Abstract
Coronary artery disease (CAD) is an inflammatory process that results in buildup of atherosclerosis, typically lipid-rich plaque in the arterial wall. Progressive narrowing of the vessel wall and subsequent plaque rupture can lead to myocardial infarction and death. Recent advances in intravascular fluorescence imaging techniques have provided exciting coronary artery-targeted platforms to further characterize the molecular changes that occur within the vascular wall as a result of atherosclerosis and following coronary stent-induced vascular injury. This review will summarize exciting recent developments in catheter-based imaging of coronary arterial-sized vessels; focusing on two-dimensional near-infrared fluorescence imaging (NIRF) molecular imaging technology as an approach to specifically identify inflammation and fibrin directly within coronary artery-sized vessels. Intravascular NIRF is anticipated to provide new insights into the in vivo biology underlying high-risk plaques, as well as high-risks stents prone to stent restenosis or stent thrombosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is characterized by cholesterol-driven attraction of inflammatory cells within the arterial wall that results in the formation of lipid-laden intraluminal plaque, smooth muscle proliferation, and progressive narrowing of the vessel lumen. The formation of high-risk vulnerable plaques prone to rupture followed by activation of platelets and formation of mural thrombi can lead to arterial occlusion and fatal myocardial infarction. The complex molecular and cellular inflammatory cascade is orchestrated by the recruitment of T lymphocytes and macrophages to nascent atheroma and their paracrine inflammatory effects on endothelial and smooth muscle cells.
While a wealth of information has been unraveled by structural imaging approaches [1], the underlying biology of atheroma is invisible to structural imaging. To fulfill this knowledge gap in living subjects, molecular imaging of atherosclerosis has become an important clinical and research tool that allows in vivo visualization of atherosclerosis biology in nondestructive fashion [2–6].
Imaging of atherosclerosis using molecular imaging probes coupled with noninvasive and invasive imaging systems can now provide visualization of the in vivo changes that occur within the vessel wall during important clinical syndromes such as myocardial infarction, stroke, and ischemic limbs. The development of advanced catheter-based imaging approaches that provide high resolution images of arterial inflammation including protease activity in atheroma or fibrin deposition has been recently enabled by translatable intravascular near-infrared fluorescence (NIRF) molecular imaging [7]. Recently, new integrated high-resolution molecular-structural imaging systems combining NIRF and optical frequency domain imaging (OFDI) have been developed and used in vivo to visualize inflammation in atherosclerosis and microthrombosis after coronary stent deployment. In addition, use of novel molecular imaging agents which target specific disease processes in atherosclerosis have expanded the application of these advanced catheter-based imaging systems.
The following review will describe some of the new and exciting molecular imaging techniques and clinical applications of such advances.
Noninvasive Molecular Imaging of CAD
Although a noninvasive molecular imaging approach for coronary artery disease would be optimal for a clinical screening standpoint, the small caliber of the coronary arteries with superimposed cardiac and respiratory motion often demands higher-resolution approaches than afforded by noninvasive imaging. In the larger carotid arteries however, the development of nano-based particle-enhanced magnetic resonance imaging [8] (Fig. 1) and fluorine-18-fluorodeoxyglucose positron emission tomography (FDF-PET) has enabled the in vivo detection of inflamed, macrophage-rich plaques in carotid atherosclerosis [9] (Fig. 2). Preliminarily, PET molecular imaging has been recently applied to the coronary arteries. In a pilot study, FDG accumulation was observed in culprit lesions as well as in the ascending aorta and left main arteries in patients with recent acute coronary syndrome (ACS) [10] (Fig. 3). In addition, the use of 18 F-NaF (sodium fluoride), an established radioisotope previously used to image bone formation, has allowed detection of plaque osteogenic activity [11•]. Compared to FDG, NaF allows improved PET-CT signal-to-background by avoiding high myocardial background signal that typifies 18 F-FDG studies. However as stated above, noninvasive imaging of coronary atheroma, although technically feasible, is limited by spatiotemporal resolution. The development of catheter-based intravascular imaging systems that detect molecular probes that target inflammation have helped to overcome the limitations of noninvasive imaging in atherosclerosis.
