Abstract
Starch-based hydrogels are natural polymeric structures of high scientific interest in the food, pharma, and cosmetic sectors. In this work, the effects of the starch source (rice, corn, wheat, and tapioca starch) and processing time (600 MPa for 5 and 15 min) on gel formation and on the physical characteristics of the structures formed were evaluated. At the pressure level utilized, all the starches were completely gelatinized regardless of the processing time tested. The hydrogels obtained displayed a shear-thinning and gel-like behavior (G' > G"). HPP, under the processing conditions tested, was more effective in producing hydrogels based on tapioca and rice than on corn and wheat starch. Rice, wheat, and corn starch HPP hydrogels showed a cream-like structure, whereas those based on tapioca starch evidenced a more compact structure. With a HPP processing time of 15 min, tapioca and rice starch HPP hydrogels displayed higher viscosity, G', and firmness, suggesting an overall structural reinforcement. However, with 15 min of processing time, the lightness and whiteness of rice, wheat, and corn starch HPP hydrogels were negatively influenced, presumably as a consequence of the increased amount of water absorbed in starch granules. These results suggest that both the starch source and processing time play an important role in the formation of gels from starch suspensions and affect the physical characteristics of HPP hydrogels. Finally, the natural products obtained might be suitable for use in several applications where either creamy or gummy structures are desirable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starch represents a versatile and inexpensive material of potential use in polymer technology and numerous other food and non-food applications. Among staple foods rich in starch, rice, wheat, corn, and tapioca (or cassava) are the most abundantly produced and traded worldwide [21]. However, often, these crops show defects hindering their utilization in food processing lines and are discarded. Starches contained in defective crops can be recovered and used to produce starch-based products and reduce environmental burdens. The implementation of eco-efficient processes of starches could positively affect crop production sustainability. Among newer applications of these natural biopolymers, the production of starch-based biodegradable packages has been proposed to replace or lower the use of petroleum-based plastics [42]. In addition, the production of starch-based hydrogels is promising and gaining attention. Hydrogels are 3D polymeric hydrophilic networks able to absorb and retain significant amounts of water. The most relevant characteristics of starch-based hydrogels are their safety, biocompatibility, and biodegradability [25, 29, 45, 48, 49, 54, 58, 62].
Hydrogel structures form by applying physical or chemical stresses to starch suspensions, which induce water penetration into the starch granules forming a strong and stable network [9]. Biduski et al. [9] investigated the characteristics of starch-based hydrogels produced by alkaline or thermal gelatinization, utilizing native or cross-linked rice starch with different amylose content (8%, 20%, and 32%). These authors concluded that the gelatinization method and the starch amylose content influenced the hydrogel’s physical properties. Alkaline (NaOH 50%, 55 °C, 0.5 h) gelation, consisting of several processing steps lasting more than 24 h, was the most suitable method to obtain well-structured stable gels with cross-linked rice starch (20% amylose content). Other authors reported that the well-established physical or chemical cross-linking and graft polymerization methods produced hydrogels [2, 18, 29]. However, their main limitations were long processing times, high energy consumption, and safety issues during the synthesis process.
Different technologies for hydrogel formation have been proposed to overcome the limits of conventional methods, including high-pressure processing (HPP), which has been applied mainly for food preservation, causing no or minimal damages to the sensorial and nutritional properties of products. Moreover, HPP has been proposed for the recovery of bioactive compounds from food by-products, the enhancement of bioaccessibility and bioavailability of food micronutrients, the reduction of food allergenicity, the preservation of lipids, and the reduction of salt in foods [5]. HPP was also proven very promising for the modification or gelatinization of starch suspensions for starch-based hydrogel production [10,11,12,13, 16, 19, 31, 33, 37, 38, 40, 41, 52, 55]. By modifying the non-covalent intermolecular interactions, HPP can cause the disordering of food biopolymers, including proteins and starches, until inducing protein unfolding or a complete starch gelatinization [4, 34].
HPP-assisted gelatinization is strongly influenced by the starch source, starch/water ratio, pressure level, temperature, and processing time [6, 53]. The structural properties of pressure-induced gels are different from those of thermal gels, mainly due to the pressure stabilization of hydrogen bonds during the gelatinization process, absence of extensive shearing (e.g., stirring), and a reduction in the extent of retrogradation [16, 28, 40]. Starch-based HPP hydrogels displayed excellent mechanical and rheological properties such as less rigidity, highly structured, and promising stability and functionalization [37, 38].
In the last 20 years, many efforts have been made to unravel the role of physicochemical characteristics of starches and HPP conditions on the gelatinization process. However, the physical properties of hydrogels require further investigation to support their industrial-scale use [13, 56]. Therefore, in this paper, the effects of the starch source and two processing times on gel physical characteristics and formation were investigated. Furthermore, the purpose of this investigation was the successful production of structured and stable starch-based HPP hydrogels and to present how the structure and functionality of these novel structures are influenced by the utilization of different starch sources and two pressure-inducing gelatinization conditions, namely, 600 MPa for 5 and 15 min.
