wall molecular features, including the state of inflammation of the vessel wall, and other specific molecular components such as fibrin deposition.
The combination of NIRF with OCT has recently been illustrated, and a dual-modality catheter has been developed to have the same size and imaging speed with respect to conventional OCT [47•]. The core technology for simultaneous OCT-NIRF imaging is obtained by replacing the single-mode fiber used for standalone OCT catheters with a double clad optical fiber, capable of transmitting OCT and NIRF light at the same time, with no significant changes to catheter optics [47•, 48]. For the detection of a specific molecule, NIRF molecular imaging agents can be injected prior to intervention. Among multiple agents with different molecular targets, the detection of fibrin deposition with OCT-NIRF to monitor BMS and DES healing process has been demonstrated [49]. Figure 7 shows an example of OCT-NIRF imaging of fibrin deposition. In this study, authors demonstrated that NIRF molecular imaging can improve the detection of unhealed stents complementing standalone OCT imaging. Regulatory approval for the use of NIRF molecular agents will be required for the clinical translation of OCT- and NIRF-targeted molecular imaging.
An example of NIRF molecular imaging of fibrin deposition on metallic stents obtained in vivo, using an injectable molecular agent. In this study, OCT-NIRF was used to visualize drug-eluting stents (DES) more elevated fibrin deposition with respect to bare metal stent (BMS) at day 7 after implantation in a rabbit model. Image (a) shows the 2D NIRF fluorescence map for the entire vessel segment along the longitudinal direction (i.e. the vertical axis corresponds to catheter rotation between 0° and 360°, and the horizontal axis is the pullback direction). A higher signal (yellow color) corresponds to a high fibrin deposition. High correlation with fluorescence reflectance imaging (c) and fluorescence microscopy (d) of the excised vessel can be observed. e, f Representative cross-sectional imaging from the BMS and DES stent edges, respectively. Histological cross sections using Carstairs’ stain (g, h) demonstrates good correlation with fibrin-negative and fibrin-positive OCT-NIRF images. Scale bars are equal to 1 mm. This figure is reproduced with permission from an article published by Hara et al. in the European Heart Journal [49]
Similarly to NIRF, the detection of vessel wall auto-fluorescence (NIRAF) has been investigated. Recent studies showed feasibility of NIRAF excited in the visible 630–650 nm range and collected in the near-infrared between 700 and 900 nm for ex vivo human coronary plaques. Authors demonstrated that NIRAF is elevated in lesions that contain a necrotic core and suggested a possible correlation between near-infrared autofluorescence, plaque inflammation, and oxidative stress [50]. The advantage of using plaque autofluorescence is that the injection of a fluorescent molecular agent is not required and endogenous tissue fluorescence is used. This allowed to translate dual-modality OCT-NIRAF technology to the clinic performing a study in a series of 12 patients undergoing PCI (Fig. 8). The results of this studies demonstrated for the first time that dual-modality microstructural and fluorescence imaging can be safely conducted in patients and that NIRAF provides complementary information to OCT structural imaging [51•]. Further research needs to be conducted to elucidate the molecular mechanisms that originate NIRAF signal and the clinical significant of these results.
Clinical OCT-NIRAF (a), fluoroscopy, imaging of atherosclerosis. b 2D auto-fluorescence longitudinal map, depicting the distribution of the NIRAF signal within a long segment of the LAD. A high signal is observed in the proximal segment of the artery, and the cross-sectional OCT-NIRAF images depict a case of plaque rupture (c–h). OCT images show the presence of a large TCFA, having a high NIRAF signal co-localized with the plaque rupture location. This image is reproduced with permission from an article by Ughi et al., published in the Journal of American College of Cardiology Cardiovascular Imaging [51•]
μOCT
The resolution of conventional OCT for intravascular imaging is limited to approximately 10–15 μm. Micro-OCT (or μOCT) has been proposed to improve the spatial resolution of OCT demonstrating imaging at a resolution of approximately 2 μm [52]. Images acquired in ex vivo coronary plaques illustrated the capability of μOCT to visualize features that are not visible to traditional OCT, providing additional cellular and chemical information of atherosclerosis (Fig. 9). Visualization of foam cells or macrophages, cholesterol crystals, smooth muscle cells, fibrin, platelet aggregation, and microcalcifications has been demonstrated (Fig. 9). Additionally, the unique resolution of μOCT has been shown to provide an improved visualization of intracoronary stents, highlighting previously unseen details of stent endothelial coverage, fibrin accumulation, and drug-eluting stent polymer.
