Abstract
Attention-deficit/hyperactivity disorder (ADHD) is highly associated with the delayed sleep phase disorder, a circadian rhythm sleep–wake disorder, which is prevalent in 73–78% of children and adults with ADHD. Besides the delayed sleep phase disorder, various other sleep disorders accompany ADHD, both in children and in adults. ADHD is either the cause or the consequence of sleep disturbances, or they may have a shared etiological and genetic background. In this review, we present an overview of the current knowledge on the relationship between the circadian rhythm, sleep disorders, and ADHD. We also discuss the various pathways explaining the connection between ADHD symptoms and delayed sleep, ranging from genetics, behavioral aspects, daylight exposure, to the functioning of the eye. The treatment options discussed are focused on improvement of sleep quality, quantity, and phase-resetting, by means of improving sleep hygiene, chronotherapy, treatment of specific sleep disorders, and by strengthening certain neuronal networks involved in sleep, e.g., by sensorimotor rhythm neurofeedback. Ultimately, the main question is addressed: whether ADHD needs to be redefined. We propose a novel view on ADHD, where a part of the ADHD symptoms are the result of chronic sleep disorders, with most evidence for the delayed circadian rhythm as the underlying mechanism. This substantial subgroup should receive treatment of the sleep disorder in addition to ADHD symptom treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep problems are often regarded as a comorbidity in psychiatric disorders. Since the first publications on the connection between sleep problems and attention-deficit/hyperactivity disorder (ADHD) in the early 1970s (Conners 1970), many studies have followed. In the past decade, knowledge has moved ahead more quickly with many pivotal publications on this topic. Recent systematic reviews by Diaz-Roman et al. showed that there is an association between ADHD and both subjective and objective sleep disturbances in both children and in adults (Diaz-Roman et al. 2016, 2018). Another recent systematic review of 22 studies by Coogan and McGowan (2017) showed consistent evidence for the association between ADHD and a later chronotype or a delayed sleep. One study even implicated a causative role of the circadian rhythm and sleep problems in a subgroup of patients with ADHD (Arns and Kenemans 2014).
This review aims to present the current insights into the role of the circadian rhythm and sleep in ADHD, and an overview of past and recent studies on this topic. The first paragraphs give a general introduction to the basic science of sleep, circadian rhythm, and the consequences of sleep and circadian rhythm problems. From there, the genetic, etiological, and functional connection between ADHD circadian rhythm misalignment and sleep problems is discussed. The final paragraphs focus on diagnostic, treatment, and prevention possibilities, and recommendations for future studies. Ultimately, we evaluate whether the time has come to redefine the current view on ADHD. We present our hypothesis, based on the current insights, stating that in patients with ADHD, at least a part of the symptoms of ADHD are the result of chronic sleep disorders, with most evidence for the delayed circadian rhythm as the underlying mechanism.
Sleep basics
Humans spend about one-third of their lives in a sleeping state, although the function and implications of this ‘inactive state’ are not fully understood to date. However, we do know what happens if we don’t sleep. From case studies and experiments, it is known that sleep is needed for the restoration of bodily functions, memory consolidation, and elevation of mood, cognitive function, and general health, and plausibly for healthy brain development (Kurth et al. 2016). In this paragraph, some of the basics of sleep science are elaborated.
The two-process model
A well-known, validated, and accepted model in sleep medicine is the two-process model by Borbély (1982). This model postulates the sleep pressure Process-S and the circadian Process-C, see Fig. 1. Both Process-S and Process-C, and especially their interaction, play a crucial role in sleep–wake regulation and optimal vigilance (i.e., alertness) regulation. Throughout this review, the two-process model of sleep will be used to explain some of the mechanisms involved in sleep deprivation and disorders.
Process-S reflects the increase in sleep pressure, or drowsiness, and is a function of the duration of wakefulness which starts accumulating after waking up in the morning (Achermann et al. 1993). This drowsiness can be quantified using the electroencephalogram (EEG) and is often reflected as frontal theta activity, a slow EEG rhythm (Arns and Kenemans 2014). This slow theta activity builds up during the day and shows a gradual decline during sleep.
The circadian Process-C reflects the individuals’ biological clock, which fluctuates with a cycle of about 24 h (hence the term ‘circadian’). Figure 1 depicts how processes S and C interact. The larger the distance between processes S and C, the higher the sleep pressure, indicating the most likely moment of sleep initiation.
Sleep stages
Normal sleep consists of several consecutive sleep stages that occur in a cyclic pattern of approximately 90 min per sleep cycle. The most widely used guidelines for the definition of sleep stages are those by the American Academy of Sleep Medicine (AASM) (Berry et al. 2015). The AASM recommends the subdivision of the following sleep stages: REM (rapid eye movement), N1 (non-REM stage 1), N2, and N3, where N1–N3 is graded from light to deep sleep respectively. N1 is also referred to as drowsiness or shallow sleep and is characterized by low-amplitude and mixed frequency brain activity as quantified by EEG. N2 is the transition phase from N1 to N3 and is characterized by the typical N1 EEG signal, plus short bouts of high-voltage activity or high amplitude (sleep spindles and K-complexes, respectively). N3 is the deep sleep phase, which is characterized by high-amplitude slow wave EEG. The REM sleep phase is distinguished by low muscle tone (except for the tiny muscles such as present in the eye) and is also referred to as the ‘dream’ phase of sleep.
From the start to the end of a night’s sleep, the relative amount of time spent in N3 (deep) sleep declines per cycle, while the relative duration of REM sleep increases over the sleep cycles. The first one or two sleep cycles are therefore regarded as ‘restorative’ sleep, while the last sleep cycles are more dominated by dreaming. Sleep also changes over the course of life. In a meta-analysis of 65 studies among 3577 healthy sleepers from age 5 up to 102 years old, it was reported that time spent in sleep stadia N1 and N2 increases with age, while time in N3 and REM sleep decreases (Ohayon et al. 2004).
Circadian rhythm and chronotype
Many people are known as typical ‘morning’ or ‘evening’ people, also referred to as chronotypes, but most people fall in between these chronotypes (Roenneberg et al. 2003). A person’s chronotype is to a large part genetically determined (Brown et al. 2008). Chronotype can alter somewhat over the lifespan and differs most between genders in adolescence (Paine et al. 2006; Roenneberg et al. 2004). For instance, a higher percentage of adolescents are classified as evening chronotype relative to adults, of which more boys than girls (Roenneberg et al. 2003). Among the general population, 2–26% of adults are evening chronotypes (Paine et al. 2006; Taillard et al. 2004). This wide prevalence range is due to the different age ranges examined and to the different methods used for assessment of chronotype.
The circadian rhythm is regulated by the suprachiasmatic nuclei (SCN) in the brain. The SCN synchronizes to endogenous clock signals such as various hormonal statuses and to the external environment making use of so-called zeitgebers, such as daylight, environmental temperature, and food availability (Roenneberg et al. 2007). A crucial zeitgeber is daylight intensity reaching the retina of the eye, which gives the SCN information about the time of the day, thereby leading to photoentrainment of the internal clock system.
The SCN orchestrates many complex ‘timed’ internal systems such as body temperature, endocrine functions, and blood pressure through autonomous rhythms. The individual’s sleep/wake cycle is also directed by the SCN. Internal and external zeitgebers are translated to information about the time of the day by the SCN. Human photoentrainment is predominantly linked to dusk (Kantermann et al. 2007), when daylight intensity is diminished and its color spectrum shifts from blue to red. That is when the SCN signals the pineal gland to produce melatonin, the ‘sleep hormone’ (Benarroch 2011). The rise of the endogenous melatonin concentration is often used as the phase marker of the circadian rhythm (Process-C in Fig. 1) and can be measured in blood and saliva. The time of the day that the melatonin concentration in saliva reaches the threshold of 3 pg/ml is termed the dim-light melatonin onset (DLMO) (Lewy and Sack 1989). Sleep is typically initiated 2–3 h after the time of DLMO (Lewy 2007). The most widely used biomarkers for the circadian phase are the DLMO, the core-body temperature, and objective actigraphy measures.
Impact of sleep disturbances
Disturbance of sleep can have consequences, as we all experience at least occasionally. While occasional sleep deprivation is evident to have detrimental effects on cognitive functioning (Slama et al. 2017), comparable effects have been found after sustained sleep restriction (Van Dongen et al. 2003), i.e., limiting the amount of time in bed. In a sleep restriction study, participants were allowed to sleep for 6 h for 2 weeks, which led to a decline of sustained attention and working memory that was equal to two nights of complete sleep deprivation. In contrast to participants that were sleep-deprived, sleep-restricted participants were unaware of their cognitive deficits. Similar findings have been reported after 5–7 days of sleep restriction (Axelsson et al. 2008; Belenky et al. 2003). These studies also showed that these cognitive deficits, most specifically inattention, needed more days of normal sleep in order to fully recover than the duration of the initial sleep restriction (Axelsson et al. 2008; Belenky et al. 2003). Sleep restriction studies have also been conducted in children, albeit not as extensively as in adults. In general, sleep restriction studies in healthy children have demonstrated impairments of attention (Fallone et al. 2001, 2005; Sadeh et al. 2003; Beebe et al. 2008) and more impaired behavior regulation after 1 week of sleep restriction (Belenky et al. 2003). Thus, core symptoms of ADHD such as inattention and externalizing behavior can be induced in healthy children through sleep restriction (Fallone et al. 2001; Golan et al. 2004), suggesting a role for chronic sleep debt in the etiology of ADHD.
