Abstract
Intracoronary near-infrared spectroscopy (NIRS)-IVUS is a direct coronary imaging catheter for the identification and quantification of lipid core plaque (LCP). NIRS is combined with traditional grayscale IVUS in a hybrid catheter that provides co-registered architectural and compositional plaque characterization data. The novel capability of the NIRS catheter component is its ability to directly identify LCP, which underlies the majority of vulnerable and unstable plaques. NIRS-IVUS has potential to optimize percutaneous coronary intervention (PCI) by delineating precise lesion length, assuring adequate stent expansion and apposition, detection of stent edge dissection, and predicting periprocedural myocardial infarction (PMI). NIRS-IVUS may also be important in the identification of vulnerable plaques at risk to cause future events.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Limitations of Coronary Angiography and Importance of Plaque Composition: Need for Improved Imaging Techniques
Catheterization laboratory decision-making in individual patients requires challenging decisions regarding revascularization strategies (i.e., percutaneous coronary intervention (PCI) versus bypass surgery) and performance of PCI (e.g., choosing target lesions, identifying length of vessel to treat, optimization of stent deployment, and prevention of distal embolization and PMI).
Based on first principles, optimal stenting would fully cover the target lesion, landing stent edges in normal zones of the vessel. However, atherosclerosis is typically a diffuse process, with disease often extending beyond the critical stenosis. Failure to cover the full length of the target diseased coronary arterial segment with drug-eluting stent (DES) has been associated with increased rates of sub-acute stent thrombosis and late restenosis [1, 2, 3••, 4••], particularly if the uncovered lesion segment is composed of lipid core plaque (LCP) [5, 6]. Unfortunately, coronary angiography alone is inadequate. Although important to delineate the presence of disease and quantification of lumen stenosis, angiography underestimates the magnitude of atherosclerosis, particularly in early disease when positive vascular remodeling maintains “normal” lumen caliber despite substantial plaque in the vascular wall. In addition, angiography does not provide data on chemical composition of plaque.
Rationale for Direct Coronary Imaging
The ideal invasive tool for coronary plaque characterization should provide a complete roadmap of atherosclerotic burden and, in particular, the morphological and compositional data of individual plaques. Specific parameters should include (1) lumen stenosis; (2) lesion length; (3) coronary flow reserve; (4) intramural plaque architecture including plaque burden, eccentricity, and remodeling; (5) lipid content; (6) fibrous cap thickness; and (7) presence of inflammation.
Plaque composition has several clinical implications. In particular, the presence of a lipid pool appears to be an important determinant of plaque instability. LCP is implicated in more rapid lesion progression, propensity to plaque rupture, acute coronary syndromes, and sudden death [7–9, 10••, 11, 12], as well as distal embolization complications during PCI [13–21]. Recognition that non-flow limiting LCP may represent vulnerable plaque has resulted in a paradigm shift with regard to the pathophysiology of coronary artery disease, with the focus no longer exclusively on lumen stenosis. This growing need for information on plaque itself in order to identify patients and lesions at risk of complications during PCI, and for future adverse cardiac events, has been the primary driver of the development of novel intracoronary imaging methods able to detect plaque composition, in particular, the presence of a lipid pool.
Diffuse Reflectance Near-Infrared Spectroscopy (NIRS)
A single modality near-infrared intravascular spectroscopy (NIRS) catheter system (LipiScan™, InfraReDx, Inc., Burlington, MA, USA) was originally developed for invasive detection of LCP. Recognizing the need for multimodality imaging, a combined NIRS-IVUS catheter (InfraReDx, Inc., Burlington, MA, USA) has recently been introduced and approved by the FDA and has a CE mark (Fig. 1). This novel catheter allows complete characterization of coronary plaques, providing simultaneous and co-registered acquisition of structural and compositional information. The complementary nature of the NIRS compositional and IVUS structural information allow more complete characterization of coronary plaques [22, 23••, 24•, 25–27]. NIRS-IVUS identification of plaque architecture and composition has the potential to improve safety and long-term outcomes of PCI by (1) delineation of lesion length and adequate longitudinal stent coverage and axial expansion; (2) identification of large LCP, which are known to increase risk of distal embolization and PMI; and (3) detection of “vulnerable” LCP, those lesions posing greatest risk for future adverse events including acute coronary syndrome (ACS) and sudden death.
