Abstract
Several multicenter trials have demonstrated the high diagnostic accuracy and clinical efficacy of modern coronary computed tomography angiography (CTA) when utilized to evaluate symptomatic patients with low-to-intermediate pretest probability of coronary artery disease (CAD). However, coronary CTA remains a purely anatomic test and further assessment with invasive coronary angiography or other non-invasive tests are occasionally required, with subsequent inherent risks and costs to patients and healthcare systems. Recently, remarkable advances in multidetector computed tomography technology has significantly improved temporal and spatial resolution of coronary CTA. In the past decade, initially in animal models and then in humans, stress myocardial perfusion imaging by computed tomography (CTP) evolved and is being increasingly studied. It is the purpose of this review to highlight recent updates in the CTP literature and try to figure how to place CTP in CAD management in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is the major cause of morbidity and mortality worldwide, particularly in western countries [1, 2]. Over the last several decades, there have been noticeable advances in percutaneous and surgical coronary revascularization. Prognosis of patients with ischemic heart disease has markedly improved, mainly in acute coronary syndromes [2–4]. However, concerning management of stable CAD, it has become clear, following the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, that some patients with stable, medically-managed ischemic heart disease may not benefit from percutaneous coronary intervention based solely on the presence of anatomic stenosis severity [5]. Medical therapy is also evolving and the benefit of revascularization is limited in many patients [5–8]. Although the idea that awareness of the degree of ischemia could select patients who would benefit most from revascularization has grown, it is not yet fully clarified [5, 9–12].
Clinical practice probably reflects the absence of a clear guidance or step-by-step plan for ischemia pursuit. Patients with suspected stable CAD are often invasively stratified using invasive coronary angiography (ICA) and a large proportion of those are found to have normal coronary arteries [13, 14]. Besides the risks and costs of an invasive approach, anatomical assessment underperforms when compared with concomitant functional assessment to guide revascularization [6–8, 13, 15].
Technological developments in cardiovascular imaging have also been staggering and cardiac CT and cardiac magnetic resonance (CMR) are established modalities broadly utilized in the clinical management of patients with established or suspected cardiovascular disease. In this pursuit, major cardiovascular imaging goals are not only to diagnose patients with coronary disease, but also to identify those who would benefit from revascularization procedures [10].
Most non-invasive techniques for the evaluation of coronary artery disease rely on functional assessment of myocardial perfusion — myocardial perfusion imaging (MPI). Noninvasive anatomical coronary evaluation is only possible by CT or CMR coronary angiography. The latter has had difficulty in adding incremental accuracy value to classical protocols for assessing ischemia, including assessment of first-pass myocardial perfusion and gadolinium delayed enhancement [16, 17]. Major drawbacks are limited spatial resolution and artifacts which, in practice, confines CMR coronary angiography to the evaluation of the origin of coronary arteries in congenital heart disease, where minimal or absent radiation exposure is more compelling [17].
On the other hand, coronary CTA has been demonstrated to be a noninvasive reference standard for diagnosis of anatomic coronary artery disease. First, the assessment of coronary calcium with non-contrast CT has shown to better reclassify asymptomatic patients regarding the risk of adverse cardiovascular events when compared with standard risk factors and risk scores alone [18]. In symptomatic patients, coronary CTA is an established, powerful tool in the evaluation and stratification of CAD, mainly in patients with intermediate to low pre-test probability, largely due to its outstanding negative predictive value [19–21]. However, calcified plaques with blooming effects and motion artifacts tend to overestimate or preclude stenosis evaluation [21, 22]. Moreover, optimal image acquisition requires lower heart rates and regular rhythm [22, 23]. Low specificity and predictive positive value limits broader application in specific subgroups of patients, mainly in those with previously known CAD or high pre-test probability [24]. Nevertheless, CTA unmatched spatial resolution makes coronary CTA an excellent diagnostic tool to visualize coronary bypasses, often missed and associated with more difficult and riskier ICA [25]. Even coronary stent visualization, which traditionally has been considered a handicap for coronary CTA (particularly with stents with diameter inferior to 2–3 mm), could no longer be a challenge in case of widespread use of the newer bio-absorbable stents [26]. Moreover, unlike other non-invasive functional tests that rely on ischemia evaluation for detection of CAD, coronary CTA can identify patients with non-obstructive or subclinical CAD. This could have a positive impact in the institution of more premature and efficacious secondary preventive measures, although clinical evidence data supporting this strategy is currently lacking [27, 28].
Another matter of concern regarding the widespread use of coronary CTA is radiation exposure. In fact, effective radiation doses are unsettled and some authors defend a higher conversion factor of 0.028 mSv x mGy-1 x cm-1 when compared to the more usual 0.014 or 0.017 used in most cardiac CT studies [29]. Improvement of acquisition protocols has led to substantial reductions in radiation doses in coronary CTA when compared to SPECT or ICA [30, 31]. CT has become an intense field of research. Novel systems with more rows of detectors and dual source systems have become the standard of care, allowing reduced radiation exposure and less dependence of image quality on heart rate, body habitus, and rhythm variability [32].