In a meta-analysis of 12 studies on 35,936 healthy children between 5 and 12 years of age, Astill and colleagues (Astill et al. 2012) demonstrated clear associations between longer sleep duration and better executive function and school performances, and also between shorter sleep and more internalizing and externalizing behavior problems. Also adolescents that go to bed late have lower school performances (Zerbini and Merrow 2017). Several studies demonstrated that when morning school-time was delayed by half an hour, sleep duration increased by 29–45 min, with subsequent reductions in daytime sleepiness, depressed mood and caffeine use (Boergers et al. 2014; Owens et al. 2010). In a multicenter study among 9000 students, it was even shown that when school started 90 min later (a shift from 7.35 to 8.55 am), the number of car crashes among teen drivers reduced by 70% (Wahlstrom et al. 2014).
A systematic review with data on 690,747 children from 20 countries showed that children nowadays on average sleep 1 h and 15 min less than a century ago (Matricciani et al. 2012) and thus may be for a larger part chronically sleep-restricted. It seems that children and adolescents today generally sleep too short, supported by a trend for increased signs of drowsiness in healthy children over 10 years’ time, as objectified using EEG (Arns et al. 2013a) (reflecting Process-S, depicted in Fig. 1).
Sleep and circadian rhythm in ADHD
In non-experimental settings, sleep disturbance is associated with behavioral characteristics of ADHD in both healthy individuals (Kass et al. 2003; Gau et al. 2007) and non-medicated individuals diagnosed with ADHD (Mahajan et al. 2010). In the following paragraphs, these associations will be further discussed.
Sleep disorders associated with ADHD
In children with ADHD, there is a vast amount of literature on the increased prevalence of various sleep disorders and sleep problems, including delayed sleep–wake disorder, insomnia, sleep-disordered breathing, increased nocturnal motor activity, restless legs, and parasomnias such as sleep anxiety and teeth clenching (Van der Heijden et al. 2005a, b; Tsai et al. 2016; Mota-Veloso et al. 2017; Melegari et al. 2016). Furthermore, a systematic review showed that children with ADHD spend relatively more time in N1 (shallow) sleep as compared to controls (Diaz-Roman et al. 2016). As a result of the lower sleep quality, children with ADHD have increased daytime sleepiness (Velez-Galarraga et al. 2016; Craig et al. 2017). Moreover, the severity of sleep problems was correlated to poorer cognitive functioning in children with ADHD (Sciberras et al. 2015). Of the adolescents with ADHD, 73% reports any sleep problem, and 42% has daytime sleepiness (Langberg et al. 2017; Hysing et al. 2016). Finally, among adolescents from the general population, more ADHD symptoms were associated with more delayed sleep, shorter sleep, longer time awake before falling asleep, more nocturnal wake time, higher sleep deficiency, and more insomnia (Hysing et al. 2016).
In adults with ADHD, sleep is also affected: 78% of them have a delayed circadian rhythm as objectively measured by actigraphy and DLMO, and an increased prevalence of short sleep as compared to healthy controls (van Veen et al. 2010; Bijlenga et al. 2013a). The Restless Legs Syndrome (RLS) is prevalent among 35–44%, and insomnia in 67% of adults with ADHD (Cortese et al. 2005; Snitselaar et al. 2015; Brevik et al. 2017). The prevalence of sleep apnea in adults with ADHD has not been established yet, but there are indications that symptoms of sleep apnea are related to ADHD symptoms (Vogel et al. 2017). For example, in sleep medicine it is taught that a cardinal feature of sleep apnea is hyperactive behavior during the day. There are also more symptoms of sleep apnea in those with ADHD as compared to controls (Bjorvatn et al. 2017). A recent longitudinal twin study showed that children with ADHD had poorer sleep quality in young adulthood, but only if their ADHD persisted (Gregory et al. 2017). Conversely, the severity of sleep problems in children with ADHD is an important predictor for the persistence of ADHD into young adulthood (Cadman et al. 2016). The two thus seem intimately intertwined across the lifespan in individuals with ADHD.
A few studies in children have reported a decrease of ADHD symptoms after treatment of specific sleep problems and disorders. These included a sleep coaching intervention for sleep-onset insomnia (Corkum et al. 2016), treatment of sleep apnea by removal of the adenoid and tonsils (Huang et al. 2007), or dopaminergic therapy for restless legs syndrome (Walters et al. 2000), suggestive of a more causative relation between the ADHD symptoms and the present sleep disorder.
The relationship between sleep problems and the two symptom domains of ADHD is not clear yet. Some studies report a relationship between sleep problems and symptoms of hyperactivity/impulsivity (Bijlenga et al. 2013a; McGowan et al. 2016), but a meta-analysis including 13 studies relates sleep problems mainly to symptoms of inattention (Lundahl et al. 2015). Our previous population study links sleep problems to both symptom domains (Vogel et al. xxxx).
Circadian rhythm and ADHD symptoms
Of all sleep problems associated with ADHD, a delayed sleep/wake cycle is the most common (i.e., a delayed circadian rhythm) (Coogan and McGowan 2017; Snitselaar et al. 2017; Kooij and Bijlenga 2013), with an objectively measured prevalence of 73–78% in both children and adults with ADHD (Craig et al. 2017; van Veen et al. 2010). Following Fig. 1, a delayed Process-C ‘pushes’ Process-S, resulting in a delayed sleep propensity and later sleep. Waking up at regular times results in shorter sleep, non-restored sleep propensity (i.e., daytime sleepiness), and accumulated sleep propensity over the days. Eventually, a chronically delayed rhythm will ‘push’ Process-S to the limit, resulting in mental and physical complaints. This is similar to the impaired attention and executive function as a potential result of the delayed rhythm and subsequent sleep restriction seen in this population. While many people from the general population have an evening chronotype, only just 0.1–3.1% fulfills diagnostic criteria for the delayed sleep phase syndrome (DSPS) (Yazaki et al. 1999; Schrader et al. 1993; Sivertsen et al. 2013). According to self-reports, the DSPS prevalence in adults with ADHD is at least 26%, which is a huge increase as compared to the general population (Bijlenga et al. 2013a). Other studies have investigated the occurrence of sleep-onset insomnia (SOI), which is difficulty falling asleep and/or a sleep-onset latency of more than 30 min. In the literature, SOI and DSPS are both used to characterize a delayed circadian rhythm in adults with ADHD. SOI is present in 72–78% of non-medicated children and adults with ADHD, using DLMO as the objective circadian marker (Van der Heijden et al. 2005b; Van Veen et al. 2010). In another study, we found that the time span between DLMO and sleep initiation was on average about an hour longer in those with ADHD and a delayed circadian rhythm, as compared to healthy controls (Bijlenga et al. 2013b). This trend is also confirmed from subjective reports, where 57% of adults and children with ADHD had SOI compared to 18% in controls (Arns et al. 2014a). This may indicate lower synaptic sensitivity to melatonin and/or perhaps a behavioral aspect leading to sleep procrastination.
The link between sleep problems and ADHD
The functional and neuroanatomical overlap between brain regions involved in attention, arousal, and sleep regulation reflects the complex relationship between ADHD and sleep (Owens 2008). Sleep problems may be causes, effects, or intrinsic features of ADHD (Hvolby 2015).
For instance, in young children we are all familiar with the hyperactive, ‘high-spirited’ behavior when they are very tired. These children compensate for their fatigue with hyperactive behavior (O’Brien 2009; Hegerl and Hensch 2014). In this example, hyperactivity is caused by sleepiness and is regarded as a vigilance autostabilization behavior (i.e., keeping yourself awake by moving/talking). A healthy adult experiencing drowsiness at home near bedtime will feel sleepy and will decide to ‘withdraw,’ seeking an environment with low external stimulation, thus increasing the probability of falling asleep. However, when this same healthy adult is driving a car experiencing the same drowsiness, he will try to avoid further drowsiness by turning up the volume of the radio, open the window and lower the temperature by turning down the heating, and so on. Hence, this healthy person will exhibit autostabilization or externalizing behavior in order to stay awake. This autostabilization behavior can thus be either adaptive (i.e., keeping oneself awake while driving a car) or maladaptive (i.e., the hyperactivity in children with ADHD and the constant mind wandering in adults with ADHD), depending on the circumstance and chronicity.
However, hyperactive behavior in the evening may also be the cause of sleep-onset problems (Cortese et al. 2009). A child exhibiting hyperactive behavior in the evening may seem full of energy and thus postpone bedtime. Also, adults may experience internal hyperactivity such as internal restlessness, many thoughts, or rumination that keeps them awake.
Another link between sleep and ADHD is that sleep disorders may also lead to symptoms, behaviors or functional impairments that mimic those in ADHD, such as concentration problems, learning impairment, problematic behavior, and emotion dysregulation (O’Brien 2009). This points to the direction that sleep problems and ADHD share intrinsic features. In a recent study among healthy individuals, the trait impulsivity was associated with objective measures of phase delay, lower sleep quality and sleep efficiency (McGowan and Coogan 2018). Furthermore, medical treatment of ADHD also impacts sleep, with limited evidence for both positive effects in children (Owens et al. 2016) and adults (Kooij et al. 2001), and negative effects in children (Becker et al. 2016; Santisteban et al. 2014). Moreover, children with ADHD with a longer sleep duration before the start of their treatment have a higher chance of better treatment response (Morash-Conway et al. 2017).