NIRS-IVUS system. NIRS-IVUS catheter system components including console, pullback and rotation device, and catheter are shown on the left hand side. a Chemogram. b Block chemogram. c Longitudinal IVUS view. d, e Cross-sections where IVUS and NIRS data are combined. NIRS = near-infrared spectroscopy, IVUS = intravascular ultrasound
NIRS Principles of Operation and Validation
In diffuse reflectance NIRS, a sample of interest is irradiated with near-infrared light and a detector measures the proportion of diffusely reflected light returned as a function of wavelength. Two quite different processes determine the amount of light that returns to the detector—scattering and absorption. Scattering is caused by alteration of its path by cellular and extracellular structures (larger than the wavelength of light) in the material. Absorption occurs when energy is absorbed by chemical bonds of the constituent molecules. Absorbed light is primarily transformed into molecular vibration energy contained within chemical bonds between atoms.
The NIRS-IVUS device comprises a scanning near-infrared laser and an ultrasound transducer coupled with a 3.2-F rapid exchange catheter (6 F guide compatible) with an entry profile of 2.4 F and a shaft profile of 3.6 F (TVC Insight™ Catheter), a pullback device (Nexus Controller™), and a console (TVC Imaging System™). The catheter can be inserted over a 0.014-in. guide wire. IVUS functions as a rotating transducer operating at 40 MHz and acquired at an automated rotational pullback speed of 0.5 mm/s together with simultaneous co-registered NIRS measurements. The majority of the NIRS tissue information is obtained from a depth of 1 mm or less in the direction from the luminal surface toward the adventitia. Overall, the system carries out more than 30,000 chemical measurements per 100 mm of the artery, which is consecutively scanned at a tissue depth of 1 mm over an area of 1–2 mm2. The radio-opaque marker on the NIRS catheter identifies the location of the catheter and imaging element in relationship to target vessel fiduciary landmarks as detected by coronary angiography (e.g., stenoses, side branches, guide catheter, etc.) with the possibility to mark and annotate anatomical landmarks at the locations of acquisition of the NIRS chemogram, thereby co-registering the two imaging modalities.
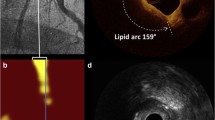
A predictive algorithm calculates the probability that LCP is present at each point along the artery. The data are immediately and automatically displayed on a two-dimensional map of the vessel called “chemogram.” The x-axis represents length of pullback in the artery and the y-axis represents degrees of rotation; a color scale from red to yellow indicates increasing algorithm probability that LCP is present (Fig. 2). The “block chemogram” provides a summary of the results for each 2 mm section of artery around its circumference at those longitudinal sections. The numerical value of each block in the block chemogram represents the 90th percentile of all pixel values in the corresponding 2 mm chemogram segment. The block chemogram is mapped to the same color scale as the chemogram and the display uses four discreet colors to aid in the visual interpretation of the algorithm probability of the presence of LCP in that 2 mm block (red: p < 0.57, orange: 0.57 ≤ p ≤ 0.84, tan: 0.84 ≤ p ≤ 0.98, yellow: p > 0.98). The lipid core burden index (LCBI) quantitates LCP in the entire scanned region, computed as the fraction of pixels that exceed an LCP probability of 0.6, multiplied by 1000.