MRI Imaging of macrophage-rich plaque within a human carotid artery using iron oxide nanoparticles. T2-weighted image of a left common carotid artery before (A, C and E) and after (B, D, and E) infusion of ultrasmall superparamagnetic iron oxide (USPIO) infusion in a patient at three different time points [0 (B), 6 (D) and 12 (F) weeks]. Yellow arrows show enhancement of signal within macrophage-rich plaque at baseline, prior to high-dose statin therapy. Over time, USPIO signal loss is lost (blue arrows), consistent with a reduction in plaque macrophages. Reproduced by permission from reference [8]
Axial positron emission tomographic (PET)-CT images of carotid plaque inflammation (metabolic signal) from 2. patients. Patient A with low fluorine-18-fluorodeoxyglucose (FDG) uptake and Patient B with high FDG uptake in the region of the carotid plaque. Reproduced by permission from reference [9]
Conjugated FDG PET/CT image of increased FDG uptake (plaque inflammation/metabolism) in the ascending aorta and in culprit LAD plaque (arrow) in a 60 year old man with presumed acute coronary syndrome. Reproduced by permission from reference [10]
Intravascular High-Resolution Molecular Imaging of Atherosclerosis
NIRF Imaging: Advantages of the NIR Window
The properties of near-infrared fluorescence imaging have transformed the field of intravascular imaging in atherosclerosis due to 1) the efficient transmission of light in the NIR window, thereby increasing light penetration 2) high intrinsic sensitivity in the NIR region and lower light absorption by hemoglobin through whole blood 3) reduced auto-fluorescence of surrounding tissue, thereby improving the detection of targeted NIRF molecular imaging agents above the background. In addition, a broad array of attachment chemistries for targeted and activatable imaging agents are currently available and allow for dramatically improved sensitivity of NIRF imaging. In particular, protease-activatable probes made of NIR fluorochromes that are attached to a high molecular weight methyl poly-(ethylene glycol) (MPEG) poly-L-lysine backbone targeted to various cathepsins, matrix metalloproteinases (MMPs) and thrombin have yielded promising results by both intravital fluorescence microscopy and in vivo intravascular imaging of atherosclerosis using both 1D and 2D NIRF imaging catheters [12–14].
2D NIRF System and Applications to Atherosclerosis
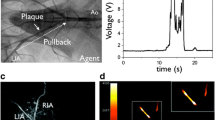
The initial feasibility of NIRF sensing was demonstrated using a spectroscopic NIRF catheter [15] but was limited by incomplete sampling and lower sensitivity. The development of a 2-dimensional (2D) NIRF imaging device and catheter has enabled the sensing of NIRF signals in vessels of diameters more typical of human coronary arteries (2.5- 3.5 mm) [14] (Fig. 4). The imaging system houses a continuous-wave laser source operating at 750 nm. The catheter contains an optical fiber within a polyethylene sheath that guides the 750 nm laser light and collects the NIRF signal emitted form the molecular probe. The fiber rotates and translates mechanically, collecting fluorescent light intensity at each position, and thus generates a 2D NIRF image. The outer diameter of the imaging catheter is 2.9 F and operates on a monorail system over a 0.014 inch guidewire, allowing easy manipulation of the catheter within coronary-sized vessels. The in vitro and in vivo performance of this catheter was tested in a rabbit model of atherosclerosis using a cysteine protease-activatable imaging reporter Prosense VM110 (Perkin Elmer, Waltham, Massachusetts). High-resolution NIRF images of vessel wall inflammation through blood were obtained with signal-to-noise ratios > 10. In addition, the 2D NIRF imaging system revealed the first real-time, in vivo illumination of edge-based, stent-induced arterial inflammation [14] (Fig. 5).
Schematic of an intravascular 2D NIRF imaging system. The tip of the fiber contains an angle-coated prism that reflects laser light into the arterial wall. The catheter couples subsequent fluorescent light back into the fiber. The light is then directed to a dichroic beam splitter that selectively reflects it into a photomultiplier tube, allowing generation of a 2D NIRF image as the catheter automatically translates and rotates. Reproduced by permission from reference [14]
NIRF imaging of stent injury induced protease inflammation. A. Ex vivo fluorescence resonance imaging (FRI) at 800 nm of increased protease activity at stent edges. B. Corresponding ex vivo intravascular NIRF pullback of stented segment. C. Ex vivo NIRF-white light fusion image of stent. D. High magnification white light image. E. High magnification NIRF image revealing signal along the greater curvature of the stent struts. Reproduced by permission from reference [14]
NIRF-OFDI System
Optical frequency domain coherence tomography (OFDI) is one of the most promising new imaging tools used to visualize the structural details of the coronary vessel wall. Recently, a dual modality catheter imaging was developed by Yoo et al. [16]. The unique imaging probe combines a double clad fiber with a single-mode core that transmits and receives OFDI light with a multi-mode light-guiding inner cladding that transmits the NIRF excitation and receives the emitted fluorescence light. A lens at the tip of the fiber provides focused, co-registered OFDI and NIRF excitation signals in the arterial wall. Three-dimensional reconstruction of OFDI and NIRF data is obtained from a helical pullback and provides highly sensitive detection of < 500 nM of NIR fluorochrome at a depth of up to 3-4 mm from the catheter in saline. There are two major advantages of the dual modal NIRF-OFDI system. First, OFDI enables quantitative NIRF imaging. This is because detection of fluorescence signal intensity is distance-dependent — this is because photon scattering and attenuation through media (e.g., saline or blood) increases with the distance traversed. In arterial context, this means that quantitative NIRF signal can only be realized if the catheter position relative to vessel wall (where the fluorochrome is localized) is known. Importantly, standalone NIRF catheters do not possess anatomical information and therefore the catheter-to-wall distance is not available. With integrated OFDI-NIRF, OFDI provides exquisite synchrounous anatomical catheter positional information and therefore enables distance correction of the NIRF signal. Distance correction is a key advance that allows improved ability to track and quantify NIRF signal differences in plaques and stents over time. The second advantage is that both the molecular NIRF and anatomical OFDI datasets can be simultaneous acquired in a single pullback, facilitating clinical translation.