The delayed circadian rhythm and ADHD have genetic associations and shared environmental factors, and may have shared etiology. Other sleep problems may also contribute to the severity of ADHD symptoms. All of these factors interact and influence each other. An important environmental factor is exposure at night to blue light sources such as LED lights and the use of light-emitting sources such as smartphones or tablets (Chaste et al. 2011; Baird et al. 2011; Bijlenga et al. 2011; Arns et al. 2013b, c), for review see (Roenneberg and Merrow 2016). Using smartphone data, researchers found that social media activities delayed bedtimes and led to shorter sleep duration (Walch et al. 2016). This may be explained by both the effect of the light emitted by the smartphone, and also by postponing sleep because of arousal from engagement in the social media activities.
Sleep problems that emerged in childhood may have had functional and neuronal consequences, for neuronal networks involved in sleep, sleep behavior, and for persistence of the ADHD symptoms in later life (Kurth et al. 2016). In order to understand the consequences of sleep problems in early childhood, longitudinal studies are needed that focus on the functional and behavioral effects of chronic sleep problems. While a minority of all patients diagnosed with ADHD do not experience sleep problems, the proposed hypothesis holds for the (larger) subgroup of patients with ADHD that has sleep problems.
Genetics
The heritability of chronotype is estimated to be approximately 50% (Kalmbach et al. 2017). Across three important genome-wide association studies (GWAS), nine genes were identified that are responsible for morningness (Jones et al. 2016; Lane et al. 2016; Hu et al. 2016), reviewed in Kalmbach et al. (2017). For the identification of genes responsible for eveningness, however, only candidate-gene studies have been performed thus far. Some of the variations in genes that are responsible for a lengthening of the sleep/wake cycle, resulting in a longer than 24-h circadian rhythm (Process-C, Fig. 1) and thus a delayed sleep, have also been linked to ADHD (Coogan and McGowan 2017; Baird et al. 2012; Xu et al. 2010). One of these is the CLOCK gene, which has been linked to ADHD, bipolar, and depressive disorder (Coogan and McGowan 2017; Xu et al. 2010; Benedetti et al. 2003). The BMAL1 and PER2 genes are also involved in both delayed sleep and in ADHD: both genes showed decreased circadian rhythmicity in ADHD subjects as compared to healthy adults (Baird et al. 2012). The alleles upstream from PAX8 are associated with sleep duration and with thyroid function, and less copies of the minor alleles are associated with both shorter sleep duration and with ADHD symptoms (Gottlieb et al. 2015).
Solar intensity and ADHD
Late chronotypes have significant variation in their average sleep duration across the year, especially from the end of March until end of October, i.e., during Daylight Saving Time (DST) they sleep less, while earlier chronotypes do not (Allebrandt et al. 2014). Intense natural light in the morning counteracts phase-delaying effects (Lewy et al. 1987). People that are typically exposed to outdoor (natural) light go to sleep earlier, and sleep more than those typically exposed to indoor (non-natural) light (Walch et al. 2016). A delayed circadian phase was advanced by morning bright light therapy in two pilot studies among adults with ADHD (Fargason et al. 2017; Rybak et al. 2006). Consistent with this, Arns et al. found that among people without Northern (i.e., Scandinavian) genetic background (hypothesized to be less susceptible to variation in sunlight intensity, as discussed in Arns et al. 2015), there is a strong geographical correlation between higher solar intensity and a lower prevalence of ADHD (Arns et al. 2013b, 2014b). This relationship is explained by the fact that sunlight intensity serves as an important cue for the brain’s circadian rhythm regulation, where high daylight intensity is a stronger cue than low daylight intensity to synchronize the circadian rhythm that also improves deep sleep (Lewy 2007; Roenneberg et al. 2007; Wams et al. 2017). Those with a genetic disposition to a lengthening of the sleep cycle may therefore profit from stronger synchronization cues such as high daylight intensity as well as dark evenings and nights, leading to a better synchronized circadian rhythm, better sleep, and less ADHD symptoms.
There are indications that there is an early ‘imprint’ or programming of the biological clock relative to light intensity or day length, which occurs in the weeks or months after birth. In mice, exposure to light in the perinatal period determines the responsiveness of its biological clock to subsequent changes in day length changes (i.e., changes of the ‘photoperiod’) (Ciarleglio et al. 2011). Also in laboratory studies in humans, there are indications of an adaptation of the circadian system according to prior light exposure (Chang et al. 2011). Besides the genetic makeup, the season of birth may also influence the development of one’s circadian system. Indeed, the prevalence of evening chronotypes in healthy individuals born in June and July is highest and lowest in December and January (Natale and Adan 1999; Natale et al. 2002), reviewed in Brooks and Canal 2013). Another study demonstrated that adolescents born in months associated with an increasing day length were later chronotypes than those born in months with decreasing day lengths (Vollmer et al. 2012). When the prevalence of ADHD was studied in relation to season of birth, Seeger et al. (2004) reported that being a 7R carrier of dopamine D4 receptors (one of the genetic risk factors associated with ADHD) (Nikolaidis and Gray 2010), and being born in spring or summer resulted in a 2.8 higher likelihood of being diagnosed with ‘hyperkinetic disorder’ (i.e., ADHD). However, in a much larger study, the hypothesized association between season of birth and ADHD was refuted after adjustment for multiple testing (Brookes et al. 2008). A note on the latter study, however, may be that the majority of the included subjects had a Northern genetic background (who are hypothesized to be less susceptible to variation in sunlight intensity, as discussed in Arns et al. 2015). This intriguing link is currently being investigated in more detail by the authors (Vollebregt and Arns 2016).
The role of the visual system
There are several studies indicating that the visual system in ADHD is also affected. In children with ADHD, 76% has reduced visual acuity, i.e., more strabismus (cross-eyedness), subnormal stereo-acuity (depth detection), convergence insufficiency, and/or smaller optic disks (Gronlund et al. 2007). The incidence of ADHD was threefold within a group of children having convergence insufficiency as compared to the general US population (Granet et al. 2005; Barnhardt et al. 2012). Another indication for visual system abnormalities in ADHD is the prevalence of as much as 83% of refractive errors in children with ADHD (Mezer and Wygnanski-Jaffe 2012). Furthermore, young adults with ADHD have more problems with depth perception, peripheral vision, and color perception, especially in the blue spectrum, as compared to matched controls (Kim et al. 2014; Banaschewski et al. 2006). Moreover, abnormalities in the visual field and the visual acuity in children with ADHD improved with ADHD medication (Martin et al. 2008). In another study, children with strabismus and increased ADHD symptoms had less ADHD symptoms after strabismus surgery, a result that gives rise to the idea that the eye problems caused or aggravated the ADHD symptoms in these children (Chung et al. 2012).
Besides the well-known rods and cones photoreceptor cells in the retina that are responsible for night and color vision, there are also retinal photoreceptor cells that are responsible for the non-image forming perception of light intensity. These are the M1-type intrinsically photosensitive retinal ganglion cells (ipRGCs), which modulate, among others, the pupillary reflex, the release of melatonin and dopamine, and project via the retinohypothalamic tract to the suprachiasmatic nuclei (SCN) (Schmidt and Kofuji 2009). Dopamine is released during daytime and inhibits melatonin secretion, and vice versa, melatonin is released in the evening and night and inhibits dopamine release (Stone et al. 2013). The photopigment melanopsin in these ipRGCs is most sensitive to blue light wavelengths (Lockley et al. 2003; Provencio et al. 2000). In addition to projection to the SCN, the ipRGCs also project to sleep-promoting neurons in the ventrolateral preoptic nucleus and superior colliculus (Lupi et al. 2008). The SCN synchronizes multiple peripheral clocks that will together drive circadian rhythmicity (Meijer et al. 2007).
The first studies linking ipRGC functioning to psychiatric disorders are relatively new. Roecklein et al. showed that patients with seasonal affective disorder (SAD) (‘winter depression’) had deviant ipRGC functioning compared to controls (Roecklein et al. 2013a, b). The SAD patients had a reduced pupil dilation after exposure to blue light, but not after red light. Roecklein et al. hypothesized that their SAD patients have a decreased blue-spectrum light sensitivity, which is responsible for a weaker circadian entrainment of the SCN to natural daylight. This could have triggered the depression during wintertime when natural daylight intensity is diminished. The prevalence of SAD is almost ten times as high among adults with ADHD as compared to the general population (Mersch et al. 1999; Amons et al. 2006). The functioning of the ipRGCs is hypothesized also to be suboptimal in ADHD. In our preliminary web survey, 69% of adults with ADHD reported oversensitivity of their eyes to bright light, versus 24% in those without ADHD (Kooij and Bijlenga 2014). Respondents with ADHD also reported to wear sunglasses significantly more hours during all seasons as compared to the control group, thereby possibly further compromising synchronization of the biological clock to daylight. This result supported the idea that the oversensitivity to light in the ADHD population reflects a deviant retinal development or functioning. This hypothesis is currently under further investigation by the authors, see the online Dutch trial register www.trialregister.nl, #NTR4337.
Retinal dopamine and melatonin
The ipRGCs have connections with the amacrine cells that produce dopamine, also located in the retina (Stone et al. 2013; Mendoza and Challet 2014). Retinal dopamine dysfunctioning has been hypothesized to play a role in the regulation of neurodevelopmental growth of the eye, leading to refractive errors, which may explain the increased prevalence of refractive errors that were found in ADHD (Mezer and Wygnanski-Jaffe 2012; Stone et al. 2013). Interestingly, ADHD is considered a neurodevelopmental disorder that is associated with low dopamine levels in certain brain areas (Sikstrom and Soderlund 2007), and the retina is basically an outgrowth of brain tissue (Erskine and Herrera 2014). The dopaminergic DRD4 gene is heavily involved in converting light to electrical signals in the retina, and its transcription exhibits a strong circadian pattern in rodents (Kim et al. 2010). A DRD4 7R allele is one of the proposed genetic risk factors of ADHD (Nikolaidis and Gray 2010). Compared to other DRD4 genotypes, carriers of the 7R genotype have less ability to reduce the light-sensitive second messenger cyclic adenosine monophosphate (cAMP) level with illumination (Asghari et al. 1995). Furthermore, 7R-carriers reported higher daytime sleepiness than non-carriers (Jawinski et al. 2016).