Sample NIRS scan. The chemogram displays the NIRS findings obtained during rotation and pullback of the imaging element within the coronary artery of a patient. The x-axis represents millimeter of pullback, and the y-axis represents degrees of rotation from 0 to 360. Yellow indicates a high probability that LCP is present at the interrogated site. MaxLCBI4mm is determined by defining the intervention region, computing LCBI for all 4 mm sub-segments within the intervention region, and identifying the maximum LCBI subsegment. NIRS = near-infrared spectroscopy, LCP = lipid core plaque, LCBI = lipid core burden index
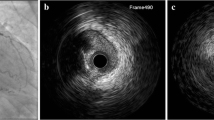
The initial calibration and validation studies for NIRS detection of LCP were performed ex vivo in intact and perfused human coronary arteries, the signals compared against the autopsy gold standard of LCP (fibroatheroma >60° in circumferential extent, >200 μm in thickness on a cross-sectional histologic specimen, with a fibrous cap having a mean thickness of <450 μm) [23••] (Fig. 3). A receiver-operating characteristic curve for detection of LCP of 0.83 (95 % confidence interval 0.70 to 0.93) was observed, which satisfied the primary endpoint of the study. Clinical validation studies demonstrated that NIR signals obtained in patients have spectra that are similar to those obtained in autopsy specimens [27]. Based on these studies, the NIRS catheter received US FDA approval and CE mark for detection of LCP of interest in the coronary arteries and measurement of LCBI.
Histologic validation of NIRS. A NIRS scan correlates well to histologic findings in coronary artery from an 85-year-old male with a history of myocardial infarction. The chemogram shows prominent lipid core signal, occupying 180° in the middle coronary segment. The NIRS signals at the coronary edges correctly indicate absence of LCP. NIRS = near-infrared spectroscopy, LCP = lipid core plaque. (Reproduced, with permission, from Gardner et al. JACC Cardiovasc Imaging. 2008; 1:638-48)
NIRS-IVUS: “Value Added” Clinical Applications
To fully appreciate the potential clinical value of NIRS-IVUS imaging, it is essential first to consider the growing body of evidence demonstrating that PCI performed employing IVUS achieves superior outcomes compared to angiographic guidance alone. A fundamental precept of optimal PCI is to cover the full length of target lesions. IVUS commonly documents that vessels appearing angiographically normal often harbor extensive plaque; thus, stenting based on the angiogram by itself may result in “geographic miss.” Such incomplete lesion coverage is associated with increased stent complications including stent thrombosis and restenosis [1, 3••, 4••, 28]. In contrast, IVUS-guided PCI reduces such complications. A recent meta-analysis encompassing 11 studies in 19,619 patients documented the superiority of direct coronary imaging to facilitate optimal performance of PCI compared with angiography-guidance [4••]. Further, the recent ADAPT DES trial [3••] showed IVUS guidance changed the procedure in >75 % cases, resulting in longer, appropriately sized stents. Most important, IVUS guidance improved clinical outcomes, with a 33 % reduction in myocardial infarction (MI), 50 % reduction in stent thrombosis, and 38 % reduction in target vessel revascularization (TVR).
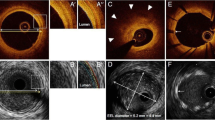
In aggregate, these observations emphasize the benefits of co-registered data on plaque composition as well architecture provided by multimodality NIRS-IVUS imaging. The IVUS component delineates plaque burden and documents optimal stent expansion and edge dissections. The “value added” of NIRS is to delineate the length of the lesion composed of LCP, which may be critical to identify the location and length of artery to be stented. Recent clinical cases (Fig. 4) document that placement of the ends of a stent over an LCP could result in high frequency of stent thrombosis [5, 6]. Employing NIRS, Dixon et al. demonstrated that in 16 % of cases, LCP extended beyond the angiographic margins of the target lesion [29]. A subsequent study employing NIRS-IVUS documented significant plaque burden (by IVUS) and LCP (by NIRS) beyond the angiographic margins in 80 % of target coronary lesions (Hanson et al., unpublished) (Fig. 5). Thus, NIRS-IVUS may help to avoid placement of the ends of a stent in a LCP by accurately and precisely guiding full coverage of LCP. Long-term studies are required to determine whether NIRS-informed stent length decisions result in better clinical outcomes.