Examples of μOCT ex vivo imaging, showing the benefits of higher image resolution for the visualization of the fine details of vessel wall features. a and b compare conventional OCT imaging to μOCT for a calcific plaque. d Ex vivo μOCT imaging of a necrotic core fibroatheroma, showing evidence of foam cell or macrophages infiltration (arrows and image inlet). e Illustration of μOCT imaging of smooth cells and f drug-eluting stent, showing details of the polymer covering the metallic strut (image inlet). These images are adapted with permission from an article from Liu et al., published in the journal Nature Medicine [52]
Key features of μOCT are the use of a very broad bandwidth laser (having a wavelength approximately between 650 and 950 nm) and sample arm optics with high numerical aperture (NA) [52]. The current challenges for the translation of this novel technology to the clinic are the miniaturization of the complex optics required by μOCT in a probe having both a size <3F and reduced rigid length, making it suitable for navigation and deliverability in coronary arteries. Additionally, as a high NA produces a short depth of focus (DOF), a solution for catheter optics capable of extending the DOF to more than 1 mm is necessary to obtain sufficient image field-of-view for in vivo applications. Recent studies illustrated the development of a μOCT imaging probe using a cylindrical waveguide to divide the wavefront to provide multiple circular propagation modes and achieve a field-of-view of approximately 1 mm [53]. A second study proposed the use of a binary spatial filter and was able to achieve similar results [54]. First in vivo studies using μOCT endovascular imaging technologies are expected in a near-future.
Conclusion
It is fair to say that OCT has revolutionized the field of intracoronary imaging over the course of the last decade. With major advances in stent optimization strategy, insights into different morphologies of acute coronary syndromes, and mechanisms of intracoronary stent and scaffold failure, OCT has become an indispensable tool for the interventional cardiologist as well as the clinical researcher in the field of coronary artery disease.
With respect to technological innovations, several breakthroughs in combined imaging modalities, as illustrated with the in vivo in human application of OCT-NIRAF, are to be expected for the next years.
Abbreviations
- BMS:
-
Bare metal stent
- BVS:
-
Bioresorbable vascular scaffold
- ACS:
-
Acute coronary syndrome
- DAPT:
-
Dual antiplatelet therapy
- DES:
-
Drug-eluting stent
- EEL:
-
External elastic lamina
- ISR:
-
In-stent restenosis
- IVUS:
-
Intra-vascular ultra sound
- LDL:
-
Low-density lipoprotein
- MACE:
-
Major adverse cardiovascular events
- OCT:
-
Optical coherence tomography
- PCI:
-
Percutaneous coronary intervention
- PCSK-9:
-
Proprotein convertase subtilisin/kexin type 9
- ST:
-
Stent thrombosis
- STEMI:
-
ST segment elevation myocardial infarction
- TCFA:
-
Thin-cap fibro atheroma
- VLST:
-
Very late stent thrombosis
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Ali ZA, Maehara A, Genereux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet (London, England). 2016;388(10060):2618–28. This trial has established a firm methodology for analysis of OCT images pre and post stent implantation. The large number of patients to which it proved to be applicable makes this trial the standard for further research and clinical work in PCI optimization using OCT.
• Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, et al. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur Heart J. 2016;38(11):792–800. This study is a seminal work in an area considered complex by many interventional cardiologists
Prati F, Uemura S, Souteyrand G, Virmani R, Motreff P, Di Vito L, et al. OCT-based diagnosis and management of STEMI associated with intact fibrous cap. JACC Cardiovascular imaging. 2013;6(3):283–7.
Souteyrand G, Amabile N, Mangin L, Chabin X, Meneveau N, Cayla G, et al. Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J. 2016;37(15):1208–16.
Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia-Garcia HM, Yamaji K, et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133(7):650–60.
Adriaenssens T JM, Godschalk T, Malik N, Alfonso F, Xhepa E, De Cock D, Komukai K, Tada T, Cuesta J, Sirbu V, Feldman L, Neumann FJ, Goodall A, Heestermans T, Buysschaert I, Hlinomaz O, Belmans A, Desmet W, ten Berg J, Gershlick A, Massberg S, Kastrati A, Guagliumi G, Byrne R, editor Optical Coherence Tomography Findings in Patients Presenting with Definite Coronary Stent Thrombosis. Results from the PREvention of ST by an Interdisciplinary Global European effort (PRESTIGE) OCT study. Transcatheter Technology Conference 2015; 2015.
Nakazawa G, Otsuka F, Nakano M, Vorpahl M, Yazdani SK, Ladich E, et al. The pathology of neoatherosclerosis in human coronary implants bare-metal and drug-eluting stents. J Am Coll Cardiol. 2011;57(11):1314–22.
Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-optimisation of percutaneous coronary intervention (CLI-OPCI) study. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2012;8(7):823–9.
Wijns W, Shite J, Jones MR, Lee SW, Price MJ, Fabbiocchi F, et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J. 2015;36(47):3346–55.
Ino Y, Kubo T, Matsuo Y, Yamaguchi T, Shiono Y, Shimamura K, et al. Optical coherence tomography predictors for edge restenosis after Everolimus-eluting stent implantation. Circulation Cardiovascular interventions. 2016;9(10):e004231.
Higuma T, Soeda T, Abe N, Yamada M, Yokoyama H, Shibutani S, et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovascular interventions. 2015;8(9):1166–76.
Hu S, Wang C, Zhe C, Zhu Y, Yonetsu T, Jia H, et al. Plaque erosion delays vascular healing after drug eluting stent implantation in patients with acute coronary syndrome: an in vivo optical coherence tomography study. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2017;89(S1):592–600.
Hou J, Xing L, Jia H, Vergallo R, Soeda T, Minami Y, et al. Comparison of intensive versus moderate lipid-lowering therapy on fibrous cap and atheroma volume of coronary lipid-rich plaque using serial optical coherence tomography and intravascular ultrasound imaging. Am J Cardiol. 2016;117(5):800–6.
Kim SJ, Lee H, Kato K, Yonetsu T, Xing L, Zhang S, et al. Reproducibility of in vivo measurements for fibrous cap thickness and lipid arc by OCT. JACC Cardiovascular imaging. 2012;5(10):1072–4.
Imanaka T, Fujii K, Hao H, Shibuya M, Saita T, Kawakami R, et al. Ex vivo assessment of neointimal characteristics after drug-eluting stent implantation: optical coherence tomography and histopathology validation study. Int J Cardiol. 2016;221:1043–7.
Kim C, Kim BK, Lee SY, Shin DH, Kim JS, Ko YG, et al. Incidence, clinical presentation, and predictors of early neoatherosclerosis after drug-eluting stent implantation. Am Heart J. 2015;170(3):591–7.
Kuroda M, Otake H, Shinke T, Takaya T, Nakagawa M, Osue T, et al. The impact of in-stent neoatherosclerosis on long-term clinical outcomes: an observational study from the Kobe University Hospital optical coherence tomography registry. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2016;12(11):e1366–e74.
Taniwaki M, Windecker S, Zaugg S, Stefanini GG, Baumgartner S, Zanchin T, et al. The association between in-stent neoatherosclerosis and native coronary artery disease progression: a long-term angiographic and optical coherence tomography cohort study. Eur Heart J. 2015;36(32):2167–76.
Bennett J, Hiltrop N, Triantafyllis A, Adriaenssens T, Desmet W, Sinnaeve P, et al. Intraluminal scaffold dismantling: the downside of positive remodeling? J Am Coll Cardiol. 2016;67(22):2702–4.
Mangiameli A, Fajadet J, Dumonteil N. Bioresorbable vascular scaffold fracture in the overlapping zone as cause of accelerated neoatherosclerosis and in-scaffold very-late thrombosis. The international journal of cardiovascular imaging. 2017;33(1):1–3.