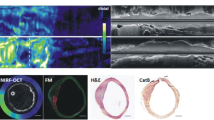
The NIRF-OFDI catheter was first validated in vitro using models of stent microthrombosis. Cadaveric human coronary arteries containing Cy7-labeled fibrin-coated stents were imaged using this novel dual imaging catheter. NIRF cylinidrical rendering of fibrin signal acquired by the dual imaging catheter correlated strongly with corresponding FRI results [16] (Fig. 6). To demonstrate the in vivo potential of the dual imaging catheter, stented iliac arteries of New Zealand White (NZW) rabbits were imaged in vivo. Three-dimensional rendering of combined OFDI and NIRF signals of fluorescent-labeled fibrin-rich microthrombi within coronary stents provided high-resolution OFDI images of metallic struts covered with Cy7-labeled fibrin within thrombus. The catheter was also validated in vivo using the cysteine protease-activatable NIRF probe within inflammatory plaque of atherosclerotic NZW rabbits.
OFDI imaging of thrombus within a coronary stent implanted into a cadaveric coronary artery. The stent contains NIR fluorescent fibrin. A. OFDI Cross-sectional image with thrombus (yellow). B. OFDI cross sectional image with stent struts and micro-thrombi (yellow). C. NIRF cylindrical rendering of fibrin signal acquired by the dual-modality imaging catheter. D. Matching NIR FRI with Cy7 filter set confirms the in vivo findings. Reproduced by permission from reference [16]
While the above demonstrated the ability to detect fluorescent fibrin attached to stents ex vivo, an i.v. injectable, in vivo fibrin sensor for intravascular NIRF imaging was not available until the development of FTP11-CyAm7, a new fibrin-targeted NIRF agent that was recently developed [17•, 18]. This NIRF molecular imaging agent can now targets fibrin deposition within unhealed coronary stents and enable in vivo imaging. Localization of the agent can be imaged in vivo using the NIRF-OFDI imaging catheter described above (Fig. 7). This new technology enables simultaneous colocalization of microstructural and molecular detail of coronary stent healing and atherosclerotic plaque, which provides useful information for future understanding of these important biological processes, and to potentially the risk of stent thrombosis.
In vivo NIRF-OFDI imaging of fibrin deposition with unhealed coronary stents, 7 days after implantation into a rabbit iliac artery. The injectable fibrin-targeted NIRF agent FTP-Cy7 was i.v. injected 2 hours prior to imaging. In vivo NIRF-OFDI signal demonstrates microscopic fibrin deposition overlying and in between stent struts of. Reproduced by permission from reference [18]
The NIRF-OFDI catheter is based on a clinical OFDI platform, and therefore is under active development for clinical testing in human coronary artery disease subjects by 2016.
New NIRF Imaging Agents for Atherosclerosis: ICG
While Prosense VM110 is an excellent sensor for imaging arterial inflammatory protease activity, it is not yet available for clinical use. The identification of a clinical NIRF imaging agent for molecular imaging would greatly advance intracoronary applications. Recently, ICG, an FDA-approved amphiphilic NIR fluorochrome currently used in outpatient imaging of retinal and choroidal vasculature, has been discovered to be a promising targeted imaging agent for the detection of lipid- and macrophage- rich plaque [19]. ICG binds to acetylated LDL and due to its lipophilic properties, it accumulates within macrophages in atheroma. A recent study demonstrated the colocalization of ICG within lipid and macrophage-rich rabbit atheroma. The NIR fluorescence signal emitted by ICG was detected in vivo using a 2D NIRF intravascular imaging catheter. In vivo sensing of ICG localized within atheroma of ICG-injected atherosclerotic rabbits. ICG is clinically approved for use and is a promising new agent that may accelerate the clinical application of intravascular NIRF imaging of high-risk plaques.
