Abstract
Meat is the main protein source of the human diet in many cultures. Because of the increasing population growth and welfare, the conventional meat industry cannot follow consumer demands worldwide. Besides, some of the environmental, sustainability-related, and ethical concerns associated with the traditional meat industry have directed scientists to develop new strategies to tackle these negative effects. Culturing meat from cell culture is an emerging bioprocess that will revolutionize the industrial animal agriculture. Many tissue engineering techniques can be utilized for this rising field, although its further development faces important cell culture challenges as well as scale-up limitations. The invention of innovative tools for large-scale in vitro meat production will concurrently advance the technology for biomedical and therapeutic applications. This review highlights vital factors and fundamental cell biology parameters for designing a bioprocess to produce an environmentally friendly meat product that will be accepted by consumers. New applications of current biomedical products and concepts will form the groundwork for future academic research and novel designs enabling large-scale production of cultured meat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meat is an essential part of the human diet in many cultures, and because of the increasing population growth and welfare, the meat industry cannot follow consumer demands worldwide. Additionally, limited land resources and several adverse effects of conventional meat production (e.g., the poor nutritional value of meat, foodborne diseases, extensive use of antibiotics, and greenhouse gas emissions) have compelled scientists to develop innovative techniques that will tackle the negative consequences of traditional meat production through livestock [6, 70, 104]. The meat industry also results in substantially increased land and water use, as livestock farming also requires farmland to produce animal feed (e.g., corn and soy). To further illustrate (Fig. 1), according to research from Bard College, production of beef (per calorie) requires 160 times more land than potatoes, wheat, and rice [126]. Aside from that, the production of popular protein sources results in the formation of about 11 times more greenhouse gases than the production of rice. Having the above-mentioned in mind, a switch to cultured or engineered meat, referred to also as lab-grown or clean meat, from its “traditional” production, would lessen or even detain the effects of global warming. Furthermore, since this technology does not rely on the “sacrifice” of animals to produce food, it would also be more acceptable from the ethical point of view for the well-being of animals.
Nutritional and environmental consequences associated with feed-to-food conversion between meat production and plant-based alternatives. Livestock takes up about 77% of global agriculture land yet produces 17% of the world’s supply of calories. Crops occupy less than 25% of global agriculture land for production of 83% global food caloric supply. Farm-based meat production contributes 4 times more to the total greenhouse gas emissions than plant-based food
Many of the crucial technologies for the realization of large-scale cultured meat production are the same as those that have been pioneered for other large-scale cell culture applications (e.g., antibody therapeutics, cell-based therapy, regenerative medicine) [118, 130]. For the most part, edible animal meat is derived from skeletal muscle tissue; in vitro cultured meat production relies on techniques and strategies developed in skeletal muscle tissue engineering. Research conducted in the mentioned field has elucidated multiple fundamental mechanisms of skeletal muscle repair and identified various types of cells and regulatory factors, which play a crucial role in muscle regeneration [109]. Those findings can also be applied for creating artificially engineered meat in vitro. Proof of concept for the feasibility to grow meat in vitro (beef patty) was provided in 2013 [105]. However, it was not a consumer-available product. Although the science behind skeletal muscle tissue engineering is steadily increasing [17, 69, 102], the technology behind the production of cultured meat is still in its infancy [4, 104, 130]. Several technical challenges are facing its industrial-scale production before it can be introduced into the market in an appreciable quality and quantity and at a reasonable price [4, 29, 36, 130, 131].
Skeletal Muscle Tissue Engineering for Meat Production
The process of growing tissue-engineered meat starts with the acquisition of desired cell or tissue types. Because mature skeletal muscle cells lack proliferation capability, stem cells (mesenchymal) are the most common primary source of myoprogenitors [102]. Despite exhibiting some limitations regarding their regeneration potential, which is restricted only to minor damages [119, 140, 144], the regenerative abilities of these stem cells (satellite cells) and their potential for proliferation and differentiation present important foundations for skeletal muscle tissue engineering.