Hiltrop N, Jorge C, Bennett J, Adriaenssens T. Late neoatherosclerotic scaffold failure: an unexpected achilles heel for current bioresorbable scaffold technology? Int J Cardiol. 2016;223:133–5.
• Raber L, Brugaletta S, Yamaji K, O’Sullivan CJ, Otsuki S, Koppara T, et al. Very late scaffold thrombosis: intracoronary imaging and histopathological and spectroscopic findings. J Am Coll Cardiol. 2015;66(17):1901–14. An important description and illustration of mechanisms of very late scaffold failure.
• Karanasos A, Van Mieghem N, van Ditzhuijzen N, Felix C, Daemen J, Autar A, et al. Angiographic and optical coherence tomography insights into bioresorbable scaffold thrombosis: single-center experience. Circulation Cardiovascular interventions. 2015;8(5):e002474. This study was the first larger patient number series dealing with OCT mechanisms of scaffold failure.
Sotomi Y, Suwannasom P, Serruys PW, Onuma Y. Possible mechanical causes of scaffold thrombosis: insights from case reports with intracoronary imaging. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2017;12(14):1747–56.
Kim JS, Hong MK, Shin DH, Kim BK, Ko YG, Choi D, et al. Quantitative and qualitative changes in DES-related neointimal tissue based on serial OCT. JACC Cardiovascular imaging. 2012;5(11):1147–55.
Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371(23):2155–66.
Hashikata T, Tojo T, Namba S, Kitasato L, Hashimoto T, Kameda R, et al. Neointimal coverage of zotarolimus-eluting stent at 1, 2, and 3 months’ follow-up: an optical coherence tomography study. Heart Vessel. 2016;31(2):206–11.
Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the international Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59(12):1058–72.
Mintz GS. Clinical utility of intravascular imaging and physiology in coronary artery disease. J Am Coll Cardiol. 2014;64(2):207–22.
Mintz GS. Intravascular imaging of coronary calcification and its clinical implications. JACC Cardiovascular imaging. 2015;8(4):461–71.
Li X, Li J, Jing J, Ma T, Liang S, Zhang J, et al. Integrated IVUS-OCT imaging for atherosclerotic plaque characterization. IEEE journal of selected topics in quantum electronics : a publication of the IEEE Lasers and Electro-optics Society. 2014;20(2):7100108.
Li BH, Leung AS, Soong A, Munding CE, Lee H, Thind AS, et al. Hybrid intravascular ultrasound and optical coherence tomography catheter for imaging of coronary atherosclerosis. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2013;81(3):494–507.
Wang T, Pfeiffer T, Regar E, Wieser W, van Beusekom H, Lancee CT, et al. Heartbeat OCT: in vivo intravascular megahertz-optical coherence tomography. Biomedical optics express. 2015;6(12):5021–32.
Li J, Ma T, Mohar D, Steward E, Yu M, Piao Z, et al. Ultrafast optical-ultrasonic system and miniaturized catheter for imaging and characterizing atherosclerotic plaques in vivo. Sci Rep. 2015;5:18406.
Tian J, Dauerman H, Toma C, Samady H, Itoh T, Kuramitsu S, et al. Prevalence and characteristics of TCFA and degree of coronary artery stenosis: an OCT, IVUS, and angiographic study. J Am Coll Cardiol. 2014;64(7):672–80.
Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106(13):1640–5.
van Soest G, Goderie T, Regar E, Koljenovic S, van Leenders GL, Gonzalo N, et al. Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. J Biomed Opt. 2010;15(1):011105.
Ughi GJ, Adriaenssens T, Sinnaeve P, Desmet W, D’Hooge J. Automated tissue characterization of in vivo atherosclerotic plaques by intravascular optical coherence tomography images. Biomedical optics express. 2013;4(7):1014–30.
Miyamoto Y, Okura H, Kume T, Kawamoto T, Neishi Y, Hayashida A, et al. Plaque characteristics of thin-cap fibroatheroma evaluated by OCT and IVUS. JACC Cardiovascular imaging. 2011;4(6):638–46.