Materials and Methods
Materials
Rice (S7260) (17.7% amylose content, 96.5% purity in dry weight basis), wheat (S5127) (26.96% amylose content, 99% purity in dry weight basis), and corn (S4126) (21.17% amylose content, 97% purity in dry weight basis) starch powders were purchased from Sigma Aldrich (Steinheim, Germany). Tapioca starch (20.2% amylose content, 92.2% purity in dry weight basis) was obtained from Rudolf Sizing Amidos do Brazil (Ibirarema, Sao Paulo, Brazil).
Samples’ Preparation
Based on previous experimental results (data not shown), a concentration of 20% (w/w) was utilized to avoid sedimentation and obtain proper gel structuring under HPP. Starch (wet basis) was suspended in distilled water and dissolved under gentle mixing. To ensure sample homogeneity and avoid particles settling, starch suspensions were prepared immediately before HPP treatments.
HPP Treatments
In each experiment, 10 g of the starch suspension was deaerated, thoroughly mixed, packed in flexible pouches, sealed, and then pressure treated in a lab-scale high-pressure unit with a vessel capacity of 50 mL (U-22, Institute of High-Pressure Physics, Polish Academy of Sciences, Unipress Equipment Division, Poland). Based on previous observations (patent pending) and literature findings [55], the treatment conditions were 600 MPa, applied at a compression rate of 8.4 MPa/s for 5 and 15 min with an initial temperature of 25 °C and at decompression time of less than 15 s. The estimated temperature increase due to pressure build-up was 2–3 °C/100 MPa [20]. Processed samples were stored (< 4 h) at room temperature until further analysis. All experiments were done in triplicate.
Samples’ Characterization
Degree of Gelatinization
The degree of gelatinization of samples was detected by measuring the loss of the optical birefringence of starch granules under polarized light (20×). An inverted light microscope Nikon Eclipse (TE 2000S, Nikon Instruments Europe B.V., Netherlands) equipped with a polarization filter, coupled to a DS Camera Control Unit (DS-5 M-L1, Nikon Instruments Europe B.V, The Netherlands), was used for image acquisition and analysis. Before each measurement, a small amount of the gelatinized sample was placed between a microscope slide and a cover glass.
Swelling Power
The swelling power was determined according to the method reported by Kusumayanti et al. [35] with slight modifications. Samples of HPP hydrogels were centrifuged (PK130R, ALC, Winchester, Virginia) at 1351 g for 10 min, the supernatant was removed, and the pellet was weighed before and after drying for 6 h at 105 °C. Swelling power, i.e., the ratio of the wet pellet over the dry weight of starch in the hydrogel sample, was evaluated as follows:
Efficiency Index (EI)
An efficiency index, defined as an empirical parameter to assess the extent of gel formation under the HPP conditions utilized, was evaluated as follows:
where hydrogel formed refers to the drained weight of the structured material.
Macroscopic Analysis
The macroscopic analysis of HPP hydrogels was performed by a digital camera (Sony Corp, Tokyo, Japan) in angular mode. Original pictures without editing and filtering are reported.
Color Measurements
Lightness (L*), redness (a*), and yellowness (b*) of HPP hydrogels were determined by the colorimeter CR-400 (Konica Minolta Inc., Tokyo, Japan). The color difference (ΔΕab*) was calculated according to Eq. 3, as reported by Bodart et al. [14].
where ΔL*, Δa*, and Δb* were the difference between two samples in L*, a*, and b*, respectively.
To account for the effects of HPP processing time on hydrogels, the color index ΔE was evaluated according to a modified form of Eq. 3, as follows:
while the color differences among HPP hydrogels obtained with different starches at the two processing times (5 min and 15 min) were evaluated according to the modified forms of Eq. 3 as follows:
The whiteness index (WI) was calculated according to Eq. 4 reported by Kaur et al. [32].
At least ten measurements were done on each sample.
Rheology
The rheology of HPP hydrogels was determined utilizing a controlled stress and strain rheometer AR 2000 (TA Instruments, New Castle, Delaware, USA), equipped with a Peltier plate and a circulating water bath (DC10-Haake K10, Karlsruhe, Germany). A plate-cone geometry (40-mm diameter, 2°) with a fixed gap of 52 μm was used. The HPP hydrogel samples (1 g, n = 3) were loaded in the center of the Peltier plate and left undisturbed for 120 s at 25 °C, allowing stress relaxation and temperature equilibration.
Flow Curves
Flow curves of hydrogels samples (n = 3) were obtained by varying the shear rate from 0.1 to 100 s−1 at 25 °C.
Data of apparent viscosity (η) were collected and interpreted by using the manufacturer’s software (Trios v5.0.0.44608, TA Instruments-Waters LLC, New Castle, Delaware, USA).
Frequency Sweep Tests
Small deformation rheological properties of the samples (n = 3) were obtained from frequency sweep tests recorded in the range from 0.1 to 100 rad/s at 25 °C within the linear viscoelastic region of the processed samples (3% of strain). The viscoelastic parameters such as the storage or elastic modulus, G', and the loss or viscous modulus, G", were recorded and calculated using the manufacturer’s software abovementioned.
Texture Profile Analysis (TPA)
The texture profile analysis (TPA) of all HPP hydrogels was performed using a TA.XT2 texture analyzer (Stable Micro Systems, Surrey, UK) equipped with a load 5-kg cell connected to a microcomputer. Hydrogel samples (6 g, n = 3) were loaded into a cylindrical cell (24-mm height and 25-mm ID), and compression-decompression cycles were carried out using a cylindrical probe (10-mm diameter) at room temperature and a rate of 1 mm/s up to attaining 50% of sample deformation. The compression runs were repeated twice, with a decompression rate of 1 mm/s and a delay of 5 s between two bites, to generate force-time curves. Hardness, adhesiveness, springiness, chewiness, cohesiveness, and gumminess of hydrogels were calculated from the compression data recorded.