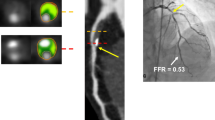
Similarly to ICA, an inherent limitation of coronary CTA is an anatomy-based diagnosis without information of the hemodynamic consequences of detected lesions [33]. FAME and DEFER trials demonstrated that anatomy-guided revascularization was inferior to ischemia-guided functional approach to revascularization, even when quantitative coronary angiography (QCA) was used [6–8]. Fractional flow reserve (FFR) in invasive studies should now be the reference standard against which all the other ischemia tests should be compared [34]. It takes into account collateral flow and it is not dependent on heart rate, blood pressure, and ventricular function [35]. Yet, FFR is inseparable from ICA, being guided by anatomical references, meaning that apparently discrete but significant lesions could pass undetected [36].
A “one-stop” noninvasive test that could provide simultaneous anatomical and functional data would represent a major breakthrough in CAD management. CMR is the most complete exam, providing information on myocardial perfusion under pharmacological vasodilator stress, myocardial scar and volumetric biventricular function [37–39]. Nevertheless, CMR coronary angiography, despite some positive value encountered in small single center studies, does not currently represent a viable approach for CAD anatomic evaluation [17]. Hybrid approaches like PET MPI/coronary CTA are an interesting way to overcome this need. In fact, it seems to be associated with high sensitivity and specificity, but is hampered by radiation exposure, high costs and residual clinical access [40]. In current practice, a two step strategy may be wiser: an appropriate subset of patients would be first guided to an anatomical based test like coronary CTA with a high predictive negative value; then, only patients with positive or doubtful results would be guided to myocardial perfusion tests or to ICA, according to pre-test risk and clinical presentation [34]. Under current knowledge, it should be emphasized that even patients directly guided by ICA should have functional assessment by FFR, if feasible [34].
For CT enthusiasts, however, there are alternative and evolving pathways to unify anatomical and functional assessment of coronary arteries. Perhaps the most intriguing is FFRCT, where flow dynamics are applied to a standard coronary CTA acquisition data [41]. The promise of a non-invasive FFR is very appealing, although it is only in a developing state and precise knowledge of its technical assumptions are limited to a few centers [42]. Transluminal attenuation gradient (TAG) quantification is another method that attempts to estimate the physiologic significance of anatomic stenosis on coronary CTA. Early literature regarding TAG, however, suggests that currently it may have decreased diagnostic performance as compared to FFRCT [43]. It is calculated by the linear regression coefficient between luminal attenuation and axial distance from the coronary ostium. Another possible approach is CT perfusion (CTP). Several single center studies and one multi-center study have demonstrated that CTP is feasible and adds incremental value to coronary CTA, with integrated protocols performing reasonably well against more established techniques [44]. The purpose of this article is to review the most relevant and recent CTP studies in clinical grounds (see Tables 1, 2, and 3).
Technical Considerations and First Studies
In appropriate patients, coronary CTA protocols can limit data acquisition to only one diastolic phase by prospective ECG-triggering in order to reduce radiation exposure. On the contrary, CTP protocols mandate a larger acquisition time window to observe contrast wash-in and wash-out in the myocardium. This is done at rest and under stress conditions, just as in CMR protocols [69].
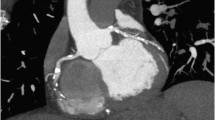
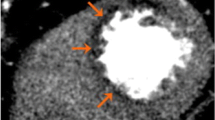
The main principle of CTP is the direct relationship between myocardial attenuation and amount of iodine contrast [69]. By usage of thicker reconstructions (8–10 mm) of multiplanar reformation with narrow window width (100-300 HU), perfusion defects in CTP can be visualized with better equilibrium between sensitivity and signal-noise ratio when compared to wider width and minimum or maximum-intensity projections [69]. However, beam-hardening artifacts originated by high density nearby structures are common and affect visual and automated analysis, even when attenuation correction software is used. Beam-hardening artifacts are possibly the greatest obstacle in CTP data interpretation and — even with extensive training — can be mistaken as perfusion defects [69].
The first studies conducted by Kurata and Kido et al. demonstrated CT feasibility for myocardial perfusion assessment but found many limitations, partially related with the reduced temporal resolution of 16-slice MDCT [45, 46].