Acute stent thrombosis at the site of lipid core plaque. This patient previously underwent stenting of the right coronary artery for acute inferior myocardial infarction, and developed acute stent thrombosis 8 h after primary PCI. Repeat coronary angiography documented thrombotic occlusion of the stent, which was treated with aspiration thrombectomy. Angiogram after thrombectomy is shown in inset angiogram (stent spanned from points 2 to 4). The proximal stent edge was found to be embedded within lipid core plaque (red arrows corresponding to point 4 on angiogram, cross-sectional IVUS image, and green line on NIR chemogram). PCI = percutaneous coronary intervention, NIR = near infrared, deg = degrees. (Reproduced, with permission, from Sakhuja et al. Circulation. 2010; 122:2349-50)
Lesion length by NIRS-IVUS. a Angiogram documents a complex lesion in the circumflex artery. b–f IVUS and g NIRS documented the extent and location of plaque burden and lipid content, respectively. Both heavy plaque burden and lipid core are present well beyond angiographic lesion borders. IVUS = intravascular ultrasound, NIRS = near-infrared spectroscopy
Prevention of Periprocedural MI
PCI in native vessels is complicated by periprocedural MI (PMI) in up to 15 % of cases. PMI is predominantly attributed to distal embolization (DE) of lipid core plaque contents, may result in acute “no-reflow”, and is associated with adverse long-term [13–21]. Pre-PCI identification of such plaques at high risk of embolization could lead to the use of preventive strategies (e.g., distal embolic protection device) to improve procedural safety.
NIRS can identify LCP lesions at embolic risk. Recent studies demonstrate that NIRS documented large LCP was associated with high risk of distal embolization and PMI [13, 14]. The COLOR Registry of patients undergoing NIRS assessment prior to PCI [14] documented that the extent of LCP in the treated region calculated by the LCBI for each 4 mm longitudinal segment (maxLCBI4mm) predicted risk, with PMI developing in 50 % of LCP with a maxLCBI4mm of ≥500 (22.6 % of the lesions) and in only 4.2 % of LCP with a low maxLCBI4mm (p = 0.0002) (Fig. 6). PMI has been associated with disappearance of yellow LCP after stenting (Fig. 7), presumably due to release of LCP contents and distal embolization.
Lipid core plaque and periprocedural myocardial infarction. Box plot of maxLCBI4mm grouped by occurrence of periprocedural myocardial infarction. Black circles show the actual data points. Boxes have lines at the lower quartile, median (center line), and upper quartile values. Whiskers extend 1.5× the group interquartile range. Outliers (crosses) are data with values beyond the ends of the whiskers. Box notches indicate a robust estimate of the uncertainty about the medians, and lack of overlap of the notches visually shows that the two group medians differ at the 5 % significance level. LCBI = lipid core burden index. (Reproduced, with permission, from Goldstein et al. Circ Cardiovascular Interv. 2011; 4:429-37)
Case of periprocedural myocardial infarction. Pre- and post-PCI angiogram frames and chemograms for a patient experiencing PMI. The black arrows indicate the location on the angiogram of the proximal and distal boundaries of the PCI location and correspond to the boundaries of the chemogram segment. Pre-PCI maxLCBI4mm was 591 at the region indicated by the 4 mm mark. The post-PCI chemogram shows substantial reduction in LCP (maxLCBI4mm reduced to 189 in the matched region). Post-PCI reduction of “Yellow” associated with PMI likely indicates downstream embolization of LCP contents. PCI = percutaneous coronary intervention, PMI = periprocedural myocardial infarction, LCBI = lipid core burden index. (Reproduced, with permission, from Goldstein et al. Circ Cardiovascular Interv. 2011; 4:429-37)
These results generated the hypothesis that embolic protection devices (EPD) may prevent distal embolization of coronary plaque contents and reduce PMI in native coronary arteries. Prior studies of the use of EPD during coronary stenting without direct imaging guidance failed to show clinical benefit. Future studies are needed to validate the benefits of a selective strategy of EPD use during coronary stenting based on invasively proven LCP content.