Statistical Analysis
Results were analyzed by statistical descriptive analysis (mean ± SD), one-way ANOVA, and post hoc comparison using the Fisher least significant difference (LSD) test to determine significant differences amongst experiments (p value was < 0.05). All analyses were performed using Statgraphics Centurion XVI Statistical Software (Statistical Graphics Corp., Hendon, Virginia).
Results and Discussion
Gel Formation
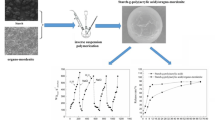
Figure 1 shows the polarized micrographs of starch suspensions, untreated and pressurized at 600 MPa at two processing times (5 min and 15 min). This visual observation can be easily used to define a gelatinization index of starch samples. As expected, untreated starch granules (control) showed complete birefringence (Fig. 1, left side). Pressure treatments at 600 MPa for 5 and 15 min caused the total loss of birefringence, indicating a complete gelatinization of the starch suspensions. These findings were in good agreement with those reported by other authors on rice, corn, wheat, and tapioca starch suspensions, pressurized at 600 MPa from 5 min to 30 min [6, 16, 17, 27, 31, 39, 52, 55].
Starch gelatinization after HPP treatment was also assessed based on the swelling power and the efficiency index (Table 1). From the data reported in Table 1, it can be observed that the values of EI and SP of corn, rice, and wheat starch hydrogels were not influenced by the two processing times evaluated (p > 0.05). However, tapioca starch hydrogels evidenced a higher SP at a processing time of 15 min (p < 0.05), suggesting that tapioca starch granules are able to hold more water even when the complete gelatinization is achieved. This swelling behavior can be attributed to a higher capacity of these granules to swell and solubilize than cereal starches granules [8] and to the absence of amylose-lipid complex formation [31]. Katopo et al. [31] reported that under HPP treatment, amylose from cereal starches instead of amylose from tapioca starch interacted with lipids, developing a helical complex that holds amylopectin molecules restricting the dispersion and swelling of the starch granules.
In all cases, the two indexes strongly depended on the starch source (p < 0.05). SP and EI values of tapioca and rice starch hydrogels were higher than those of corn and wheat starch. This result is in agreement with those reported by Stute et al. [55] describing the less water absorption capacity of some type of starch granules, such as corn and wheat, while others, such as tapioca starch granules, were characterized by an extensive swelling during HPP treatments. The different macromolecular architecture, branching, and molecular weight [8], as well as starch solubility and granule size, must be considered as important factors during hydrogel formation under static processes such as HPP [37]. Moreover, the amylose content of rice starch used in this study (17.7%) was lower than that of wheat (26.96%) and corn starch (21.2%), having this different composition and the amylose-lipid complex formation detrimental effects on granules swelling under HPP [52] and, consequently, hindering HPP hydrogels production. This study suggests that gel formation parameters, namely, EI and SP, were more affected by the starch source than by HPP processing time [10, 11, 16, 52].
Macroscopic Analysis
As shown in Fig. 2, at the two processing times investigated, HPP hydrogels were highly homogeneous and had good structural integrity. Corn, rice, and wheat hydrogels had a cream-like appearance, while those based on tapioca had a rubber-like structure. Katopo et al. [31] reported similar results when treating starch suspensions (25% w/w) for 5 min at 600 MPa, obtaining spreadable and adhesive corn and rice hydrogels and hard and less adhesive tapioca hydrogels. The native crystalline structure of starch affected hydrogels formation by pressure [31, 55]. As detected by X-ray diffraction [31], tapioca starch showed a C-type spatial configuration (a mixture of A-type and B-type pattern), whereas rice, corn, and wheat starch presented an A-type spatial configuration. Starch gelatinization by HPP treatments is caused by water forced into starch granules due to compressive forces [41]. Taking into account that A-type starches show densely packed structures (seven double helices with a staggered monoclinic lattice) with a significant less space available for water compared to B-type and C-type starches [60, 61], it can be assumed that these structural differences may, in part, influence the different physical appearance of the starch-based HPP hydrogels obtained and in agreement with SP results (Table 1). Furthermore, the creamy-like appearance observed on corn, rice, and wheat starch hydrogels can be related to the higher number of granules per gram of starch compared to tapioca starch, as reported by BeMiller and Whistler [8]. Visual observation confirmed that corn, rice, and wheat hydrogels were lighter than tapioca hydrogels, especially at 600 MPa for 5 min.
It can be hypothesized that tapioca starch had a stronger starch profile due to the higher length of the chains, a higher molecular weight of amylose, and a good association between amylose and amylopectin under HPP treatments [7, 31], resulting in HPP hydrogels that are more compact, highly structured, and able to entrap more water [1, 59].
The results of the macroscopic evaluation suggest that the physical appearance of hydrogels produced under pressure was strongly influenced by the starch source, which should be taken into account to forecast future applications of these structures as a smart carrier of compounds for the development of novel foods which will benefit of their creamy or gummy consistency. However, further research is required to determine the mechanisms and the key factors influencing the structuring of these innovative materials.