Dopamine and melatonin have opposing roles in the regulation of the circadian rhythm (Mendoza and Challet 2014; Mundey et al. 2005; Iuvone et al. 1978). While dopamine is mainly synthesized and released in the early morning and during the day, melatonin is released in the late afternoon or early evening and peaks at night (Iuvone et al. 2005; Doran et al. 1990). Dopamine has an inhibitory effect on melatonin release, and vice versa (Green and Besharse 2004). The dopaminergic system is understood to be under profound circadian control (Parekh et al. 2015), and impaired retinal dopamine synthesis results in circadian rhythm fluctuations (Wirz-Justice 1984). The hypothetically impaired functioning of the ipRGCs in ADHD subgroups may have its reflections on the melatonin and dopamine-producing cells in the retina, thereby having a role in the circadian misalignment as seen in the majority of ADHD patients.
Dopamine also plays a crucial role in sleep regulation. For instance, dopamine neurons in the ventral tegmental area (VTA) have a higher number of active dopamine cells after REM sleep deprivation and during recovery than in normal sleep (Maloney et al. 2002). Given that dopamine plays such a crucial role in the circadian rhythm and sleep regulation, the relationship becomes plausible between specific sleep disorders and a neurodevelopmental disorder associated with a dysregulated dopamine functioning, such as ADHD (French and Muthusamy 2016).
Sleep problems and ADHD: two sides of the same coin?
Symptoms of ADHD, a delayed circadian rhythm, and sleep disorders are thus intertwined by various pathways. They seem to share a genetic and etiological background and may profit from a common treatment. However, results from studies investigating such common treatment are yet scarce.
Importance of recognition and diagnosis
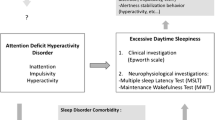
The screening, diagnostic assessment, and treatment of sleep disturbances, besides that of ADHD, is of great importance. Sleep problems and ADHD independently affect the quality of life and social functioning, at least in children (Craig et al. 2017). As the prevalence of sleep disorders is very high in ADHD, those diagnosed with ADHD should be routinely screened for delayed sleep problems and other sleep disorders. Various screening questionnaires are available, such as the Holland Sleep Disorders Questionnaire (HSDQ, also available in other languages including English), which screens for circadian rhythm sleep disorders, sleep-related movement disorders, insomnia, hypersomnia, parasomnia, and sleep-related breathing disorders (Kerkhof et al. 2013). Most patients with ADHD will screen positive for at least the delayed sleep phase disorder (DSPS), which should be followed up by a targeted diagnostic assessment. Besides questions on their sleep times and habits on nights before work days and free days, this may include using objective measures such as wrist actigraphy and/or the determination of the DLMO in saliva for confirmation of a delayed circadian rhythm.
Treatment options
Treatment of sleep disorders should be conducted alongside the treatment of ADHD and comorbid disorders. Sleep problems associated with mood or anxiety disorders may diminish with the treatment of those disorders. Sleep problems due to a chaotic lifestyle, which is generally characteristic to ADHD, may be reduced by the medical and psychological treatment of ADHD itself. Psycho-education on sleep hygiene may increase awareness of factors affecting sleep and improve the environmental and behavioral aspects that promote better sleep. The preferred treatment for primary insomnia that may also apply for secondary insomnia is a cognitive behavioral treatment for insomnia (CBTi), which encompasses sleep hygiene, stimulus control, sleep restriction, cognitive therapy, and relaxation training. CBTi is proven highly effective for symptoms of insomnia and improvement of sleep quality, as recently shown in a meta-analysis including 87 randomized controlled trials (van Straten et al. 2018). Moreover, it is safe, has no side effects, and is therefore preferred over sleep medication (Anderson 2018).
Sleep hygiene
Sleep hygiene consists of lifestyle measures that promote better sleep, such as having a comfortable sleeping area, not using caffeine in the evening, and maintaining a fixed sleep time schedule. Children with ADHD have worse sleep hygiene than controls (van der Heijden et al. 2018), and vice versa a bad sleep hygiene is related to more self-reported sleep problems in adolescents with ADHD (Martin et al. 2018). Sleep hygiene should be part of standard psycho-education in all patients with problems falling asleep, maintaining sleep, early awakening, or low sleep quality (Chen et al. 2010). Sleep hygiene involves directions for the timing and amount of caffeine and use of other substances, how to better entrain the internal circadian rhythm to the external clock time, e.g., by use of bright light in the morning and during the day but not late in the evening or at night, how to increase sleep pressure, e.g., by getting up on time in the morning and exercising, and how to keep the body comfortable enough to be able to sleep, e.g., by increasing skin temperature by taking a shower before bed. An open-label randomized controlled trial among children with ADHD showed that sleep hygiene education significantly decreased sleep problems. More interestingly, the symptoms of ADHD also decreased, and daily functioning and quality of life were increased, up to six months after the intervention (Hiscock et al. 2015). Bad sleep hygiene is however not the only reason why those with ADHD have problems to fall asleep (van der Heijden et al. 2006), as already discussed.
Chronotherapy
Chronotherapy for a delayed sleep phase involves phase-resetting of the internal clock by the use of exogenous melatonin in the late afternoon or evening, and/or by bright light therapy in the early morning (Kooij and Bijlenga 2013). Melatonin can be used as either a phase-advancing agent in low dosage (e.g., 0.5 mg) in the late afternoon or early evening, or as a sleep-inducing agent in higher dosage (e.g., 3–5 mg) about an hour before bedtime. A meta-analysis of nine studies including adults and children with DSPS showed that melatonin treatment, at various dosages and at various administration times, advanced the DLMO by more than an hour, and the sleep-onset time by 40 min (van Geijlswijk et al. 2010).
An overview on the use of melatonin in pediatric neurology concluded that it is safe and most effective as chronotherapy (Bruni et al. 2015). In 101 children with ADHD and chronic sleep-onset insomnia, treatment during 4 weeks with 3–6 mg of melatonin versus placebo before bedtime, advanced their sleep-onset time with on average 27 min, and increased sleep duration with on average 20 min (Van der Heijden et al. 2007). However, no effect was found on problem behavior, cognitive performance, or quality of life. In the follow-up study after 3.7 years, 65% of these children still used melatonin daily. Of them, 88% reported no sleep-onset problems anymore, 71% reported improved behavior, and 61% reported improved mood (Hoebert et al. 2009). Discontinuation of treatment resulted in a delay of sleep onset in most children, suggesting clinical benefit on ADHD symptoms can be achieved, albeit requires a longer duration of normal sleep.
Light therapy in the morning is indicated as chronotherapy for SAD, which has shown to advance a delayed circadian rhythm as well (Lewy et al. 1984). A small study showed that there is an additive effect of light therapy to the treatment with melatonin alone to advance the circadian rhythm (Paul et al. 2011). Two recent pilot studies also showed promising results for the treatment of ADHD in adults using bright light therapy (Fargason et al. 2017; Rybak et al. 2006). Both studies showed that the improvement in ADHD symptoms was related to the advancement of the circadian rhythm. These results are promising for further investigation in larger studies.
Treatment of other sleep disorders
Patients with a delayed circadian rhythm can be easily treated by the therapist with chronotherapy. Primary or secondary insomnia symptoms can be treated with CBTi. However, those screened positive for other sleep disorders should be further investigated by specialists in a sleep laboratory. Assessment and treatment of major sleep disorders generally take a few months, and depending on the diagnosis, may range from a behavioral intervention to pharmacological treatment, and even surgery. For example, the treatment of obstructive sleep apnea depends on its cause and may include a posture training, diet, continuous positive airway pressure (CPAP), a tongue-retaining device, and/or surgery in case of physiological malformations underlying the apnea. After the treatment of the sleep disorder, the severity of the ADHD symptoms may be re-evaluated.
Neurofeedback
Neurofeedback is a method where EEG activity is fed back in real time in order to induce self-regulation over specific brain activity, based on learning principles and operant conditioning. Several studies have demonstrated that applying this technique for a specific frequency band, namely sensorimotor rhythm neurofeedback (SMR, a 12–15 Hz rhythm found on central lateralized sites) results in increased sleep spindle density during sleep (Sterman et al. 1970; Hoedlmoser et al. 2008), decreased sleep latency (Hoedlmoser et al. 2008), and increased total sleep time (Hoedlmoser et al. 2008; Cortoos et al. 2010). Sleep spindles occur during light and deep sleep where they protect from waking due to external stimuli, thus facilitating the process of falling asleep. After melatonin administration, more sleep spindles are found and a recent polysomnographic study found that children with ADHD exhibited reduced activity in this same 12–15 Hz sigma band during sleep, reflective of reduced sleep spindles (Saletin et al. 2017). Another recent study in a group of ADHD patients showed that SMR neurofeedback resulted in a normalized sleep-onset. Also, those with a normalized sleep-onset latency had improved attention after treatment (Arns et al. 2014a).