Identification of Vulnerable Plaque
Acute coronary syndromes (ACS) are caused by rupture of macrophage-rich, inflamed thin-capped fibroatheroma (TCFA) with superimposed thrombus formation [11]. Although focal flow limiting coronary stenoses are the targets for revascularization to relieve myocardial ischemia, ruptured plaques most commonly arise from precursor “vulnerable plaques” (VP) that, prior to disruption, are typically non-flow limiting, asymptomatic, and would not be detectable by invasive flow interrogation with FFR or stress testing. Further, it is now clear that the majority of patients with ACS harbor multiple unstable plaques [30], consistent with the concept that plaque instability is a multifocal process and supporting efforts to identify non-flow limiting lesions that are at higher risk for adverse events.
Plaque composition influences lesion stability. In contrast to fibrous plaques, which are thought to be more stable, lipid core plaques (LCP) are associated with rapid progression of atherosclerosis, plaque rupture, and ACS. Therefore, detection of LCP in vivo might be an important step to identify patients and lesions at risk for adverse cardiac events.
Autopsy studies of patients succumbing to acute MI and sudden coronary death document that the most common underlying morphology is an inflamed thin-capped fibroatheroma (TCFA), characterized histologically by (1) thin fibrous cap (<65 μm), (2) large necrotic lipid pool, and (3) enzymatically active macrophages near or within the fibrous cap [11] (Figs. 1 and 2). Direct intracoronary imaging has potential to detect VPs. By IVUS, unstable plaques are typically bulky and eccentric. They also may exhibit ulceration, intimal flap, and thrombus [7–9, 12]. Culprit lesions causing ACS cases manifest more positive remodeling by IVUS than culprit lesions causing stable angina [7, 12]. The landmark PROSPECT trial [10••] employing IVUS-Virtual Histology (VH) is the first study to prospectively demonstrate that non-flow limiting plaques with certain morphologic features predict subsequent events; in this seminal investigation, lesions with plaque burden >70 %, minimal luminal area <4 mm2, and delineated thin-capped fibroatheroma by VH were at highest risk for adverse events.
Given its validated capabilities for detection of LCP, it therefore follows that delineation of plaque architecture and composition by NIRS-IVUS has the potential to detect VP. An important recent study in patients with ST elevation myocardial infarction (STEMI) by Madder et al. [24•] showed that the culprit lesion by NIRS is typically a large LCP (Fig. 8), and that a maxLCBI4mm >400 discriminated culprit from non-culprit lesions (Fig. 9). NIRS has also demonstrated large LCP in culprit segments in a small group of sudden cardiac death survivors [31]. The concept that bulky LCP is a signature of lesions causing STEMI is consonant with extensive ex vivo autopsy data [1, 3••, 4••, 28, 32, 33]. It has also been shown by NIRS that 80 % of target lesions responsible for ACS were caused by LCP [25]; in addition, ACS patients were more likely to harbor LCP remote from the culprit site (Fig. 10). Given the potential impact of plaque composition on PCI procedural outcome and concern over LCP serving as the substrate for future adverse cardiac events, these findings may have important clinical implications. Interestingly, in that study, the target lesion in patients with chronic stable angina was also commonly a LCP. Given that unstable versus stable angina is a clinical distinction, it may well be the case that some clinically “stable” patients harbor lipid-rich potentially unstable plaques that may be the precursor for future ACS.