Color Measurements
Table 2 showed the color parameters of HPP hydrogels based on the different starches and processed at 600 MPa for 5 and 15 min. The starch source and processing time (p < 0.05) influenced the color parameters of starch-based HPP hydrogels. All HPP hydrogels obtained in this study had positive values of L*, in the range between 45 and 66, indicating that white and bright components were predominant in all cases. Corn starch hydrogels were the brightest, followed by wheat, rice, and tapioca hydrogels (p < 0.05). At a processing time of 15 min, a darkening of corn, rice, and wheat starch hydrogels has been detected related to a significant decreasing of L* values (~ 20%), whereas the lightness of tapioca hydrogels increased by 9% (p < 0.05). The amount of water entrapped inside these structures increased at a processing time of 15 min, and a reduced amount of water was present on the surface reflecting the light, resulting in less bright creamy hydrogels and more translucent tapioca starch hydrogels. WI values, which showed the same trend, confirmed these findings.
With respect to the parameter a*, corn, wheat, and rice hydrogels evidenced negative values, indicating a slight tendency to the greenness of these samples (the greenest was corn, followed by wheat and rice (p < 0.05)). On the contrary, tapioca hydrogels had positive values of the parameter a* indicating an increased redness (p < 0.05). At processing times of 15 min, a* values significantly increased in all samples (p < 0.05), namely, up to + 85% in rice, + 55% in wheat, + 28% in tapioca, and + 6% in corn hydrogels.
A higher tendency to yellowness components was evidenced by corn and tapioca starch hydrogels with higher b* values for tapioca starch hydrogels. Negative b* values were detected for rice and wheat hydrogels, accounting for the tendency to blueness, being wheat hydrogels the bluest. These results can be attributed to the different levels of yellow pigments present in the starches, being wheat starch, for instance, a characteristic starch devoid of yellow pigments such as xanthophyll (lutein) and flavonoids, whereas in corn and tapioca starch, those pigments are more abundant [8]. A significant increase (p < 0.05) of b* values in all samples produced at 600 MPa for 15 min have been identified, with an increment of the yellowness components on corn and tapioca starch hydrogels and a reduction of the blueness components on rice and wheat starch hydrogels.
To better understand if the color differences of hydrogels, which are affected both by the two processing times and starch source, could be detected by the human eye, the values of the parameter ΔΕab* were calculated. The human eye perceives the color differences if ΔΕab* values are > 3 [46]. For the sake of completeness, ΔΕab* values were obtained either by comparing the colors of the hydrogels made with the same starch and different processing time (data reported in Table 2) and by comparing the hydrogels made with different starches and the same processing time (data reported in Table 3). Processing time strongly affected the color perception of starch-based HPP hydrogels (p < 0.05) (Table 2). ΔΕab* values were much higher than 3, confirming that with a processing time of 15 min, the human eye could detect color differences. Wheat, corn, and rice starch hydrogels showed significant net color changes (ΔΕab* > 11). Data from Table 3 allowed concluding that the color parameters of rice and wheat starch hydrogels were similar (lower ΔΕab* values) for both processing time. Moreover, ΔΕab* values of tapioca hydrogels processed for 5 min were higher than those of tapioca hydrogels obtained at 15 min processing time, the latter having similar color characteristics of wheat, corn, and rice starch hydrogels. These results, confirmed by visual observations, could be explained by the different physical structures of HPP hydrogels (as reported in the upcoming sections) that determined their color profiles.
Rheology
To further unveil to which extent HPP processing time and starch source impacted mechanical characteristics of these structures, flow behavior (Fig. 3) and viscoelastic properties (Fig. 4) of hydrogels were determined. Figure 3 shows the viscosity of starch-based HPP hydrogels, formed at 600 MPa, and 5 or 15 min, as a function of the shear rate. Independently of the starch source and for both processing times, all curves exhibit non-Newtonian behavior (shear-thinning). Other authors already reported this rheological behavior of starch-based hydrogels [30, 63]. Hydrogels are characterized by shear-dependent flow behavior and can flow under the action of high shear forces. These forces cause irreversible changes, or fractures, in the gel network, determining the reorganization of the structure and reduced intermolecular resistance to flow [50, 51].
Tapioca hydrogels offered the highest resistance to flow, followed by corn, wheat, and rice starch hydrogels (p < 0.05). The different shapes of the viscosity curves confirmed the different flow behavior of HPP hydrogels. In the entire range of shear rate applied, tapioca starch hydrogels showed the highest viscosity values, confirming that strong gummy structures are more resistant to flow [24]. The viscosity of corn, rice, and wheat starch hydrogels was much lower, confirming that these spreadable materials do not resist to flow and had a weak structure. The flow profiles of HPP hydrogels obtained at 600 MPa and 15 min were higher than those of gels produced at 600 MPa and 5 min, confirming that a longer HPP processing time gave rise to stronger structures with higher viscosity since the starch-starch and starch-water interactions are more likely to occur.
As shown in Fig. 4, the mechanical profiles of all HPP hydrogels produced in this work were typical of strong and stable gel structures, with the elastic response one order of magnitude higher than the viscous response (G' > G"). Both moduli were almost independent on frequency, confirming that, under pressure, starch particles formed stable, continuous, and well-structured cross-linked gel networks [22, 36].