Conclusions
Intravascular molecular imaging of atherosclerosis is an exciting area of on-going research and is ripe for clinical application in human subjects. The lack of FDA approved molecular-targeted imaging agents has been a major barrier to translating this technology into clinical practice. Fortunately, ICG is currently FDA approved and is a clinically relevant imaging agent in atherosclerosis. Furthermore, new clinical NIRF imaging agents developed the cancer arena [20] may be applicable to atherosclerosis. We anticipate that intravascular molecular imaging with dual modality NIRF catheters will be clinically tested in human coronary arteries within the next 2 years.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–35.
Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;116(9):1052–61.
Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451(7181):953–7.
Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012;111(2):231–44.
Osborn EA, Jaffer FA. The advancing clinical impact of molecular imaging in CVD. JACC Cardiovasc Imaging. 2013;6(12):1327–41.
Verjans JW, van de Borne SW, Hofstra L, Narula J. Molecular imaging of myocardial remodeling after infarction. Methods Mol Biol. 2011;680:227–35.
Calfon MA, Vinegoni C, Ntziachristos V, Jaffer FA. Intravascular near-infrared fluorescence molecular imaging of atherosclerosis: toward coronary arterial visualization of biologically high-risk plaques. J Biomed Opt. 2010;15(1):011107.
Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ JMUK-I, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53(22):2039–50.
Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, et al. In vivo 18 F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48(9):1818–24.
Rogers IS, Nasir K, Figueroa AL, Cury RC, Hoffmann U, Vermylen DA, et al. Feasibility of FDG imaging of the coronary arteries: comparison between acute coronary syndrome and stable angina. JACC Cardiovasc Imaging. 2010;3(4):388–97.
Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383(9918):705–13. This is the first non invasive imaging method able to identify and localize ruptured and potenitally high-risk coronary plaques using 18F-NaF PET-CT of plaque osteogenesis.
Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, et al. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115(17):2292–8.
Jaffer FA. Intravital fluorescence microscopic molecular imaging of atherosclerosis. Methods Mol Biol. 2011;680:131–40.
Jaffer FA, Calfon MA, Rosenthal A, Mallas G, Razansky RN, Mauskapf A, et al. Two-dimensional intravascular near-infrared fluorescence molecular imaging of inflammation in atherosclerosis and stent-induced vascular injury. J Am Coll Cardiol. 2011;57(25):2516–26.
Jaffer FA, Vinegoni C, John MC, Aikawa E, Gold HK, Finn AV, et al. Real-time catheter molecular sensing of inflammation in proteolytically active atherosclerosis. Circulation. 2008;118(18):1802–9.
Yoo H, Kim JW, Shishkov M, Namati E, Morse T, Shubochkin R, et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat Med. 2011;17(12):1680–4.
Hara T, Bhayana B, Thompson B, Kessinger CW, Khatri A, McCarthy JR, et al. Molecular imaging of fibrin deposition in deep vein thrombosis using fibrin-targeted near-infrared fluorescence. JACC Cardiovasc Imaging. 2012;5(6):607–15. First intravenous injectable NIRF fibrin-targted agent for intravascular NIRF imaging.
Hara T, McCarthy JR, Hamidi E, Erdem S, Mauskapf A, Bouma B, Tearney G, Jaffer F. Intravascular Optical Molecular Imaging of fibrin deposition to assess vascular healing after coronary stent implantation. Circulation. [Abstract]. 2012;126.
Vinegoni C, Botnaru I, Aikawa E, Calfon MA, Iwamoto Y, Folco EJ, et al. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci Transl Med. 2011;3(84):84ra45.
van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–9.
Compliance with Ethics Guidelines
Conflict of Interest
Marcella Calfon Press declares no conflict of interest.
Farouc A. Jaffer has received research grants from Merck, Kowa, and Siemens.
Funding sources
NIH R01 HL108229, American Heart Association Founders Grant-in-Aid #13GRNT17060040.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Intravascular Imaging
Rights and permissions
About this article
Cite this article
Press, M.C., Jaffer, F.A. Molecular Intravascular Imaging Approaches for Atherosclerosis. Curr Cardiovasc Imaging Rep 7, 9293 (2014). https://doi.org/10.1007/s12410-014-9293-x
Published:
DOI: https://doi.org/10.1007/s12410-014-9293-x