Phipps JE, Hoyt T, Vela D, Wang T, Michalek JE, Buja LM, et al. Diagnosis of thin-capped Fibroatheromas in intravascular optical coherence tomography images: effects of light scattering. Circulation Cardiovascular interventions. 2016;9(7):e003163.
White S, van Soest G, Johnson T. Development of tissue characterization using optical coherence tomography for defining coronary plaque morphology and the vascular responses after coronary stent implantation. Curr Cardiovasc Imaging Rep. 2014;7:9311. doi:10.1007/s12410-014-9311-z.
Fard AM, Vacas-Jacques P, Hamidi E, Wang H, Carruth RW, Gardecki JA, et al. Optical coherence tomography--near infrared spectroscopy system and catheter for intravascular imaging. Opt Express. 2013;21(25):30849–58.
Oemrawsingh RM, Cheng JM, Garcia-Garcia HM, van Geuns RJ, de Boer SP, Simsek C, et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J Am Coll Cardiol. 2014;64(23):2510–8.
Waxman S, Dixon SR, L’Allier P, Moses JW, Petersen JL, Cutlip D, et al. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. JACC Cardiovascular imaging. 2009;2(7):858–68.
Madder RD, Goldstein JA, Madden SP, Puri R, Wolski K, Hendricks M, et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovascular interventions. 2013;6(8):838–46.
Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30(7):1282–92.
• Yoo H, Kim JW, Shishkov M, Namati E, Morse T, Shubochkin R, et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat Med. 2011;17(12):1680–4. This publication is to be considered pioneering work in the field of combined structural and molecular imaging in vivo.
Ughi GJ, Verjans J, Fard AM, Wang H, Osborn E, Hara T, et al. Dual modality intravascular optical coherence tomography (OCT) and near-infrared fluorescence (NIRF) imaging: a fully automated algorithm for the distance-calibration of NIRF signal intensity for quantitative molecular imaging. The international journal of cardiovascular imaging. 2015;31(2):259–68.
Hara T, Ughi GJ, McCarthy JR, Erdem SS, Mauskapf A, Lyon SC, et al. Intravascular fibrin molecular imaging improves the detection of unhealed stents assessed by optical coherence tomography in vivo. Eur Heart J. 2015;48(16):4229–37.
Wang H, Gardecki JA, Ughi GJ, Jacques PV, Hamidi E, Tearney GJ. Ex vivo catheter-based imaging of coronary atherosclerosis using multimodality OCT and NIRAF excited at 633 nm. Biomedical optics express. 2015;6(4):1363–75.
• Ughi GJ, Wang H, Gerbaud E, Gardecki JA, Fard AM, Hamidi E, et al. Clinical characterization of coronary atherosclerosis with dual-modality OCT and near-infrared Autofluorescence imaging. JACC Cardiovascular imaging. 2016;9(11):1304–14. This study represents the first-in-man study using multi-modality optical coherence tomography in combination with molecular imaging in the coronary arteries.
• Liu L, Gardecki JA, Nadkarni SK, Toussaint JD, Yagi Y, Bouma BE, et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat Med. 2011;17(8):1010–4. Innovative technology, with astonishing high-detail images on coronary artery plaques and stent struts.
Yin BCK, Liang C-P, et al. μOCT imaging using depth of focus extension by self-imaging wavefront division in a common-path fiber optic probe. Opt Express. 2016;24:5555. doi:10.1364/OE24005555.
Kim J, Xing J, Nam HS, Song JW, Kim JW, Yoo H. Endoscopic micro-optical coherence tomography with extended depth of focus using a binary phase spatial filter. Opt Lett. 2017;42(3):379–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Giovanni J. Ughi declares that he has no conflict of interest.
Tom Adriaenssens reports consultancy fees from Abbott SJ Medical.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Adriaenssens, T., Ughi, G.J. Recent Advances in the Field of Optical Coherence Tomography. Curr Cardiovasc Imaging Rep 10, 23 (2017). https://doi.org/10.1007/s12410-017-9418-0
Published:
DOI: https://doi.org/10.1007/s12410-017-9418-0