The starch source and processing time affected the mechanical properties of all samples. In the frequency range investigated, the mechanical profile of tapioca starch hydrogels was stronger than that of rice, wheat, and corn starch hydrogels, accounting for a stronger gel network. Moreover, at 600 MPa for 15 min, G' values of all HPP hydrogels increased due to the increasing stability of the internal structure. In gels obtained with physical methods, polymer chains are physically cross-linked by hydrogen bonds, and crystalline segments and hydrophobic interactions cooperate to produce molecular connections of different strength, stability, and spatial distribution [36]. Based on our results, and considering the physical governing principles of HPP, it can be hypothesized that in high-pressure-induced starch gels [34, 53], the physical non-covalent interactions are increased and become stronger at longer processing times, resulting in higher G' and G" values. This is consistent with results recently published by Larrea-Wachtendorff et al. [38], describing similar behavior of corn and rice starch HPP hydrogels treated at 600 MPa for 20 min and with the findings of Buckow et al. [16], showing the time-dependence of HPP gel formation.
Texture Profile Analysis (TPA)
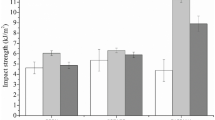
Tapioca hydrogels had higher firmness (p < 0.05) and negligible adhesiveness (p < 0.05) than corn, rice, and wheat starch hydrogels (Fig. 5). The differences in firmness were even more remarkable on samples processed for 15 min, confirming that structural reinforcement of hydrogels occurred. This is in agreement with rheology measurements. Despite the reduced firmness of “cream-like” corn, wheat, and rice starch hydrogels, with a processing time of 15 min, a slight but not negligible increase of firmness was observed in rice gels (p < 0.05), while no significant differences were detected in corn and wheat starch hydrogels.
Cohesiveness is related to the strength of internal bonds in gels [26]. The values of this parameter in rice, tapioca, and wheat starch hydrogels were not affected by the processing time (p > 0.05), except for corn starch hydrogels, whose cohesiveness increased, reaching the same values detected in rice hydrogels for a treatment time of 15 min. All HPP hydrogels produced in this work showed excellent cohesiveness (0.6–0.8), and the values detected are in good agreement with those of other authors, who measured similar values of this parameter on the development of gels for innovative food applications such as gummy candies [3] and surimi sausage [43], and for novel non-food applications such as long-acting drug delivery [57], topical creams [23], and quercetin-loaded gels [26].
Adhesiveness, defined as the force required to detach a probe from a sample, can be assumed as the force necessary to remove a bolus adhering to the palate during mastication [15], a cosmetic product from the skin, or a pharmaceutical product from the mucosa [44]. By applying a processing time of 15 min, the adhesiveness of corn and rice hydrogels increased from 0.63 and 0.49 [N*s] to 0.81 and 0.85 [N*s] (p < 0.05), respectively. Wheat starch hydrogels presented a good adhesiveness (0.37 [N*s]), regardless of the two processing times utilized (p > 0.05). Adhesiveness values of the spreadable HPP hydrogels were in good agreement with those found for skin photoprotection starch-based emulsions and drug delivery systems for topical use [44, 47]. Moreover, data on firmness were used to evaluate gumminess and chewiness. The gumminess is the energy required to disintegrate a semisolid food and make it ready for swallowing. The chewiness is the work needed to reduce the consistency of food, making it suitable for swallowing [15]. The values of chewiness confirmed that, among the starches utilized in this work, two types of gels were obtained under pressure: easily swallowing corn, rice, and wheat starch hydrogels and firm tapioca hydrogels. A higher chewing force was needed to make the latter gels ready for swallowing.
In conclusion, the results of texture and rheology were coherent and demonstrated that HPP processing time and starch source influenced the physical and mechanical properties of starch-based HPP hydrogels and should be considered during the design of innovative products such as baby foods, gluten-free pastes, and gummy candies, among others.
Conclusions
This investigation demonstrated that HPP treatments at 600 MPa for 5 and 15 min can be used to fabricate stable starch-based hydrogels. Corn, rice, tapioca, and wheat starch were completely gelatinized under the processing conditions investigated, being rice and tapioca the most suitable starches to produce hydrogels by the HPP treatments used. HPP hydrogels based on corn, rice, and wheat starch featured “soft” gel structures, characterized by low hardness (N) and viscosity (η), and high adhesiveness (-N/s), whiteness (WI), and lightness (L*). Due to their good spreadability, these hydrogels were very similar to creams. Tapioca starch, instead, formed HPP hydrogels with a gummy or rubbery compact structure resistant to flow, characterized by high hardness (N) and viscosity (η), and a translucid color. The mechanical properties of all hydrogels produced at 600 MPa were improved by utilizing processing times of 15 min. However, this processing time had detrimental effects on the color of hydrogels, particularly lightness (L*) and whiteness (WI). Notwithstanding the results of this research could allow forecasting new potential applications of starch-based HPP hydrogels, further investigations are needed to assess the physical and microbiological stability of these structures as well as their performance during shelf life.