Taking advantage of CT superior spatial resolution against SPECT, George et al. were the first to quantify transmural differences (transmural perfusion ratio, TPR) of myocardial perfusion in humans with helical 64-slice or 256-slice CT scanners acquisition [47]. Forty patients with abnormal SPECT were included, 26 of whom submitted to ICA. CTP was integrated with coronary CTA and compared against anatomical assessment by ICA (critical luminal stenosis if QCA >50 %) combined with functional assessment by SPECT. It was found to have similar accuracy to detect flow-limiting stenosis. On a per-patient basis, coronary CTA/CTP showed sensitivity, specificity, PPV, and NPV of 86 %, 92 %, 92 %, and 85 %, respectively. On a per-vessel basis, coronary CTA/CTP showed sensitivity, specificity, PPV, and NPV of 79 %, 91 %, 75 %, and 93 %, respectively. An important limitation of this study was its heterogeneous protocol in which only the 256-slice CT group carried out rest imaging; there was no assessment of perfusion reversibility in 64-slice CT group and coronary CTA was of limited quality. Another limitation was beta-blocker administration previously to stress test that might have affected the vasodilator response to adenosine, lowering the ability to detect perfusion defects. Nevertheless, the potential beta-blockers effects upon vasodilator perfusion imaging are still unknown [70].
Although exercise is the preferred stress method in MPI, only vasodilator stress was used in the CTP studies published so far. To date, most used adenosine as a vasodilator stressor aiming to reveal perfusion deficits in regions supplied by functionally significant coronary lesions. Cury et al. also demonstrated feasibility of dipyridamole as a vasodilator agent in 36 patients with a previous positive SPECT result [54, 71].
Hardware and Protocols Evolution
In less modern equipment, ECG-gated helical scanning achieves full cardiac coverage by scanning the entire heart in multiple heart-beats, but carries the risk of attenuation and misalignment artifacts, as well as the impossibility of a full quantitative analysis of myocardial perfusion [69]. Despite static acquisition limitations, the difference in contrast wash-out between ischemic and normal myocardium seems to be relatively constant after a minimum delay of 12 seconds [72]. This could be employed as an optimal window time in CTP protocols using this approach. However, CT hardware industry has maintained a steep evolution. Recent addition of more rows of detectors and dual-source systems (DSCT) enabled covering the entire myocardium with a single rotation in most patients with excellent temporal resolution.
Blankstein et al. applied the best DSCT temporal resolution to obviate the need for beta blockade [50]. This comprehensive protocol also included scar assessment using computed tomography delayed enhancement (CTDE), taking advantage of iodinated contrast media pharmacokinetics [73]. Thirty four patients with high-risk features, recently submitted to SPECT and ICA or oriented to ICA were included. Lower tube-voltage (100 kV) was used in non-obese patients and prospective ECG-triggering was implemented for rest imaging, allowing for a mean effective dose of 12.7 mSv, similar to that obtained in SPECT. On a per-patient basis, CTP against QCA showed a sensitivity, specificity, PPV, and NPV of 92 %, 67 %, 89 %, and 75 %, respectively. CTP showed sensitivity and specificity equivalent to SPECT; for both modalities, when anatomical threshold of 70 % rather than 50 % by QCA was used, there was an increase in sensitivity and a decrease in specificity. All patients who had evidence of CTDE had rest CTP defects. The following studies have shown the same advantages in similar populations using similar acquisition protocols [51, 53]. Tamarappoo et al. prospectively studied 32 patients with stress perfusion defects and showed that a visual or a semi-automated perfusion deficits assessment by DSCT correlated well against an automated computer-based analysis of SPECT results [52]. However, this software-based approach was wearisome, requiring frequent manual correction.
As the quest to lower radiation doses is always evolving, George et al. showed high accuracy of semi-quantitative TPR analysis by CTP to detect myocardial ischemia when compared against coronary CTA and SPECT, with lower radiation and contrast dose in a 320-slice CT protocol [65•]. Rest scan was performed first, whereby in future absent or non-obstrutive lesions in coronary CTA would not be submitted to unneeded CTP radiation. Initial rest scan was highly sensitive for the detection of previous myocardial infarction. Previous beta-blocker and hypothetical contrast contamination could affect stress analysis but, even so, CTP seems to have a tendency to false positives despite beta-blocker use. By a high-pitch step-and-shoot 128-slice DSCT-CTP low-dose protocol that included perfusion analysis of gadolinium fist-passage and CTDE, Feuchtner et al. showed that coronary CTA/CTP performed well when compared with a CMR comprehensive protocol [56]. Choo et al. and Nasis et al. used, respectively, a helical 128-slice DSCT CTP without rest scan and ECG-based tube current modulation and a prospective 320-slice CT acquisition at a single time point, to attain low radiation doses and found similar diagnostic accuracy [62, 66].