Lipid core plaque in STEMI culprit lesions. a Angiogram and chemogram from patient with acute inferior STEMI due to thrombotic occlusion of the RCA; the chemogram documents the underlying culprit is a large LCP. b Composite of eight other cases of acute STEMI with corresponding angiograms and chemograms documenting that the underlying culprit is a large LCP. STEMI = ST elevation myocardial infarction. (Reproduced, with permission, from Madder et al. JACC Cardiovasc Interv. 2013; 6(8):838-46)
Detection of STEMI culprit segments by NIRS and IVUS. a MaxLCBI4mm (red), PB (green), and calcification (black) by IVUS are shown. MaxLCBI4mm was significantly more discriminatory than calcification in distinguishing culprit from non-culprit segments in STEMI culprit vessels (AUC: 0.90 vs. 0.72; p = 0.016) and performed similar to PB (AUC: 0.90 vs. 0.86; p = 0.44). b The ability of NIRS and IVUS to identify STEMI culprit segments when admixed with histology-negative autopsy specimens. MaxLCBI4mm (red), PB (green), and calcification (black) by IVUS are shown. MaxLCBI4mm was significantly more discriminatory at identifying STEMI culprit segments than were PB (AUC: 0.97 vs. 0.83; p = 0.015) or calcification (AUC: 0.97 vs. 0.79; p = 0.002). PB and calcification were not significantly different (p = 0.17). STEMI = ST elevation myocardial infarction, NIRS = near-infrared spectroscopy, IVUS = intravascular ultrasound, PB = plaque burden, AUC = area under curve, ROC = receiver-operating curve, LCBI = lipid core burden index. (Reproduced from Madder et al. JACC Cardiovasc Interv. 2013; 6(8):838-46)
Target lesion lipid core plaque and remote, non-target lipid core plaque in patients with acute coronary syndrome. a Angiography in a 49-year-old male patient with unstable angina demonstrates target lesions in the mid-circumflex coronary artery (short white arrow) and the first obtuse marginal artery (asterisk). Near-infrared spectroscopy of the circumflex coronary artery reveals LCP within the mid-circumflex target lesion (long white arrow) and a remote, non-target LCP in the proximal circumflex coronary artery at the site of a minimal angiographic stenosis (black arrow). b Angiography in a 57-year-old male patient with unstable angina reveals a target lesion in the mid-right coronary artery (short white arrows). Near-infrared spectroscopy reveals extensive LCP along the length of the target lesion (long white arrows) and remote, non-target LCP at the site of mild angiographic stenosis (black arrow). LCP = lipid core plaque. (Reproduced, with permission, from Madder et al. Circ Cardiovasc Interv. 2012; 5(1):55-61)
Implications for Strategies to Prevent MI and Sudden Death
On the basis of pathophysiological “first principles,” it is virtually certain that the LCP observed in STEMI, sudden cardiac death, and other ACS cases did not develop overnight. In aggregate, these findings are important for predictive and preventive purposes, supporting the concept that it is possible that the large LCP observed at culprit sites were present and detectable before thrombus formation and the acute coronary event. Taken together, these observations support the concept that a strategy of preventive stenting of NIRS-IVUS delineated vulnerable LCPs may reduce adverse clinical events. The first step in validating this hypothesis is establishing that lesions so characterized are “at risk” for events. Several large natural history studies of non-flow limiting LRP detected by NIRS-IVUS are now underway. The Lipid-Rich Plaque study (LRP, NCT02033694) is another natural history investigation that will enroll 9000 patients presenting for coronary angiography where an IVUS and/or NIRS evaluation is planned or could be added as part of a clinically indicated evaluation; the non-index culprit lesion related to major adverse cardiac events will be investigated as an assessment of primary outcome. The prospective, multicenter PROSPECT II trial in Scandinavia (NCT02171065) and other countries is designed to collect post-PCI NIRS-IVUS data in 900 patients with ACS and composite of major cardiovascular events and determine the prognostic value of LCBI >400 evaluate over 2 years of follow-up; it will include a randomized sub-study trial of stenting of such LCP lesions of intermediate stenosis.