SMR neurofeedback is hypothesized to train the sleep spindle network, resulting in long-term potentiation (LTP) that increases synaptic strengths, and the likelihood of future activation of this network (Arns and Kenemans 2014; Sterman and Egner 2006). In line with the finding that cognitive deficits need a period of normal sleep to recover from sleep restriction (Axelsson et al. 2008; Belenky et al. 2003), a recent meta-analysis demonstrated that the effects of neurofeedback on inattention in ADHD further improved to an average of six months after treatment, whereas this was not the case in the non-active control conditions, nor in conditions involving psychostimulant medication treatment (Van Doren et al. 2018).
Time to redefine ADHD?
In this review we aimed to clarify the link between sleep problems and ADHD symptoms. There are multiple indications that treating those sleep problems reduces ADHD symptoms. The main current scientific consensus is that a dopamine and/or norepinephrine deficit is the neurochemical basis of ADHD that is associated with the main clinical problems of hyperactive, impulsive, and inattentive behavior (e.g., Sharma and Couture 2014). However, ADHD might be better conceptualized as a ‘heterogenous’ disorder from the neurobiological perspective, where at least several subtypes with different etiology exist, most clearly evidenced by the fact that none of the current neurobiological treatments have perfect efficacy. In line with this notion of neurobiological heterogeneity, it makes more sense to aim to explain this neurobiological heterogeneity, in order to develop more specific treatments. We therefore propose a novel hypothesis: ADHD symptoms resulting from a chronic sleep disorder, with most evidence for the delayed sleep phase disorder, in a large group of patients with ADHD. Chronic circadian sleep disorders that may have a large genetic component, almost always lead to poor sleep quality and/or quantity, with presumed suboptimal development or function of the dopaminergic system and thus to ADHD-like symptoms such as concentration problems, inattention, impulsivity, and hyperactivity. This may also be true for other sleep disorders, but those have been studied less.
However, it is yet unknown whether the (chronic) sleep problems are the sole cause of ADHD symptoms, if there are other underlying mechanisms to the ADHD symptoms, or if the causation in patients is heterogeneous (i.e., the etiology of the ADHD symptoms is different across patients). More research is needed to disentangle these issues and to verify our hypothesis. Future longitudinal studies may investigate the relationship between sleep and ADHD over the course of life.
In line with our hypothesis, we propose an additional diagnostic presentation category referred to as ADHD-SOM (derived from ‘somnus,’ i.e., sleep). In this group, a part of the ADHD symptoms are the result of chronic sleep disorders.
This suggestion can be embedded in current clinical practice and research. According to the DSM-5, for every diagnosis made, other explanations for the symptoms should be ruled out (APA 2013). We therefore propose clinicians to incorporate assessments that quantify sleep and any sleep problems, thereby ruling those out as the sole cause of the ADHD symptoms. This may be achieved using screening questionnaires such as HSDQ and PSQI, and the assessment of DLMO and/or actigraphy. It is essential to rule out or acknowledge the presence of a circadian rhythm sleep disorder, or sleep disorders such as insomnia, restless legs, or sleep-disordered breathing. When confirmed after further diagnostic assessment, treatment should focus on both ADHD and the sleep problem. The severity of both disorders and the preference of the patient determine the order of the treatments. The assumption that with better sleep, the symptoms of ADHD diminish does not imply that ‘standard treatment’ of ADHD is less important. When we consider ADHD-SOM as a novel presentation within the diagnosis, sleep treatment, such as chronotherapeutic treatment to get the delayed rhythm stabilized, may be necessary. Our clinical experience tells us that combined ADHD treatment and chronotherapy in ADHD patients with a delayed circadian rhythm adds to better outcomes of the ADHD treatment intervention as a whole, using dopamine suppletion (stimulants) as the ‘day agent’ and melatonin as the ‘night hormone,’ as the two naturally have opposing effects with negative feedback loops (Stone et al. 2013). The additive effect of the treatment of any sleep disorder to the ADHD treatment outcomes should be further investigated.
In summary, our plea for a redefinition of part of the ADHD symptoms as the result of a chronic sleep disorder is based on the following pieces of evidence that have been discussed throughout this manuscript:
-
1.
The consistent findings of increased prevalences of various sleep disorders in ADHD populations across studies.
-
2.
Solid scientific evidence for a strong relationship between symptoms of ADHD and a delayed circadian, with 73–78% of patients with ADHD having a delayed circadian rhythm.
-
3.
Sleep restriction studies and cross-sectional studies show that shorter sleep is associated with impaired sustained attention and executive functioning.
-
4.
Genetic associations between ADHD and a delayed circadian rhythm
-
5.
A higher ADHD prevalence in countries and geographical areas with lower solar intensities and thus less entrainment to the day and night by the central biological clock.
-
6.
Possible indications of a lower functioning of photosensitive retinal cells that are key for optimal entrainment of the circadian rhythm to the natural day and night cycle 7. Indications of an effect of light therapy both on a phase advance of the circadian rhythm and on the symptoms of ADHD.
-
7.
The central role of dopamine in ADHD, sleep, and retinal circadian alignment.
-
8.
First indications of the short- and long-term effects of sleep improvement (by sleep hygiene measures, melatonin, light therapy, and SMR neurofeedback in delayed sleep; adenotonsillectomy in sleep apnea, and drug treatment in restless legs syndrome) on the reduction of the severity of ADHD symptoms.
Finally, we propose some scientific direction for future studies:
-
1.
The longitudinal relationship between sleep and ADHD over the lifespan.
-
2.
The functioning of the retinal photosensitive cells of ADHD patients.
-
3.
The additive effect of chronotherapy for the delayed sleep phase disorder to an existing ADHD treatment regime.
-
4.
The effect of treatments for other sleep disorders on ADHD symptomatology.
References
Achermann P, Dijk DJ, Brunner DP, Borbély AA (1993) A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull 31:97–113
Allebrandt KV, Teder-Laving M, Kantermann T, Peters A, Campbell H, Rudan I et al (2014) Chronotype and sleep duration: the influence of season of assessment. Chronobiol Int 31:731–740
Amons PJ, Kooij JJ, Haffmans PM, Hoffman TO, Hoencamp E (2006) Seasonality of mood disorders in adults with lifetime attention-deficit/hyperactivity disorder (ADHD). J Affect Disord 91:251–255
Anderson KN (2018) Insomnia and cognitive behavioural therapy-how to assess your patient and why it should be a standard part of care. J Thorac Dis 10:S94–S102
APA (2013) Diagnostic and statistical manual of mental disorders (DSM-5), 5th edn. American Psychiatric Association, Washington, DC
Arns M, Kenemans JL (2014) Neurofeedback in ADHD and insomnia: vigilance stabilization through sleep spindles and circadian networks. Neurosci Biobehav Rev 44:183–194
Arns M, Conners CK, Kraemer HC (2013a) A decade of EEG theta/beta ratio research in ADHD: a meta-analysis. J Atten Disord 17:374–383
Arns M, van der Heijden KB, Arnold LE, Kenemans JL (2013b) Geographic variation in the prevalence of attention-deficit/hyperactivity disorder: the sunny perspective. Biol Psychiatry 74(8):585–590
Arns M, van der Heijden KB, Eugene Arnold L, Swanson JM, Leon Kenemans J (2013c) Reply to: Attention-deficit/hyperactivity disorder and solar irradiance: a cloudy perspective. Biol Psychiatry 76(8):e21–e23
Arns M, Feddema I, Kenemans JL (2014a) Differential effects of theta/beta and SMR neurofeedback in ADHD on sleep onset latency. Front Hum Neurosci 8:1019
Arns M, van der Heijden KB, Eugene Arnold L, Leon Kenemans J (2014b) Reply to: The geographic variation in the prevalence of attention-deficit/hyperactivity disorder the United States is likely due to geographical variations of solar ultraviolet B doses and race. Biol Psychiatry 75:e3–e4
Arns M, Swanson JM, Arnold LE (2015) ADHD prevalence: altitude or sunlight? Better understanding the interrelations of dopamine and the circadian system. J Atten Disord 22(2):163–166. https://doi.org/10.1177/1087054715599574
Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH (1995) Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem 65:1157–1165
Astill RG, Van der Heijden KB, Van Ijzendoorn MH, Van Someren EJW (2012) Sleep, cognition, and behavioral problems in school-age children: a century of research meta-analyzed. Psychol Bull 138(6):1109
Axelsson J, Kecklund G, Åkerstedt T, Donofrio P, Lekander M, Ingre M (2008) Sleepiness and performance in response to repeated sleep restriction and subsequent recovery during semi-laboratory conditions. Chronobiol Int 25:297–308
Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J (2011) Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry 17(10):988
Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J (2012) Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Mol Psychiatry 17:988–995
Banaschewski T, Ruppert S, Tannock R, Albrecht B, Becker A, Uebel H et al (2006) Colour perception in ADHD. J Child Psychol Psychiatry 47:568–572
Barnhardt C, Cotter SA, Mitchell GL, Scheiman M, Kulp MT, Group CS (2012) Symptoms in children with convergence insufficiency: before and after treatment. Optom Vis Sci Off Publ Am Acad Optom 89:1512–1520
Becker SP, Froehlich TE, Epstein JN (2016) Effects of methylphenidate on sleep functioning in children with attention-deficit/hyperactivity disorder. J Dev Behav Pediatr 37:395–404
Beebe DW, Fallone G, Godiwala N, Flanigan M, Martin D, Schaffner L et al (2008) Feasibility and behavioral effects of an at-home multi-night sleep restriction protocol for adolescents. J Child Psychol Psychiatry 49:915–923
Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP et al (2003) Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res 12:1–12
Benarroch EE (2011) The melanopsin system: phototransduction, projections, functions, and clinical implications. Neurology 76:1422–1427
Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E et al (2003) Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am J Med Genet B Neuropsychiatr Genet 123B:23–26
Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL et al (2015) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.2. American Academy of Sleep Medicine, Darien
Bijlenga D, van der Heijden KB, Breuk M, van Someren EJW, Lie MEH, Boonstra AM et al (2011) Associations between sleep characteristics, seasonal depressive symptoms, lifestyle, and adhd symptoms in adults. J Atten Disord 17(3):261–275
Bijlenga D, van der Heijden KB, Breuk M, van Someren EJ, Lie ME, Boonstra AM et al (2013a) Associations between sleep characteristics, seasonal depressive symptoms, lifestyle, and adhd symptoms in adults. J Atten Disord 17:261–274
Bijlenga D, Van Someren EJW, Gruber R, Bron TI, Kruithof IF, Spanbroek ECA et al (2013b) Body temperature, activity and melatonin profiles in adults with attention-deficit/hyperactivity disorder and delayed sleep: a case-control study. J Sleep Res 22:607–616
Bjorvatn B, Brevik EJ, Lundervold AJ, Halmoy A, Posserud MB, Instanes JT et al (2017) Adults with attention deficit hyperactivity disorder report high symptom levels of troubled sleep, restless legs, and cataplexy. Front Psychol 8:1621
Boergers J, Gable CJ, Owens JA (2014) Later school start time is associated with improved sleep and daytime functioning in adolescents. J Dev Behav Pediatr 35:11–17
Borbély AA (1982) A two process model of sleep regulation. Hum Neurobiol 1:195–204
Brevik EJ, Lundervold AJ, Halmoy A, Posserud MB, Instanes JT, Bjorvatn B et al (2017) Prevalence and clinical correlates of insomnia in adults with attention-deficit hyperactivity disorder. Acta Psychiatr Scand 136(2):220–227
Brookes KJ, Neale B, Xu X, Thapar A, Gill M, Langley K et al (2008) Differential dopamine receptor D4 allele association with ADHD dependent of proband season of birth. Am J Med Genet B Neuropsychiatr Genet 147B:94–99
Brooks E, Canal MM (2013) Development of circadian rhythms: role of postnatal light environment. Neurosci Biobehav Rev 37:551–560
Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe A et al (2008) Molecular insights into human daily behavior. Proc Natl Acad Sci USA 105:1602–1607
Bruni O, Alonso-Alconada D, Besag F, Biran V, Braam W, Cortese S et al (2015) Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc 19:122–133
Cadman T, Findon J, Eklund H, Hayward H, Howley D, Cheung C et al (2016) Six-year follow-up study of combined type ADHD from childhood to young adulthood: predictors of functional impairment and comorbid symptoms. Eur Psychiatry 35:47–54
Chang AM, Scheer FA, Czeisler CA (2011) The human circadian system adapts to prior photic history. J Physiol 589:1095–1102
Chaste P, Clement N, Botros HG, Guillaume J-L, Konyukh M, Pagan C et al (2011) Genetic variations of the melatonin pathway in patients with attention-deficit and hyperactivity disorders. J Pineal Res 51(4):394–399
Chen PH, Kuo HY, Chueh KH (2010) Sleep hygiene education: efficacy on sleep quality in working women. J Nurs Res (JNR) 18:283–289
Chung SA, Chang YH, Rhiu S, Lew H, Lee JB (2012) Parent-reported symptoms of attention deficit hyperactivity disorder in children with intermittent exotropia before and after strabismus surgery. Yonsei Med J 53:806–811
Ciarleglio CM, Axley JC, Strauss BR, Gamble KL, McMahon DG (2011) Perinatal photoperiod imprints the circadian clock. Nat Neurosci 14:25–27
Conners CK (1970) Symptom patterns in hyperkinetic, neurotic and normal children. Child Dev 41:667–682
Coogan AN, McGowan NM (2017) A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. Atten Defic Hyperact Disord 9(3):129–147
Corkum P, Lingley-Pottie P, Davidson F, McGrath P, Chambers CT, Mullane J et al (2016) Better nights/better days-distance intervention for insomnia in school-aged children with/without ADHD: a randomized controlled trial. J Pediatr Psychol 41:701–713
Cortese S, Konofal E, Lecendreux M, Arnulf I, Mouren MC, Darra F et al (2005) Restless legs syndrome and attention-deficit/hyperactivity disorder: a review of the literature. Sleep 28:1007–1013
Cortese S, Faraone SV, Konofal E, Lecendreux M (2009) Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adoles Psychiatr 48:894–908
Cortoos A, De Valck E, Arns M, Breteler MHM, Cluydts R (2010) An exploratory study on the effects of tele-neurofeedback and tele-biofeedback on objective and subjective sleep in patients with primary insomnia. Appl Psychophysiol Biofeedback 35:125–134
Craig SG, Weiss MD, Hudec KL, Gibbons C (2017) The functional impact of sleep disorders in children with ADHD. J Atten Disord. https://doi.org/10.1177/1087054716685840
Diaz-Roman A, Hita-Yanez E, Buela-Casal G (2016) Sleep characteristics in children with attention deficit hyperactivity disorder: systematic review and meta-analyses. J Clin Sleep Med 12:747–756
Diaz-Roman A, Mitchell R, Cortese S (2018) Sleep in adults with ADHD: systematic review and meta-analysis of subjective and objective studies. Neurosci Biobehav Rev 89:61–71. https://doi.org/10.1016/j.neubiorev.2018.02.014
Doran AR, Labarca R, Wolkowitz OM, Roy A, Douillet P, Pickar D (1990) Circadian variation of plasma homovanillic acid levels is attenuated by fluphenazine in patients with schizophrenia. Arch Gen Psychiatry 47:558–563
Erskine L, Herrera E (2014) Connecting the retina to the brain. ASN Neuro 6:1759091414562107
Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA (2001) Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Percept Mot Skills 93:213–229
Fallone G, Acebo C, Seifer R, Carskadon MA (2005) Experimental restriction of sleep opportunity in children: effects on teacher ratings. Sleep 28:1561–1567
Fargason RE, Fobian AD, Hablitz LM, Paul JR, White BA, Cropsey KL et al (2017) Correcting delayed circadian phase with bright light therapy predicts improvement in ADHD symptoms: a pilot study. J Psychiatr Res 91:105–110
French IT, Muthusamy KA (2016) A review of sleep and its disorders in patients with Parkinson’s disease in relation to various brain structures. Front Aging Neurosci 8:114
Gau SS, Kessler RC, Tseng WL, Wu YY, Chiu YN, Yeh CB et al (2007) Association between sleep problems and symptoms of attention-deficit/hyperactivity disorder in young adults. Sleep 30:195–201
Golan N, Shahar E, Ravid S, Pillar G (2004) Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactive disorder. Sleep 27:261–266
Gottlieb DJ, Hek K, Chen TH, Watson NF, Eiriksdottir G, Byrne EM et al (2015) Novel loci associated with usual sleep duration: the CHARGE Consortium Genome-Wide Association Study. Mol Psychiatry 20:1232–1239
Granet DB, Gomi CF, Ventura R, Miller-Scholte A (2005) The relationship between convergence insufficiency and ADHD. Strabismus 13:163–168
Green CB, Besharse JC (2004) Retinal circadian clocks and control of retinal physiology. J Biol Rhythms 19:91–102
Gregory AM, Agnew-Blais JC, Matthews T, Moffitt TE, Arseneault L (2017) ADHD and sleep quality: longitudinal analyses from childhood to early adulthood in a twin cohort. J Clin Child Adolesc Psychol 46:284–294
Gronlund MA, Aring E, Landgren M, Hellstrom A (2007) Visual function and ocular features in children and adolescents with attention deficit hyperactivity disorder, with and without treatment with stimulants. Eye 21:494–502
Hegerl U, Hensch T (2014) The vigilance regulation model of affective disorders and ADHD. Neurosci Biobehav Rev 44:45–57. https://doi.org/10.1016/j.neubiorev.2012 (Epub Oct 22)
Hiscock H, Sciberras E, Mensah F, Gerner B, Efron D, Khano S et al (2015) Impact of a behavioural sleep intervention on symptoms and sleep in children with attention deficit hyperactivity disorder, and parental mental health: randomised controlled trial. BMJ 350:h68
Hoebert M, van der Heijden KB, van Geijlswijk IM, Smits MG (2009) Long-term follow-up of melatonin treatment in children with ADHD and chronic sleep onset insomnia. J Pineal Res 47:1–7
Hoedlmoser K, Pecherstorfer T, Gruber G, Anderer P, Doppelmayr M, Klimesch W et al (2008) Instrumental conditioning of human sensorimotor rhythm (12–15 Hz) and its impact on sleep as well as declarative learning. Sleep 31:1401–1408