Dual-source Systems Specifics Acquisitions
Unique specificities of dual-source systems allowed development of unique techniques to study myocardial perfusion. Ho et al. were the first to describe the accuracy of a 128-slice DSCT with novel dynamic shuttle mode in 35 patients with fixed or reversible defects in recent SPECT [55]. Dynamic acquisition allows better capture of transient non uniform contrast distribution and blood flow quantification through the myocardium based on maximum slope of attenuation signal in myocardium and descending aorta. This is achieved by rapidly alternating between two table positions in prospectively ECG-triggered axial imaging with a 73 mm coverage, with one full scan every two heart beats, or every four beats if heart rate >63 bpm. On a per-segment basis, CTP against SPECT showed sensitivity, specificity, PPV, and NPV of 83 %, 78 %, 79 %, and 82 % and against QCA, 95 %, 65 %, 78 %, and 79 %, respectively. However, great inter-individual variations of basal myocardial blood flow prevented usage of a universal threshold, and a dynamic rest scan was needed, resulting in a higher radiation dose than static protocols. Also using dynamic shuttle mode, Kim et al. found incremental value of CTP to coronary CTA for significant CAD detection by QCA [61]. A major handicap that hampers dynamic shuttle-mode is the 73 mm limited anatomical coverage that may preclude interpretation of some myocardial segments [57, 59, 60•].
Besides shorter temporal resolution, the two x-ray sources can deliver different energy levels in dual-source systems. Based in this principle, dual-energy mode (DECT) takes advantage of different material spectral characteristics when penetrated by different x-ray energy levels to map iodine concentration within myocardium after contrast injection. Ko SM et al. introduced DECT for detection of perfusion defects in patients with CAD in DSCT-coronary CTA [58, 74]. They also used CMR-MPI as recent studies had gathered bulk evidence that render CMR-MPI as the actual benchmark for functional ischemia assessment [37–39]. Against CMR-MPI, DECT had sensitivity, specificity, PPV and NPV of 89 %, 78 %, 74 %, and 91 % in a per segment basis, and 91 %, 72 %, 82 %, and 88 % in a per vessel basis, respectively.
Validation against FFR
Since it became clear that functional significance extrapolated from anatomical evaluation of stenotic lesions is flawed, FFR is the reference standard for most recent studies involving ischemia assessment. Bamberg et al. were the first to demonstrate that CTP using MBF reclassification of coronary CTA depicted lesions provided incremental diagnostic value having FFR as reference [57]. Greif et al. found similar results in 65 patients with high prevalence of known CAD [63]. Ko BS et al. compared CTP against FFR in 40 symptomatic patients using a 320-slice CT protocol and were the first to compare visual and semi-automated CTP analysis [64]. In their study, visual CTP provided superior incremental value to coronary CTA compared to TPR, and both demonstrated increased specificity but lower sensitivity.
Bettencourt et al. designed the largest single-center CTP study published to date and were the first to directly compare CTP against CMR-MPI, having FFR as the reference standard [49•]. A pool of 101 patients with intermediate to high pre-test probability were referred to a low radiation dose coronary CTA and CTP integrated protocol with a retrospective static acquisition using a 64-slice CT and to a CMR-MPI comprehensive protocol with delayed enhancement analysis. Adding CTP to coronary CTA increased diagnostic accuracy, mainly because of a significant increase in specificity, and performed well against CMR-MPI in this population and using a standard 64-slice CT commonly seen in clinical practice.
Expanding Clinical Grounds
Until recently, CTP was mainly tested in suspected or known CAD in an outpatient basis. However, acute chest pain is a major cause of emergency department visits and frequently challenges the correct diagnosis [49•]. Hospital stays are frequently prolonged. On the other hand, failure to diagnose myocardial ischemia is associated with poor prognosis [75]. Weininger et al. innovated studying DSCT-CTP in acute chest pain with visual assessment of dynamic shuttle mode and dual-energy CTP [57]. With SPECT and MRI as the reference standards, both methods correlated well. Nevertheless, the number of patients included was rather low.
Another groundbreaking study was performed by Magalhães et al. that firstly reported incremental CTP value in patients with previous coronary stents clinically referred to ICA [48]. Rief et al. also sought to determine the incremental value of CTP for evaluation of coronary stents patency, a major limitation for coronary CTA [67•]. Ninety one patients with coronary stents oriented to ICA were recruited. Isolated coronary CTA NPV was excellent, but 15 % of all stents were non-diagnostic. With lesions >50 % by QCA as reference standard, 320-slice CT rest-stress protocol improved diagnostic accuracy, mainly by coronary CTA reclassification of non-diagnostic segments, particularly in stents with <3.0 mm diameter. Indeed, the use of CTP better predicted subsequent need for revascularization in case of intra-stent restenosis. This combined protocol had significant lower effective radiation exposure when compared to ICA.