Additional Clinical Uses
Observations in Peripheral Vascular Disease
Recent NIRS-IVUS observations in peripheral vessels demonstrate that nearly all severely stenotic symptomatic superficial femoral artery (SFA) plaques are fibrocalcific, with only one-third of such lesions also containing LCP. These findings may have implications pertinent to similarities and differences in the pathophysiology of peripheral arterial disease (PAD) compared to coronary artery disease and PAD interventional management (Zacharias et al., unpublished).
Application for Assessment of Pharmacological Agents
NIRS has potential applicability in the assessment of novel anti-atherosclerotic medications by providing a surrogate endpoint for plaque regression/stabilization studies. In particular, the ability of NIRS-IVUS to assess the lipid content as well as volume of plaques may be an effective means of identifying the beneficial effect of an agent. The YELLOW (reduction in yellow plaque by aggressive lipid lowering therapy) study in patients with multivessel CAD undergoing PCI and a subsequent follow-up angiogram and NIRS interrogation at 7 weeks recently demonstrated a reduction of coronary LCP with high-dose rosuvastatin therapy [34]. In The European Collaborative Project on Inflammation and Vascular Wall Remodeling in Atherosclerosis—Intravascular Ultrasound Study (AtheroRemoIVUS), a prospective observational study, NIRS imaging was performed in a non-culprit coronary artery in 203 patients referred for angiography due to stable angina pectoris (SAP) or ACS [2]. A fourfold increase in major adverse cardiac and cerebrovascular events during 1-year follow-up was observed in patients with a lipid core burden index (LCBI) above the median. Accordingly, NIRS-IVUS also has potential for assessment of other promising anti-atherosclerotic agents.
Conclusions
Traditional imaging techniques have not been able to provide accurate, easily obtainable information about the structure and composition of plaque, which are critical to understand the coronary artery disease state. The presence or absence of lipid core is one of the most important compositional parameters related to both the safety of stenting and the risk of rupture of a given plaque. Intracoronary NIRS-IVUS has been developed and rigorously validated as an accurate method for detection of lipid core plaque and co-registered structural measurement provided by IVUS in a single catheter. Early clinical use shows promise for the use of NIRS-IVUS to assist with common decisions to optimize PCI by determination of length of artery to stent, proof of optimal stent expansion/apposition, detection of edge dissection, and identification of lesions at higher risk of distal embolization and PMI. Longer term studies are needed to assess the utility of NIRS-guided therapy to detect vulnerable plaques and guide preventive stenting to reduce future coronary events.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Liu X et al. Intravascular ultrasound assessment of the incidence and predictors of edge dissections after drug-eluting stent implantation. JACC Cardiovasc Interv. 2009;2(10):997–1004.
Oemrawsingh RM et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J Am Coll Cardiol. 2014;64(23):2510–8.
Witzenbichler B et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129(4):463–70. This is one of the largest studies documenting that IVUS-guidance of PCI leads to changes in the stenting procedure that may lead to improved clinical outcomes.
Zhang Y et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8(7):855–65. This large meta-analysis compiles the available randomized and non-randomized data and shows that IVUS-guidance of PCI results in improved clinical outcomes.
Sakhuja R et al. Residual thrombogenic substrate after rupture of a lipid-rich plaque: possible mechanism of acute stent thrombosis? Circulation. 2010;122(22):2349–50.
Waxman S et al. A case of lipid core plaque progression and rupture at the edge of a coronary stent: elucidating the mechanisms of drug-eluting stent failure. Circ Cardiovasc Interv. 2010;3(2):193–6.
Fujii K et al. Intravascular ultrasound assessment of ulcerated ruptured plaques: a comparison of culprit and nonculprit lesions of patients with acute coronary syndromes and lesions in patients without acute coronary syndromes. Circulation. 2003;108(20):2473–8.
Jang IK et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111(12):1551–5.
Kubo T et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50(10):933–9.
Stone GW et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35. This seminal study suggests that there are features of plaque instability assessed by virtual histology IVUS that may predict downstream acute coronary events in the non-culprit arteries in patients presenting with acute myocardial infarction.
Virmani R et al. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 Suppl):C13–8.