Skeletal muscle tissue (Fig. 2) comprises aligned myofibers formed through myoblasts fused into elongated multinucleated myotubes. The centralized position of their nuclei characterizes newly formed myotubes. As the myotubes mature to shape myofibers, their nuclei settle on positions at the cell’s periphery. Each myofiber is surrounded by extracellular connective tissue, and numerous myofibers are assembled to form a skeletal muscle. Connective tissue provides a supportive framework that maintains muscle shape and enables synergic contraction of myofibers during movement. Specific alignment of myofibers dictates force generation, and on top of that, connective tissue helps maintain muscle shape and allows myofibers to contract synergically during movement [22, 109, 140].
Skeletal muscle tissue. Skeletal muscle consists of muscle fibers that are arranged in regular bundles enclosed by a dense connective tissue epimysium. From epimysium, a thin septum of connective tissue extends inward (i.e., perimysium), wrapping each fascicle of fibers. Individual muscle fibers (elongated multinuclear cells) is surrounded by a delicate connective tissue, the endomysium, composed of a basal lamina synthesized by the muscle fiber and reticular fibers and fibroblasts [56]
In general, the skeletal muscle can regenerate in response to damage by activating satellite cells, which repose beneath the basal lamina of adult skeletal muscles. As part of the normal physiological response to trauma and injury, satellite cells proliferate and differentiate into myoblasts, which subsequently fuse to form multinucleated myofibers. They usually remain quiescent in the basal lamina until various growth factors and signaling pathways activate them. These primary cells are identified through the expression of Pax7, a transcriptional factor responsible for the regulation of myogenic proliferation. They can be harvested from adult muscle and successfully grown in vitro. Myogenic differentiation is regulated by Myf5 and MyoD, which are transcription factors expressed by myoblasts (and thus, both are identification criteria for them); at this stage, the cell is “committed” to become a muscle fiber [119]. To use tissue engineering either for various applications in regenerative medicine or for cultured meat production, some requirements need to be met. A cell source that can proliferate indefinitely, while it simultaneously has the potential to differentiate into functional skeletal muscle tissue, needs to be embedded (and precisely positioned, as well as oriented) in a three-dimensional (3D) matrix. The latter must allow muscle growth while, at the same time, facilitates the delivery of oxygen and nutrients, as well as enables cell waste removal. To obtain mature, functional muscle fibers, muscle cells need to be cultured in a bioreactor that provides constant (and appropriate) biochemical and biophysical stimuli [7, 70].
The most straightforward in vitro tissue engineering approach to generate mature and contractile muscle constructs is to culture cells on a biomaterial substrate until they have evolved into a functional tissue. However, this approach faces several critical obstacles: (1) fabricating a large muscle construct while maintaining high myofiber packing density and alignment, (2) providing sufficient vascularization to maintain cellular viability in such a large construct for longer periods, and (3) fabricating engineered tissues that can generate physiologically relevant contraction forces. Even though the cells may be the protagonist for skeletal muscle tissue engineering, the important role of biomaterials should not be neglected. By combining various features, like the choice of biomaterials, scaffolds’ 3D architecture, chemical composition, surface functionalization, and bioactivity modulation of cell behavior can be achieved [109].
Cell Sources
As outlined above, a vital component of skeletal muscle tissue engineering is myoprogenitor cells that can directly or indirectly re-form muscle tissue. The stem cell technology utilized for the production of engineered meat (which, as aforementioned, is a skeletal muscle) requires the following steps: (1) harvesting stem cells, (2) expansion of stem cell numbers, (3) their differentiation into myoblasts and myofibers, and (4) assembly into the final meat product. Each cell type comes with advantages over others; sourcing considerations, cost, and degree of characterization of the cell type are all relevant factors to contemplate. Since mature muscle fibers do not possess any proliferation potential, they cannot be used as a cell source for tissue-engineered constructs. Precursor cells and native regenerative cells are reliant upon numerous external factors to promote adhesion, proliferation, differentiation, and maintenance of the desired phenotype [104, 119].
One of the main objectives of bringing cultured meat to a consumer market is the long-term cultivation of cell lines. The strains utilized for fermentation in brewing will likely be used as sample guidelines for these cell lines: Cultures can be used continuously for some number of passages, but periodically they are re-seeded from frozen stocks to avoid genetic drift [130]. Numerous strategies have been introduced to maintain stemness in continuous cultures. These, among others, include the exposure of cells to hypoxic conditions [8] and modulating parameters like scaffold stiffness [79].