CORE320, the first multi-center CTP study, was recently published. It enrolled 381 patients of 16 centers who underwent combined coronary CTA-CTP and SPECT prior to ICA [68••]. Integrating coronary CTA to CTP identified flow-limiting ≥50 % stenosis by QCA with a corresponding perfusion deficit on SPECT with sensitivity, specificity, PPV and NPV of 80 %, 74 %, 65 %, and 86 %, respectively. For flow-limiting disease defined by QCA and SPECT, coronary CTA accuracy was significantly increased adding CTP at both per-patient and per-vessel analysis, especially in patients without previous history of CAD. No use of FFR as reference standard is probably the major limitation of this study, particularly when compared to more contemporary similar studies.
Conclusions
In the near future, only one non-invasive test integrating anatomical and functional assessment would possibly satisfy all clinical demands to diagnose and manage CAD. MDCT represents a clear candidate, especially with its impressive technical evolution and clinical availability. However, despite encouraging results from various studies, many limitations are still present. Studies enrolled small patient samples and most with reference bias, with a high male dominance. Moreover, many studies had suboptimal reference standards for CAD assessment. Acquisition protocols were heterogeneous and beam-hardening artifacts remain a substantial challenge. Lowering radiation exposure has been a matter of constant evolution, but is not negligible, particularly when CMR has stepped up in myocardial perfusion imaging. CTP needs to raise the level to also challenge other non-invasive tests. Comprehensive and uniform protocols as well as prognostic impact evaluations in prospective and randomized multi-center studies are needed, in conjunction with adequate training of cardiac CT readers.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO Fact sheet N°310, updated July 2013. http://www.who.int/mediacentre/factsheets/fs310/en/index.html
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics 2012 update: a report from the american heart association. Circulation. 2012;125:e2–220.
Widimsky P, Wijns W, Fajadet J, et al. Reperfusion therapy for ST elevation acute myocardial infarction in Europe: description of the current situation in 30 countries. Eur Heart J. 2010;31:943–57.
Lakkis N, Tsyboulev V, Gibson CM, Murphy SA, Weintraub WS, DiBattiste PM, et al. Outcome of patients with acute coronary syndrome admitted to hospitals with or without onsite cardiac catheterization laboratory: a TACTICS-TIMI 18 substudy. Crit Pathw Cardiol. 2002;1(4):232–7.
Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16.
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–24.
Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME study. J Am Coll Cardiol. 2010;56:177–84.
Pijls NHJ, van Schaardenburgh P, Manoharan G, Boersma E, Bech J-W, Van't Veer M, et al. Percutaneous Coronary Intervention of Functionally Nonsignificant Stenosis: 5-Year Follow-Up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–11.
Shaw LJ, Cerqueira MD, Brooks MM, Althouse AD, Sansing VV, Beller GA, et al. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol. 2012;19:658–69.
Phillips LM, Hachamovitch R, Berman DS, Iskandrian AE, Min JK, Picard MH, et al. Lessons learned from MPI and physiologic testing in randomized trials of stable ischemic heart disease: COURAGE, BARI 2D, FAME, and ISCHEMIA. J Nucl Cardiol. 2013;20(6):969–75.
Melikian N, De Bondt P, Tonino P, De Winter O, Wyffels E, Bartunek J, et al. Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc Interv. 2010;3(3):307–14.
Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the Short-Term Survival Benefit Associated With Revascularization Compared With Medical Therapy in Patients With No Prior Coronary Artery Disease Undergoing Stress Myocardial Perfusion Single Photo Emission Computed Tomography. Circulation. 2003;107:2900–7.
Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362(10):886–95.
Maron DJ, Stone GW, Berman DS, Mancini GB, Scott TA, Byrne DW, et al. Is cardiac catheterization necessary before initial management of patients with stable ischemic heart disease? Results from a Web-based survey of cardiologists. Am Heart J. 2011;162(6):1034–43.
Arora N, Matheny ME, Sepke C, Resnic FS. A propensity analysis of the risk of vascular complications after cardiac catheterization procedures with the use of vascular closure devices. Am Heart J. 2007;153:606–11.
Bettencourt N, Ferreira N, Chiribiri A, Schuster A, Sampaio F, Santos L, et al. Additive value of magnetic resonance coronary angiography in a comprehensive cardiac magnetic resonance stress-rest protocol for detection of functionally significant coronary artery disease: a pilot study. Circ Cardiovasc Imaging. 2013;6(5):730–8.
Schuetz GM, Zacharopoulou NM, Schlattmann P, Dewey M. Meta-analysis: noninvasive coronary angiography using computed tomography versus magnetic resonance imaging. Ann Intern Med. 2010;152(3):167–77.
Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308(8):788–95.
Chow BJ, Small G, Yam Y, Chen L, Achenbach S, Al-Mallah M, et al. Incremental prognostic value of cardiac computed tomography in coronary artery disease using CONFIRM: Coronary computed tomography angiography evaluation for clinical outcomes: an InteRnational Multicenter registry. Circ Cardiovasc Imaging. 2011;4:463–72.