References
Ahmed J, Singh A, Ramaswamy HS, Pandey PK, Raghavan GSV (2014) Effect of high-pressure on calorimetric, rheological and dielectric properties of selected starch dispersions. Carbohydr Polym 103:12–21. https://doi.org/10.1016/j.carbpol.2013.12.014
Ali A, Ahmed S (2018) Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J Agric Food Chem 66(27):6940–6967. https://doi.org/10.1021/acs.jafc.8b01052
Amjadi S, Ghorbani M, Hamishehkar H, Roufegarinejad L (2018) Improvement in the stability of betanin by liposomal nanocarriers: its application in gummy candy as a food model. Food Chem 256:156–162. https://doi.org/10.1016/j.foodchem.2018.02.114
Balny C (2002) High pressure and protein oligomeric dissociation. High Pressure Res 22(3–4):737–741. https://doi.org/10.1080/08957950212447
Barba FJ, Terefe NS, Buckow R, Knorr D, Orlien V (2015) New opportunities and perspectives of high pressure treatment to improve health and safety attributes of foods. A review. Food Res Int 77:725–742. https://doi.org/10.1016/j.foodres.2015.05.015
Bauer BA, Knorr D (2005) The impact of pressure, temperature and treatment time on starches: pressure-induced starch gelatinisation as pressure time temperature indicator for high hydrostatic pressure processing. J Food Eng 68(3):329–334. https://doi.org/10.1016/j.jfoodeng.2004.06.007
BeMiller J (2018) Carbohydrate chemistry for food scientists, 3rd edn. Woodhead publishing, Cambridge
BeMiller J, Whistler R (2009) Starch: chemistry and technology, 3rd edn. Academic press, New York
Biduski B, da Silva WMF, Colussi R, Halal SL d ME, Lim L-T, Dias ÁRG, Zavareze E d R (2018) Starch hydrogels: the influence of the amylose content and gelatinization method. Int J Biol Macromol 113:443–449. https://doi.org/10.1016/j.ijbiomac.2018.02.144
Błaszczak W, Valverde S, Fornal J (2005a) Effect of high pressure on the structure of potato starch. Carbohydr Polym 59(3):377–383. https://doi.org/10.1016/j.carbpol.2004.10.008
Błaszczak W, Fornal J, Valverde S, Garrido L (2005b) Pressure-induced changes in the structure of corn starches with different amylose content. Carbohydr Polym 61(2):132–140. https://doi.org/10.1016/j.carbpol.2005.04.005
Błaszczak W, Fornal J, Kiseleva VI, Yuryev VP, Sergeev AI, Sadowska J (2007) Effect of high pressure on thermal, structural and osmotic properties of waxy maize and Hylon VII starch blends. Carbohydr Polym 68(3):387–396. https://doi.org/10.1016/j.carbpol.2006.12.023
Błaszczak W, Buciński A, Górecki AR (2015) In vitro release of theophylline from starch-based matrices prepared via high hydrostatic pressure treatment and autoclaving. Carbohydr Polym 117:25–33. https://doi.org/10.1016/j.carbpol.2014.09.031
Bodart M, de Peñaranda R, Deneyer A, Flamant G (2008) Photometry and colorimetry characterisation of materials in daylighting evaluation tools. Build Environ 43(12):2046–2058. https://doi.org/10.1016/j.buildenv.2007.12.006
Bourne MC (2002) Texture, Viscosity, and Food. In: Food texture and viscosity: concept and measurement, 2nd edn. Academic Press, San Diego
Buckow R, Heinz V, Knorr D (2007) High pressure phase transition kinetics of maize starch. J Food Eng 81(2):469–475. https://doi.org/10.1016/j.jfoodeng.2006.11.027
Buckow R, Jankowiak L, Knorr D, Versteeg C (2009) Pressure−temperature phase diagrams of maize starches with different amylose contents. J Agric Food Chem 57(24):11510–11516. https://doi.org/10.1021/jf902246t
Caló E, Khutoryanskiy VV (2015) Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polym J 65:252–267. https://doi.org/10.1016/j.eurpolymj.2014.11.024
Cappa C, Lucisano M, Barbosa-Cánovas GV, Mariotti M (2016) Physical and structural changes induced by high pressure on corn starch, rice flour and waxy rice flour. Food Res Int 85:95–103
De Maria S, Ferrari G, Maresca P (2015) Rheological characterization and modelling of high pressure process bovine serum albumin. J Food Eng 153:39–44. https://doi.org/10.1016/j.jfoodeng.2014.12.013
Food and agriculture organization (FAO). FAO statistics database updated up to 2017. http://www.fao.org/faostat/en/#data/QC/. Accessed 10 Oct 2019
Fradinho P, Sousa I, Raymundo A (2019) Functional and thermorheological properties of rice flour gels for gluten-free pasta applications. Int J Food Sci Technol 54(4):1109–1120. https://doi.org/10.1111/ijfs.14001
Froelich A, Osmałek T, Snela A, Kunstman P, Jadach B, Olejniczak M, Roszak G, Białas W (2017) Novel microemulsion-based gels for topical delivery of indomethacin: formulation, physicochemical properties and in vitro drug release studies. J Colloid Interface Sci 507:323–336. https://doi.org/10.1016/j.jcis.2017.08.011