Hu Y, Shmygelska A, Tran D, Eriksson N, Tung JY, Hinds DA (2016) GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nat Commun 7:10448
Huang YS, Guilleminault C, Li HY, Yang CM, Wu YY, Chen NH (2007) Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med 8:18–30
Hvolby A (2015) Associations of sleep disturbance with ADHD: implications for treatment. Atten Defic Hyperact Disord 7:1–18
Hysing M, Lundervold AJ, Posserud MB, Sivertsen B (2016) Association between sleep problems and symptoms of attention deficit hyperactivity disorder in adolescence: results from a large population-based study. Behav Sleep Med 14:550–564
Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH (1978) Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science 202:901–902
Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS (2005) Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res 24:433–456
Jawinski P, Tegelkamp S, Sander C, Häntzsch M, Huang J, Mauche N, Scholz M, Spada J, Ulke C, Burkhardt R, Reif A, Hegerl U, Hensch T (2016) Time to wake up: no impact of COMT Val158Met gene variation on circadian preferences, arousal regulation and sleep. Chronobiol Int 33(7):893–905. https://doi.org/10.1080/07420528.2016.1178275
Jones SE, Tyrrell J, Wood AR, Beaumont RN, Ruth KS, Tuke MA et al (2016) Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLoS Genet 12:e1006125
Kalmbach DA, Schneider LD, Cheung J, Bertrand SJ, Kariharan T, Pack AI et al (2017) Genetic basis of chronotype in humans: insights from three landmark GWAS. Sleep. https://doi.org/10.1093/sleep/zsw048
Kantermann T, Juda M, Merrow M, Roenneberg T (2007) The human circadian clock’s seasonal adjustment is disrupted by daylight saving time. Curr Biol 17:1996–2000
Kass SJ, Wallace JC, Vodanovich SJ (2003) Boredom proneness and sleep disorders as predictors of adult attention deficit scores. J Atten Disord 7:83–91
Kerkhof GA, Geuke ME, Brouwer A, Rijsman RM, Schimsheimer RJ, Van Kasteel V (2013) Holland Sleep Disorders Questionnaire: a new sleep disorders questionnaire based on the International Classification of Sleep Disorders-2. J Sleep Res 22:104–107
Kim J-S, Bailey MJ, Weller JL, Sugden D, Rath MF, Møller M et al (2010) Thyroid hormone and adrenergic signaling interact to control pineal expression of the dopamine receptor D4 gene (Drd4). Mol Cell Endocrinol 314:128–135
Kim S, Chen S, Tannock R (2014) Visual function and color vision in adults with Attention-Deficit/Hyperactivity Disorder. J Optom 7:22–36
Kooij JJ, Bijlenga D (2013) The circadian rhythm in adult attention-deficit/hyperactivity disorder: current state of affairs. Exp Rev Neurother 13:1107–1116
Kooij JJ, Bijlenga D (2014) High prevalence of photophobia in ADHD. Front Neurol 10:256
Kooij J, Middelkoop HA, van Gils K, Buitelaar JK (2001) The effect of stimulants on nocturnal motor activity and sleep quality in adults with ADHD: an open-label case-control study. J Clin Psychiatry 62:952–956
Kurth S, Dean DC 3rd, Achermann P, O’Muircheartaigh J, Huber R, Deoni SC et al (2016) Increased sleep depth in developing neural networks: new insights from sleep restriction in children. Front Hum Neurosci 10:456
Lane JM, Vlasac I, Anderson SG, Kyle SD, Dixon WG, Bechtold DA et al (2016) Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat Commun 7:10889
Langberg JM, Molitor SJ, Oddo LE, Eadeh HM, Dvorsky MR, Becker SP (2017) Prevalence, patterns, and predictors of sleep problems and daytime sleepiness in young adolescents with ADHD. J Atten Disord. https://doi.org/10.1177/1087054717690810
Lewy AJ (2007) Melatonin and human chronobiology. Cold Spring Harb Symp Quant Biol 72:623–636
Lewy AJ, Sack RL (1989) The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int 6:93–102
Lewy AJ, Sack RA, Singer CL (1984) Assessment and treatment of chronobiologic disorders using plasma melatonin levels and bright light exposure: the clock-gate model and the phase response curve. Psychopharmacol Bull 20:561–565
Lewy AJ, Sack RL, Miller LS, Hoban TM (1987) Antidepressant and circadian phase-shifting effects of light. Science 235:352–354
Lockley SW, Brainard GC, Czeisler CA (2003) High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab 88:4502–4505
Lundahl A, Kidwell KM, Van Dyk TR, Nelson TD (2015) A meta-analysis of the effect of experimental sleep restriction on youth’s attention and hyperactivity. Dev Neuropsychol 40:104–121
Lupi D, Oster H, Thompson S, Foster RG (2008) The acute light-induction of sleep is mediated by OPN4-based photoreception. Nat Neurosci 11:1068–1073
Mahajan N, Hong N, Wigal TL, Gehricke JG (2010) Hyperactive-impulsive symptoms associated with self-reported sleep quality in nonmedicated adults with ADHD. J Atten Disord 14:132–137
Maloney KJ, Mainville L, Jones BE (2002) c-Fos expression in dopaminergic and GABAergic neurons of the ventral mesencephalic tegmentum after paradoxical sleep deprivation and recovery. Eur J Neurosci 15:774–778
Martin L, Aring E, Landgren M, Hellstrom A, Andersson Gronlund M (2008) Visual fields in children with attention-deficit/hyperactivity disorder before and after treatment with stimulants. Acta Ophthalmol (Oxf) 86:259–264
Martin CA, Hiscock H, Rinehart N, Heussler HS, Hyde C, Fuller-Tyszkiewicz M,McGillivray J, Austin DW, Chalmers A, Sciberras E (2018) Associations between sleep hygiene and sleep problems in adolescents with ADHD: a cross-sectional study. J Atten Disord. https://doi.org/10.1177/1087054718762513
Matricciani L, Olds T, Petkov J (2012) In search of lost sleep: secular trends in the sleep time of school-aged children and adolescents. Sleep Med Rev 16:203–211
McGowan NM, Coogan AN (2018) Sleep and circadian rhythm function and trait impulsivity: an actigraphy study. Psychiatry Res 268:251–256
McGowan NM, Voinescu BI, Coogan AN (2016) Sleep quality, chronotype and social jetlag differentially associate with symptoms of attention deficit hyperactivity disorder in adults. Chronobiol Int 33:1433–1443
Meijer JH, Michel S, Vansteensel MJ (2007) Processing of daily and seasonal light information in the mammalian circadian clock. Gen Comp Endocrinol 152:159–164
Melegari MG, Vittori E, Mallia L, Devoto A, Lucidi F, Ferri R et al (2016) Actigraphic sleep pattern of preschoolers with ADHD. J Atten Disord. https://doi.org/10.1177/1087054716672336
Mendoza J, Challet E (2014) Circadian insights into dopamine mechanisms. Neuroscience 282:230–242
Mersch PP, Middendorp HM, Bouhuys AL, Beersma DG, van den Hoofdakker RH (1999) The prevalence of seasonal affective disorder in The Netherlands: a prospective and retrospective study of seasonal mood variation in the general population. Biol Psychiatry 45:1013–1022
Mezer E, Wygnanski-Jaffe T (2012) Do children and adolescents with attention deficit hyperactivity disorder have ocular abnormalities? Eur J Ophthalmol 22:931–935
Morash-Conway J, Gendron M, Corkum P (2017) The role of sleep quality and quantity in moderating the effectiveness of medication in the treatment of children with ADHD. Atten Defic Hyperact Disord 9:31–38. https://doi.org/10.1007/s12402-016-0204-7 (Epub 2016 Aug 11)
Mota-Veloso I, Celeste RK, Fonseca CP, Soares ME, Marques LS, Ramos-Jorge ML et al (2017) Effects of attention deficit hyperactivity disorder signs and socio-economic status on sleep bruxism and tooth wear among schoolchildren: structural equation modelling approach. Int J Paediatr Dent 27(6):523–531
Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC (2005) Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep 28:1271–1278
Natale V, Adan A (1999) Season of birth modulates morningness-eveningness preference in humans. Neurosci Lett 274:139–141
Natale V, Adan A, Chotai J (2002) Further results on the association between morningness-eveningness preference and the season of birth in human adults. Neuropsychobiology 46:209–214
Nikolaidis A, Gray JR (2010) ADHD and the DRD4 exon III 7-repeat polymorphism: an international meta-analysis. Soc Cognit Affect Neurosci 5:188–193
O’Brien LM (2009) The neurocognitive effects of sleep disruption in children and adolescents. Child Adolesc Psychiatr Clin N Am 18:813–823. https://doi.org/10.1016/j.chc.2009.04.008
Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV (2004) Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27:1255–1273
Owens JA (2008) Sleep disorders and attention-deficit/hyperactivity disorder. Curr Psychiatry Rep 10:439–444
Owens JA, Belon K, Moss P (2010) Impact of delaying school start time on adolescent sleep, mood, and behavior. Arch Pediatr Adolesc Med 164:608–614
Owens J, Weiss M, Nordbrock E, Mattingly G, Wigal S, Greenhill LL et al (2016) Effect of aptensio XR (methylphenidate HCl extended-release) capsules on sleep in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 26:873–881
Paine SJ, Gander PH, Travier N (2006) The epidemiology of morningness/eveningness: influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years). J Biol Rhythms 21:68–76
Parekh PK, Ozburn AR, McClung CA (2015) Circadian clock genes: effects on dopamine, reward and addiction. Alcohol 49:341–349
Paul MA, Gray GW, Lieberman HR, Love RJ, Miller JC, Trouborst M et al (2011) Phase advance with separate and combined melatonin and light treatment. Psychopharmacology 214:515–523
Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD (2000) A novel human opsin in the inner retina. J Neurosci Off J Soc Neurosci 20:600–605
Roecklein K, Wong P, Ernecoff N, Miller M, Donofry S, Kamarck M et al (2013a) The post illumination pupil response is reduced in seasonal affective disorder. Psychiatry Res 210:150–158