Pontone G, Andreini D, Bartorelli AL, Bertella E, Cortinovis S, Mushtaq S, et al. A Long-Term Prognostic Value of CT Angiography and Exercise ECG in Patients With Suspected CAD. J Am Coll Cardiol Img. 2013;6(6):641–50.
Vanhoenacker PK, Heijenbrok-Kal MH, Van Heste R, Decramer I, Van Hoe LR, Wijns W, et al. Diagnostic performance of multidetector CT angiography for assessment of coronary artery disease: meta-analysis. Radiology. 2007;244(2):419–28.
Alkadhi H, Scheffel H, Desbiolles L, Gaemperli O, Stolzmann P, Plass A, et al. Dual-source computed tomography coronary angiography: influence of obesity, calcium load, and heart rate on diagnostic accuracy. Eur Heart J. 2008;29(6):766–76.
Vavere AL, Arbab-Zadeh A, Rochitte CE, Dewey M, Niinuma H, Gottlieb I, et al. Coronary artery stenoses: accuracy of 64-detector row CT angiography in segments with mild, moderate, or severe calcification: a subanalysis of the CORE-64trial. Radiology. 2011;261:100–8.
Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Eng J Med. 2008;359:2324–36.
Weustink AC, Nieman K, Pugliese F, Mollet NR, Meijboom WB, van Mieghem C, et al. Diagnostic accuracy of computed tomography angiography in patients after bypass grafting: comparison with invasive coronary angiography. JACC Cardiovasc Imaging. 2009;2:816–24.
Onuma Y, Dudek D, Thuesen L, Webster M, Nieman K, Garcia-Garcia HM, et al. Five-Year Clinical and Functional Multislice Computed Tomography Angiographic Results After Coronary Implantation of the Fully Resorbable Polymeric Everolimus-Eluting Scaffold in Patients With De Novo Coronary Artery Disease: The ABSORB Cohort A Trial. JACC Cardiovasc Interv. 2013;6(10):999–1009.
Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33(6):734–44.
Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, Anderson RD, et al. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166(1):134–41.
Gosling O, Loader R, Venables P, Rowles N, Morgan-Hughes G, Roobottom C. Cardiac CT: are we underestimating the dose? A radiation dose study utilizing the 2007 ICRP tissue weighting factors and a cardiac specific scan volume. Clin Radiol. 2010;65(12):1013–7.
Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301(5):500–7.
Hausleiter J, Meyer TS, Martuscelli E, Spagnolo P, Yamamoto H, Carrascosa P, et al. Image quality and radiation exposure with prospectively ECG-triggered axial scanning for coronary CTangiography: the multicenter, multivendor, randomized PROTECTION-III study. JACC Cardiovasc Imaging. 2012;5(5):484–93.
Achenbach S, Dilsizian V, Kramer CM, Zoghbi WA. The year in coronary artery disease. JACC Cardiovasc Imaging. 2009;2(6):774–86.
Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52(8):636–43.
Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34(38):2949–3003.
Balachandran KP, Berry C, Norrie J, Vallance BD, Malekianpour M, Gilbert TJ, et al. Relation between coronary pressure derived collateral flow, myocardial perfusion grade, and outcome in left ventricular function after rescue percutaneous coronary intervention. Heart. 2004;90(12):1450–4.
Fischer JJ, Samady H, McPherson JA, Sarembock IJ, Powers ER, Gimple LW, et al. Comparison between visual assessment and quantitative angiography versus fractional flow reserve for native coronary narrowings of moderate severity. Am J Cardiol. 2002;90(3):210–5.
Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379:453–60.
Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–9.
Schwitter J, Wacker CM, Wilke N, Al-Saadi N, Sauer E, Huettle K, et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34(10):775–81.
Valenta I, Dilsizian V, Quercioli A, Ruddy TD, Schindler TH. Quantitative PET/CT measures of myocardial flow reserve and atherosclerosis for cardiac risk assessment and predicting adverse patient outcomes. Curr Cardiol Rep. 2013;15(3):344.
Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013. J Am Coll Cardiol. 2013;61(22):2233–41.
Nakazato R, Park HB, Berman DS, Gransar H, Koo BK, Erglis A, et al. Noninvasive Fractional Flow Reserve Derived From Computed Tomography Angiography for Coronary Lesions of Intermediate Stenosis Severity: Results From the DeFACTO Study. Circ Cardiovasc Imaging. 2013;6(6):881–9.
Yoon YE, Choi JH, Kim JH, Park KW, Doh JH, Kim YJ, et al. Noninvasive diagnosis of ischemia-causing coronary stenosis using CT angiography: diagnostic value of transluminal attenuation gradient and fractional flow reserve computed from coronary CT angiography compared to invasively measured fractional flow reserve. JACC Cardiovasc Imaging. 2012;5(11):1088–96.