Gałkowska D, Pycia K, Juszczak L, Pająk P (2014) Influence of cassia gum on rheological and textural properties of native potato and corn starch. Starch - Stärke 66(11–12):1060–1070. https://doi.org/10.1002/star.201400078
García-Astrain C, Avérous L (2018) Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr Polym 190:271–280. https://doi.org/10.1016/j.carbpol.2018.02.086
Gokhale JP, Mahajan HS, Surana SJ (2019) Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: in vivo and in vitro studies. Biomed Pharmacother 112:108622. https://doi.org/10.1016/j.biopha.2019.108622
Hibi Y, Matsumoto T, Hagiwara S (1993) Effect of high pressure on the crystalline structure of various starch granules. Cereal Chem 70(6):671–676
Hu X, Xu X, Jin Z, Tian Y, Bai Y, Xie Z (2011) Retrogradation properties of rice starch gelatinized by heat and high hydrostatic pressure (HHP). J Food Eng 106:262–266. https://doi.org/10.1016/j.jfoodeng.2011.05.021
Ismail H, Irani M, Ahmad Z (2013) Starch-based hydrogels: present status and applications. Int J Polym Mater 62(7):411–420. https://doi.org/10.1080/00914037.2012.719141
Jiang B, Li W, Shen Q, Hu X, Wu J (2015) Effects of high hydrostatic pressure on rheological properties of Rice starch. Int J Food Prop 18(6):1334–1344. https://doi.org/10.1080/10942912.2012.709209
Katopo H, Song Y, Jane J (2002) Effect and mechanism of ultrahigh hydrostatic pressure on the structure and properties of starches. Carbohydr Polym 47(3):233–244. https://doi.org/10.1016/S0144-8617(01)00168-0
Kaur BP, Kaushik N, Rao PS, Chauhan OP (2013) Effect of high-pressure processing on physical, biochemical, and microbiological characteristics of black tiger shrimp (Penaeus monodon): high-pressure processing of shrimp. Food Bioprocess Technol 6(6):1390–1400. https://doi.org/10.1007/s11947-012-0870-1
Kawai K, Fukami K, Yamamoto K (2012) Effect of temperature on gelatinization and retrogradation in high hydrostatic pressure treatment of potato starch–water mixtures. Carbohydr Polym 87(1):314–321. https://doi.org/10.1016/j.carbpol.2011.07.046
Knorr D, Heinz V, Buckow R (2006) High pressure application for food biopolymers. Biochim Biophys Acta, Proteins Proteomics 1764(3):619–631. https://doi.org/10.1016/j.bbapap.2006.01.017
Kusumayanti H, Handayani NA, Santosa H (2015) Swelling power and water solubility of cassava and sweet potatoes flour. Procedia Environ Sci 23:164–167. https://doi.org/10.1016/j.proenv.2015.01.025
Lapasin R (2016) Rheological characterization of hydrogels. In: Matricardi P, Alhaique F, Coviello T (eds) Polysaccharide hydrogels: characterization and biomedical applications. Taylor & Francis Group, LLC, Florida, pp 83–138
Larrea-Wachtendorff D, Tabilo-Munizaga G, Ferrari G (2019) Potato starch hydrogels produced by high hydrostatic pressure (HHP): a first approach. Polymers 11(10):1673. https://doi.org/10.3390/polym11101673
Larrea-Wachtendorff D, Di Nobile G, Ferrari G (2020) Effects of processing conditions and glycerol concentration on rheological and texture properties of starch-based hydrogels produced by high pressure processing (HPP). Int J Biol Macromol 159:590–597. https://doi.org/10.1016/j.ijbiomac.2020.05.120
Li G, Zhu F (2018) Effect of high pressure on rheological and thermal properties of quinoa and maize starches. Food Chem 241:380–386. https://doi.org/10.1016/j.foodchem.2017.08.088
Li W, Bai Y, Mousaa SAS, Zhang Q, Shen Q (2012) Effect of high hydrostatic pressure on physicochemical and structural properties of rice starch. Food Bioprocess Technol 5(6):2233–2241. https://doi.org/10.1007/s11947-011-0542-6
Li W, Tian X, Liu L, Wang P, Wu G, Zheng J, Ouyang S, Luo Q, Zhang G (2015) High pressure induced gelatinization of red adzuki bean starch and its effects on starch physicochemical and structural properties. Food Hydrocoll 45:132–139. https://doi.org/10.1016/j.foodhyd.2014.11.013
Liu H, Xie F, Yu L, Chen L, Li L (2009) Thermal processing of starch-based polymers. Prog Polym Sci 34(12):1348–1368. https://doi.org/10.1016/j.progpolymsci.2009.07.001
Liu X, Ji L, Zhang T, Xue Y, Xue C (2019) Effects of pre-emulsification by three food-grade emulsifiers on the properties of emulsified surimi sausage. J Food Eng 247:30–37. https://doi.org/10.1016/j.jfoodeng.2018.11.018
Lucero MJ, Ferris C, Sánchez-Gutiérrez CA, Jiménez-Castellanos MR, de-Paz, M.-V. (2016) Novel aqueous chitosan-based dispersions as efficient drug delivery systems for topical use. Rheological, textural and release studies. Carbohydr Polym 151:692–699. https://doi.org/10.1016/j.carbpol.2016.06.006