Yamagishi M et al. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol. 2000;35(1):106–11.
Goldstein JA et al. Coronary embolization following balloon dilation of lipid-core plaques. JACC Cardiovasc Imaging. 2009;2(12):1420–4.
Goldstein JA et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardiovasc Interv. 2011;4(5):429–37.
Heusch G et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120(18):1822–36.
Hong YJ et al. Impact of plaque composition on cardiac troponin elevation after percutaneous coronary intervention: an ultrasound analysis. JACC Cardiovasc Imaging. 2009;2(4):458–68.
Kawamoto T et al. The relationship between coronary plaque characteristics and small embolic particles during coronary stent implantation. J Am Coll Cardiol. 2007;50(17):1635–40.
Kotani J et al. Plaque gruel of atheromatous coronary lesion may contribute to the no-reflow phenomenon in patients with acute coronary syndrome. Circulation. 2002;106(13):1672–7.
Mauri L, Rogers C, Baim DS. Devices for distal protection during percutaneous coronary revascularization. Circulation. 2006;113(22):2651–6.
Mehran R et al. Atherosclerotic plaque burden and CK-MB enzyme elevation after coronary interventions: intravascular ultrasound study of 2256 patients. Circulation. 2000;101(6):604–10.
Selvanayagam JB et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111(8):1027–32.
Brugaletta S et al. NIRS and IVUS for characterization of atherosclerosis in patients undergoing coronary angiography. JACC Cardiovasc Imaging. 2011;4(6):647–55.
Gardner CM et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging. 2008;1(5):638–48. This study validates NIRS for the detection of coronary artery cholesterol in vivo.
Madder RD et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2013;6(8):838–46. This study suggests that lipid core plaque is the precusor to nearly every case of ST-segment elevation myocardial infarction.
Madder RD et al. Composition of target lesions by near-infrared spectroscopy in patients with acute coronary syndrome versus stable angina. Circ Cardiovasc Interv. 2012;5(1):55–61.
Schultz CJ et al. First-in-man clinical use of combined near-infrared spectroscopy and intravascular ultrasound: a potential key to predict distal embolization and no-reflow? J Am Coll Cardiol. 2010;56(4):314.
Waxman S et al. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. JACC Cardiovasc Imaging. 2009;2(7):858–68.
Oemrawsingh PV et al. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP Study). Circulation. 2003;107(1):62–7.
Dixon SR et al. Analysis of target lesion length before coronary artery stenting using angiography and near-infrared spectroscopy versus angiography alone. Am J Cardiol. 2012;109(1):60–6.
Goldstein JA et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343(13):915–22.
Madder RD, Wohns DH, Muller JE. Detection by intracoronary near-infrared spectroscopy of lipid core plaque at culprit sites in survivors of cardiac arrest. J Invasive Cardiol. 2014;26(2):78–9.
Boden WE et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–16.
Shaw LJ et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117(10):1283–91.
Kini AS et al. Changes in plaque lipid content after short-term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J Am Coll Cardiol. 2013;62(1):21–9.
Compliance with Ethics Guidelines
Conflict of Interest
JA Goldstein has served as a consultant, received travel reimbursement, and owns stock in InfraReDx, Inc. JE Muller has served as Chairman of the Board and Chief Medical Officer, received travel reimbursement, and owns stock in InfraReDx, Inc. ID Hanson, SR Dixon, AE Abbas, and RD Safian all declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
James A. Goldstein, M.D. is a consultant for and owner of equity in InfraReDx, Inc.
James E. Muller is a current employee of InfraReDx, Inc.
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Hanson, I.D., Goldstein, J.A., Dixon, S.R. et al. Present Status and Future Direction of NIRS-IVUS Multimodality Direct Coronary Imaging. Curr Cardiovasc Imaging Rep 8, 25 (2015). https://doi.org/10.1007/s12410-015-9342-0
Published:
DOI: https://doi.org/10.1007/s12410-015-9342-0