Roecklein KA, Wong PM, Miller MA, Donofry SD, Kamarck ML, Brainard GC (2013b) Melanopsin, photosensitive ganglion cells, and seasonal affective disorder. Neurosci Biobehav Rev 37:229–239
Roenneberg T, Merrow M (2016) The circadian clock and human health. Curr Biol 26:R432–R443
Roenneberg T, Wirz-Justice A, Merrow M (2003) Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18:80–90
Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A et al (2004) A marker for the end of adolescence. Curr Biol 14:R1038–R1039
Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M et al (2007a) Epidemiology of the human circadian clock. Sleep Med Rev 11:429–438
Roenneberg T, Kumar CJ, Merrow M (2007b) The human circadian clock entrains to sun time. Curr Biol 17:R44–R45
Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD (2006) An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry 67:1527–1535
Sadeh A, Gruber R, Raviv A (2003) The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev 74:444–455
Saletin JM, Coon WG, Carskadon MA (2017) Stage 2 sleep EEG sigma activity and motor learning in childhood ADHD: a pilot study. J Clin Child Adolesc Psychol 46:188–197
Santisteban JA, Stein MA, Bergmame L, Gruber R (2014) Effect of extended-release dexmethylphenidate and mixed amphetamine salts on sleep: a double-blind, randomized, crossover study in youth with attention-deficit hyperactivity disorder. CNS Drugs 28:825–833
Schmidt TM, Kofuji P (2009) Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci Off J Soc Neurosci 29:476–482
Schrader H, Bovim G, Sand T (1993) The prevalence of delayed and advanced sleep phase syndromes. J Sleep Res 2:51–55
Sciberras E, DePetro A, Mensah F, Hiscock H (2015) Association between sleep and working memory in children with ADHD: a cross-sectional study. Sleep Med 16:1192–1197
Seeger G, Schloss P, Schmidt MH, Rüter-Jungfleisch A, Henn FA (2004) Gene–environment interaction in hyperkinetic conduct disorder (HD + CD) as indicated by season of birth variations in dopamine receptor (DRD4) gene polymorphism. Neurosci Lett 366:282–286
Sharma A, Couture J (2014) A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann Pharmacother 48:209–225
Sikstrom S, Soderlund G (2007) Stimulus-dependent dopamine release in attention-deficit/hyperactivity disorder. Psychol Rev 114:1047–1075
Sivertsen B, Pallesen S, Stormark KM, Boe T, Lundervold AJ, Hysing M (2013) Delayed sleep phase syndrome in adolescents: prevalence and correlates in a large population based study. BMC Public Health 13:1163
Slama H, Chylinski DO, Deliens G, Leproult R, Schmitz R, Peigneux P (2017) Sleep deprivation triggers cognitive control impairments in task-goal switching. Sleep 41(2):zsx200
Snitselaar MA, Smits MG, Spijker J (2015) Prevalence of restless legs syndrome in adult ADHD and its subtypes. Behav Sleep Med 29:1–9
Snitselaar MA, Smits MG, van der Heijden KB, Spijker J (2017) Sleep and circadian rhythmicity in adult ADHD and the effect of stimulants. J Atten Disord 21:14–26
Sterman MB, Egner T (2006) Foundation and practice of neurofeedback for the treatment of epilepsy. Appl Psychophysiol Biofeedback 31:21–35
Sterman MB, Howe RC, Macdonald LR (1970) Facilitation of spindle-burst sleep by conditioning of electroencephalographic activity while awake. Science 167:1146–1148
Stone RA, Pardue MT, Iuvone PM, Khurana TS (2013) Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res 114:35–47
Taillard J, Philip P, Chastang JF, Bioulac B (2004) Validation of Horne and Ostberg morningness-eveningness questionnaire in a middle-aged population of French workers. J Biol Rhythms 19:76–86
Tsai MH, Hsu JF, Huang YS (2016) Sleep problems in children with attention deficit/hyperactivity disorder: current status of knowledge and appropriate management. Curr Psychiatry Rep 18:76
Van der Heijden KB, Smits MG, Gunning WB (2005a) Sleep-related disorders in ADHD: a review. Clin Pediatr (Phila). 44:201–210
Van der Heijden KB, Smits MG, Van Someren EJ, Gunning WB (2005b) Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int 22:559–570
van der Heijden KB, Smits MG, Gunning WB (2006) Sleep hygiene and actigraphically evaluated sleep characteristics in children with ADHD and chronic sleep onset insomnia. J Sleep Res 15:55–62
Van der Heijden KB, Smits MG, Van Someren EJW, Ridderinkhof KR, Gunning WB (2007) Effect of melatonin on sleep, behavior, and cognition in ADHD and chronic sleep-onset insomnia. J Am Acad Child Adolesc Psychiatry 46:233–241
van der Heijden KB, Stoffelsen RJ, Popma A, Swaab H (2018) Sleep, chronotype, and sleep hygiene in children with attention-deficit/hyperactivity disorder, autism spectrum disorder, and controls. Eur Child Adolesc Psychiatry 27:99–111
Van Dongen HPA, Maislin G, Mullington JM, Dinges DF (2003) The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26:117–126
Van Doren J, Arns M, Heinrich H, Vollebregt M, Strehl U, Loo S (2018) Sustained effects of neurofeedback in ADHD: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. https://doi.org/10.1007/s00787-018-1121-4
van Geijlswijk IM, Korzilius HP, Smits MG (2010) The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep 33:1605–1614
van Straten A, van der Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J (2018) Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev 38:3–16
van Veen MM, Kooij JJ, Boonstra AM, Gordijn MC, van Someren EJ (2010) Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry 67:1091–1096
Velez-Galarraga R, Guillen-Grima F, Crespo-Eguilaz N, Sanchez-Carpintero R (2016) Prevalence of sleep disorders and their relationship with core symptoms of inattention and hyperactivity in children with attention-deficit/hyperactivity disorder. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc 20:925–937
Vogel SWN, Bijlenga D, Benjamins JS, Beekman ATF, Kooij JJS, Van Someren EJW (2017) Attention deficit hyperactivity disorder symptom severity and sleep problems in adult participants of the Netherlands sleep registry. Sleep Med 40:94–102
Vogel SWN, Ten Have M, Bijlenga D, de Graaf R, Beekman AT, Kooij JJ (2018) Distribution of ADHD symptoms, and associated comorbidity, exposure to risk factors and disability: results from a general population study. Psychiatry Res 267:256–265. https://doi.org/10.1016/j.psychres.2018.06.017
Vollebregt MA, Arns M (2016) Dopamine under the influence of sunlight? Transitions in solar irradiation explaining attentional performance in DRD4 7R carriers. 19th Biennial IPEG meeting. Nijmegen, The Netherlands. Neuropsychiatr Electrophysiol
Vollmer C, Randler C, Di Milia L (2012) Further evidence for the influence of photoperiod at birth on chronotype in a sample of German adolescents. Chronobiol Int 29:1345–1351
Wahlstrom K, Dretzke B, Gordon M, Peterson K, Edwards K, Gdula J (2014) Examining the impact of later school start times on the health and academic performance of high school students: a multi-site study. Center for Applied Research and Educational Improvement, University of Minnesota, St Paul
Walch OJ, Cochran A, Forger DB (2016) A global quantification of “normal” sleep schedules using smartphone data. Sci Adv 2:e1501705
Walters AS, Mandelbaum DD, Lewin DS, Kugler S, England SJ, Miller M et al (2000) Dopaminergic therapy in children with restless legs/periodic limb movements in sleep and ADHD. Pediatr Neurol 22:182–186
Wams EJ, Woelders T, Marring I, Van Rosmalen L, Beersma DG, Gordijn MC et al (2017) Linking light exposure and subsequent sleep: a field polysomnography study in humans. Sleep 40(12):zsx165
Wirz-Justice A (1984) Dopamine receptor rhythms. Biol Psychiatry 19:1274–1276
Xu X, Breen G, Chen CK, Huang YS, Wu YY, Asherson P (2010) Association study between a polymorphism at the 3′-untranslated region of CLOCK gene and attention deficit hyperactivity disorder. Behav Brain Funct 6:48
Yazaki M, Shirakawa S, Okawa M, Takahashi K (1999) Demography of sleep disturbances associated with circadian rhythm disorders in Japan. Psychiatry Clin Neurosci 53:267–268
Zerbini G, Merrow M (2017) Time to learn: how chronotype impacts education. Psych J 6:263–276
Acknowledgements
We thank the Editorial team for the invitation to contribute to the special issue in the celebration of Prof. Dr. Jan Buitelaar’s 65th birthday.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
MA reports options from Brain Resource (Sydney, Australia), is director and owner of Research Institute Brainclinics, a minority shareholder in neuroCare Group (Munich, Germany), and a co-inventor on 4 patent applications (A61B5/0402; US2007/0299323, A1; WO2010/139361 A1; WO2017/099603 A1) related to EEG, neuromodulation, and psychophysiology, but does not own these nor receives any proceeds related to these patents; Research Institute Brainclinics received research funding from Brain Resource (Sydney, Australia) and neuroCare Group (Munich, Germany), and equipment support from Deymed, neuroConn, and Magventure; however, data analyses and writing of this manuscript were unconstrained. The other authors report no conflicts of interest.
Rights and permissions
About this article
Cite this article
Bijlenga, D., Vollebregt, M.A., Kooij, J.J.S. et al. The role of the circadian system in the etiology and pathophysiology of ADHD: time to redefine ADHD?. ADHD Atten Def Hyp Disord 11, 5–19 (2019). https://doi.org/10.1007/s12402-018-0271-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12402-018-0271-z