Mahinroosta M, Jomeh Farsangi Z, Allahverdi A, Shakoori Z (2018) Hydrogels as intelligent materials: a brief review of synthesis, properties and applications. Mater Today Chem 8:42–55. https://doi.org/10.1016/j.mtchem.2018.02.004
Martínez-Cervera S, Salvador A, Muguerza B, Moulay L, Fiszman SM (2011) Cocoa fibre and its application as a fat replacer in chocolate muffins. LWT – Food Sci Technol 44(3): 729-736. https://doi.org/10.1016/j.lwt.2010.06.035
Marto J, Gouveia LF, Gonçalves L, Chiari-Andréo BG, Isaac V, Pinto P, Oliveira E, Almeida AJ, Ribeiro HM (2016) Design of novel starch-based Pickering emulsions as platforms for skin photoprotection. J Photochem Photobiol B Biol 162:56–64. https://doi.org/10.1016/j.jphotobiol.2016.06.026
McClements DJ (2017) Recent progress in hydrogel delivery systems for improving nutraceutical bioavailability. Food Hydrocoll 68:238–245. https://doi.org/10.1016/j.foodhyd.2016.05.037
Mun S, Kim Y-R, McClements DJ (2015) Control of β-carotene bioaccessibility using starch-based filled hydrogels. Food Chem 173:454–461. https://doi.org/10.1016/j.foodchem.2014.10.053
Nguyen DQ, Jensen CTB, Kristensen PG (1998) Experimental and modelling studies of the flow properties of maize and waxy maize starch pastes. Chem Eng J 70(2):165–171. https://doi.org/10.1016/S0923-0467(98)00081-5
Nurul IM, Azemi BMNM, Manan DMA (1999) Rheological behaviour of sago (metroxylon sagu) starch paste. Food Chem 64:501–505. https://doi.org/10.1016/S0308-8146(98)00145-9
Oh HE, Pinder DN, Hemar Y, Anema SG, Wong M (2008) Effect of high-pressure treatment on various starch-in-water suspensions. Food Hydrocoll 22(1):150–155. https://doi.org/10.1016/j.foodhyd.2007.01.028
Pei-Ling L, Xiao-Song H, Qun S (2010) Effect of high hydrostatic pressure on starches: a review. Starch - Stärke 62(12):615–628. https://doi.org/10.1002/star.201000001
Qi X, Wei W, Li J, Su T, Pan X, Zuo G, Zhang J, Dong W (2017) Design of Salecan-containing semi-IPN hydrogel for amoxicillin delivery. Mater Sci Eng C 75:487–494. https://doi.org/10.1016/j.msec.2017.02.089
Stute R, Heilbronn, Klingler RW, Boguslawski S, Eshtiaghi MN, Knorr D (1996) Effects of high pressures treatment on starches. Starch-Starke 48(11–12):399–408. https://doi.org/10.1002/star.19960481104
Szepes A, Makai Z, Blümer C, Mäder K, Kása P, Szabó-Révész P (2008) Characterization and drug delivery behaviour of starch-based hydrogels prepared via isostatic ultrahigh pressure. Carbohydr Polym 72(4):571–578. https://doi.org/10.1016/j.carbpol.2007.09.028
Tuğcu-Demiröz F, Acartürk F, Erdoğan D (2013) Development of long-acting bioadhesive vaginal gels of oxybutynin: formulation, in vitro and in vivo evaluations. Int J Pharm 457(1):25–39. https://doi.org/10.1016/j.ijpharm.2013.09.003
Van Nieuwenhove I, Salamon A, Adam S, Dubruel P, Van Vlierberghe S, Peters K (2017) Gelatin- and starch-based hydrogels. Part B: in vitro mesenchymal stem cell behavior on the hydrogels. Carbohydr Polym 161:295–305. https://doi.org/10.1016/j.carbpol.2017.01.010
Vittadini E, Carini E, Chiavaro E, Rovere P, Barbanti D (2008) High pressure-induced tapioca starch gels: physico-chemical characterization and stability. Eur Food Res Technol 226(4):889–896. https://doi.org/10.1007/s00217-007-0611-2
Wu HCH, Sarko A (1978a) The double-helical molecular structure of crystalline B-amylose. Carbohydr Res 61(1):7–25. https://doi.org/10.1016/S0008-6215(00)84463-8
Wu HCH, Sarko A (1978b) The double-helical molecular structure of crystalline A-amylose. Carbohydr Res 61(1):27–40. https://doi.org/10.1016/S0008-6215(00)84464-X
Xiao X, Yu L, Xie F, Bao X, Liu H, Ji Z, Chen L (2017) One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem Eng J 309:607–616. https://doi.org/10.1016/j.cej.2016.10.101
Xie F, Yu L, Su B, Liu P, Wang J, Liu H, Chen L (2009) Rheological properties of starches with different amylose/amylopectin ratios. J Cereal Sci 49(3):371–377. https://doi.org/10.1016/j.jcs.2009.01.002
Acknowledgments
Dominique Larrea-Wachtendorff is indebted to the University of Salerno, Italy, for his Ph.D. grant.
Funding
This work was financed by ProdAl Scarl, Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Larrea-Wachtendorff, D., Sousa, I. & Ferrari, G. Starch-Based Hydrogels Produced by High-Pressure Processing (HPP): Effect of the Starch Source and Processing Time. Food Eng Rev 13, 622–633 (2021). https://doi.org/10.1007/s12393-020-09264-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-020-09264-7