Bettencourt N, Rocha J, Ferreira N, Pires-Morais G, Carvalho M, Leite D, et al. Incremental value of an integrated adenosine stress-rest MDCT perfusion protocol for detection of obstructive coronary artery disease. J Cardiovasc Comput Tomogr. 2011;5(6):392–405.
Kurata A, Mochizuki T, Koyama Y, Haraikawa T, Suzuki J, Shigematsu Y, et al. Myocardial perfusion imaging using adenosine triphosphate stress multi-slice spiral computed tomography: alternative to stress myocardial perfusion scintigraphy. Circulation. 2005;69(5):550–7.
Kido T, Kurata A, Higashino H, Inoue Y, Kanza RE, Okayama H, et al. Quantification of regional myocardial blood flow using first-pass multidetector-row computed tomography and adenosine triphosphate in coronary artery disease. Circulation. 2008;72(7):1086–91.
George RT, Arbab-Zadeh A, Miller JM, Kitagawa K, Chang HJ, Bluemke DA, et al. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009;2(3):174–82.
Magalhães TA, Cury RC, Pereira AC, Moreira VM, Lemos PA, Kalil-Filho R, et al. Additional value of dipyridamole stress myocardial perfusion by 64-row computed tomography in patients with coronary stents. J Cardiovasc Comput Tomogr. 2011;5(6):449–58.
Bettencourt N, Chiribiri A, Schuster A, Ferreira N, Sampaio F, Pires-Morais G, et al. Direct comparison of cardiac magnetic resonance and multidetector computed tomography stress-rest perfusion imaging for detection of coronary artery disease. J Am Coll Cardiol. 2013;61(10):1099–107. Larger unicenter study.
Blankstein R, Shturman LD, Rogers IS, Rocha-Filho JA, Okada DR, Sarwar A, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol. 2009;54(12):1072–84.
Rocha-Filho JA, Blankstein R, Shturman LD, Bezerra HG, Okada DR, Rogers IS, et al. Incremental value of adenosine-induced stress myocardial perfusion imaging with dual-source CT at cardiac CT angiography. Radiology. 2010;254(2):410–9.
Tamarappoo BK, Dey D, Nakazato R, Shmilovich H, Smith T, Cheng VY, et al. Comparison of the extent and severity of myocardial perfusion defects measured by CT coronary angiography and SPECT myocardial perfusion imaging. JACC Cardiovasc Imaging. 2010;3(10):1010–9.
Okada DR, Ghoshhajra BB, Blankstein R, Rocha-Filho JA, Shturman LD, Rogers IS, et al. Direct comparison of rest and adenosine stress myocardial perfusion CT with rest and stress SPECT. J Nucl Cardiol. 2010;17(1):27–37.
Cury RC, Magalhães TA, Borges AC, Shiozaki AA, Lemos PA, Júnior JS, et al. Dipyridamole stress and rest myocardial perfusion by 64-detector row computed tomography in patients with suspected coronary artery disease. Am J Cardiol. 2010;106(3):310–5.
Ho KT, Chua KC, Klotz E, Panknin C. Stress and rest dynamic myocardial perfusion imaging by evaluation of complete time-attenuation curves with dual-source CT. JACC Cardiovasc Imaging. 2010;3(8):811–20.
Feuchtner G, Goetti R, Plass A, Wieser M, Scheffel H, Wyss C, et al. Adenosine stress high-pitch 128-slice dual-source myocardial computed tomography perfusion for imaging of reversible myocardial ischemia: comparison with magnetic resonance imaging. Circ Cardiovasc Imaging. 2011;4(5):540–9.
Bamberg F, Becker A, Schwarz F, Marcus RP, Greif M, von Ziegler F, et al. Detection of hemodynamically significant coronary artery stenosis: incremental diagnostic value of dynamic CT-based myocardial perfusion imaging. Radiology. 2011;260(3):689–98.
Ko SM, Choi JW, Song MG, Shin JK, Chee HK, Chung HW. Myocardial perfusion imaging using adenosine-induced stress dual-energy computed tomography of the heart: comparison with cardiac magnetic resonance imaging and conventional coronary angiography. Eur Radiol. 2011;21(1):26–35.
Wang Y, Qin L, Shi X, Zeng Y, Jing H, Schoepf UJ, et al. Adenosine-stress dynamic myocardial perfusion imaging with second-generation dual-source CT: comparison with conventional catheter coronary angiography and SPECT nuclear myocardial perfusion imaging. AJR Am J Roentgenol. 2012;198(3):521–9.
Weininger M, Schoepf UJ, Ramachandra A, Fink C, Rowe GW, Costello P, et al. Adenosine-stress dynamic real-time myocardial perfusion CT and adenosine-stress first-pass dual-energymyocardial perfusion CT for the assessment of acute chest pain: initial results. Eur J Radiol. 2012;81(12):3703–10. Evaluation of CTP diagnostic accuracy in a emergency department.
Kim SM, Choi JH, Chang SA, Choe YH. Additional value of adenosine-stress dynamic CT myocardial perfusion imaging in the reclassification of severity of coronary artery stenosis at coronary CT angiography. Clin Radiol. 2013;68(12):e659–68.
Choo KS, Hwangbo L, Kim JH, Park YH, Kim JS, Kim J, Chun KJ, Jeong DW, Lim SJ. Adenosine-stress low-dose single-scan CT myocardial perfusion imaging using a 128-slice dual-source CT: a comparison with fractional flow reserve. Acta Radiol. 2013; Apr 2. [Epub ahead of print]
Greif M, von Ziegler F, Bamberg F, Tittus J, Schwarz F, D'Anastasi M, et al. CT stress perfusion imaging for detection of haemodynamically relevant coronary stenosis as defined by FFR. Heart. 2013;99(14):1004–11.
Ko BS, Cameron JD, Meredith IT, Leung M, Antonis PR, Nasis A, et al. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: a comparison with fractional flow reserve. Eur Heart J. 2012;33(1):67–77.
George RT, Arbab-Zadeh A, Miller JM, Vavere AL, Bengel FM, Lardo AC, et al. Computed tomography myocardial perfusion imaging with 320-row detector computed tomography accurately detects myocardial ischemia in patients with obstructive coronary artery disease. Circ Cardiovasc Imaging. 2012;5(3):333–40. Rest scan performed first previous to stress; also with delayed enhancement acquisition.
Nasis A, Ko BS, Leung MC, Antonis PR, Nandurkar D, Wong DT, et al. Diagnostic accuracy of combined coronary angiography and adenosine stress myocardial perfusion imaging using 320-detector computed tomography: pilot study. Eur Radiol. 2013;23(7):1812–21.
Rief M, Zimmermann E, Stenzel F, Martus P, Stangl K, Greupner J, et al. Computed tomography angiography and myocardial computed tomography perfusion in patients with coronary stents: prospective intraindividual comparison with conventional coronary angiography. J Am Coll Cardiol. 2013;62(16):1476–85. Aditional value of CTP in patients with coronary stents.
Rochitte CE, George RT, Chen MY, Arbab-Zadeh A, Dewey M, Miller JM, et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study. Eur Heart J. 2014;35(17):1120–30. Larger study to date and first multicenter with 381 patients studied.
Techasith T, Cury RC. Stress myocardial CT perfusion: an update and future perspective. JACC Cardiovasc Imaging. 2011;4(8):905–16.
Zoghbi GJ, Dorfman TA, Iskandrian AE. The effects of medications on myocardial perfusion. J Am Coll Cardiol. 2008;52(6):401–16.
Cury RC, Magalhães TA, Paladino AT, Shiozaki AA, Perini M, Senra T, et al. Dipyridamole stress and rest transmural myocardial perfusion ratio evaluation by 64 detector-row computed tomography. J Cardiovasc Comput Tomogr. 2011;5(6):443–8.
Bischoff B, Bamberg F, Marcus R, Schwarz F, Becker HC, Reiser M. Optimal timing for first-pass stress CT myocardial perfusion imaging. Int J Cardiovasc Imaging. 2013;29(2):435–42.
Gerber BL, Belge B, Legros GJ, Lim P, Poncelet A, Pasquet A, et al. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113(6):823–33.
Ko SM, Choi JW, Hwang HK, Song MG, Shin JK, Chee HK. Diagnostic performance of combined noninvasive anatomic and functional assessment with dual-source CT and adenosine-induced stress dual-energy CT for detection of significant coronary stenosis. AJR Am J Roentgenol. 2012;198(3):512–20.
Pope JH, Aufderheide TP, Ruthazer R, Woolard RH, Feldman JA, Beshansky JR, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;81(12):3703–10.
Acknowledgments
Vitor Ramos had a grant of Portuguese Society of Cardiology.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Vítor Ramos, Nuno Dias Ferreira, and Nuno Bettencourt declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Cardiac Computed Tomography
Rights and permissions
About this article
Cite this article
Ramos, V., Ferreira, N.D. & Bettencourt, N. Integrating Anatomical and Functional Assessment of Coronary Artery Disease: Can MDCT act as the lone Gatekeeper in the near Future?. Curr Cardiovasc Imaging Rep 7, 9292 (2014). https://doi.org/10.1007/s12410-014-9292-y
Published:
DOI: https://doi.org/10.1007/s12410-014-9292-y