Cell Lines
There are two possible cell sources for the production of in vitro meat, namely, primary cells isolated from the original tissue, or established cell lines that are derived directly from native tumor tissue or artificially generated from primary cells in vitro [114, 115, 131]. The latter can be achieved in two ways. A typical strategy involves genetic or chemical induction, which reprograms the cells to proliferate indefinitely [114, 115]. Another method is to select cells that went through a spontaneous transformation and became immortal and then further culture only the selected sub-clones [131]. These immortalized cells (i.e., continuous cell lines) have already proven to be a useful alternative source for fresh tissue samples, and they could be valuable tools to increase the speed of proliferation and differentiation [115, 131].
Nevertheless, since they have undergone significant mutations to become immortal, potential implications of these processes need to be evaluated with extreme care. Besides, these cells can change genetically over multiple passages, leading to phenotypic differences among isolates and potentially misidentification. One general limitation may also be that they are not always representative of primary cells. For example, they may exhibit different growth rates; hence, cell data should be interpreted cautiously [131].
Once cell lines for in vitro meat production have been established, best practices of cell banking, derived from the use of stem cells in biomedical applications, can be utilized to enhance the stability, reproducibility, and long-term maintenance of cell stocks for cultured meat [130].
Stem Cells
Stem cells are considered the most promising cell source since they have characteristic capabilities to retain themselves in the undifferentiated form for a specific number of population doublings. Different types of stem cells can be used to create in vitro meat and meat products [6, 29, 70]. Two favorable options among them are embryonic stem cells and satellite (i.e., myosatellite) cells from native muscle tissue.
In theory, after the embryonic stem cell line is set up, its unlimited regenerative ability eliminates the need to harvest more cells from embryos. Despite their high proliferation and differentiation capacity, these cells must be specifically stimulated to differentiate into myoblasts and may inaccurately restate myogenesis. Moreover, the cell yields from harvests are usually meager [29, 36]. For embryonic stem cells to become muscle fibers, the first requirement is their differentiation into myogenic progenitor cells. One of the major challenges at this stage is achieving direct differentiation into myoprogenitor cells without the development of any other lineages. It seems that inducing myogenesis in embryonic stem cells in vitro is a tougher nut to crack than the in vivo counterpart. Zheng and colleagues demonstrated that myogenic precursor progeny from human embryonic stem cells effortlessly form myofibers when transplanted in vivo in mice after muscle damage [152].
On the other hand, in vitro formation of myofibers from the cells has proven to be strenuous. Authors presumed that in vitro system lack some important in vivo niche elements [152]. Apart from the risk of uncontrolled proliferation and differentiation, an additional issue with using embryonic stem cells as a cell source for cultured meat production is the ethical concerns [70].
The most promising and practical cell types for skeletal muscle tissue engineering, as well as for in vitro meat production, are (myo)satellite cells. However, having the drawback of being a rare muscle cell type (they make up < 5% of the total nuclei in the skeletal muscle fiber of adult rodents and humans), they are the main contributors to muscle repair and regeneration; they recapitulate myogenesis (Fig. 3) more closely than the immortal myogenic cell lines. In the event of muscle damage and subsequent transmission of biochemical signals, activated satellite cells migrate to the injured site, proliferate, undergo myogenic differentiation, and fuse to form myotubes [109]. It has been shown in vivo that satellite cell fusion causes remodeling in adult fibers. Subsequently, their competence to proliferate and fuse with adjacent fibers in uninjured muscle has been revealed, providing a mechanism for the addition of nuclei to growing fibers. The inclusion of new nuclei to growing fibers alleviates further growth in fibers’ length and perimeter [11]. On the other hand, numerous studies have demonstrated that the expansion and culturing of satellite cells in vitro, even for a few days, can significantly limit their proliferative properties. Usually, the satellite cell proliferation rate decreases with each passage until a stage, known as proliferative senescence, is reached. At this point, the cells cease to divide, plausible because of the loss of telomeric DNA that occurs with each cell division [117]. A related key finding has been made by Gilbert et al., who showed that satellite cells could maintain their in vivo-like self-renewal properties when cultured on elastic substrates [46]. Namely, when these primary cells are cultured on appropriate biomaterials (with Young’s elasticity modulus of 12 kPa), they retain their characteristic proliferative and regenerative features.
Myogenesis. a The development of skeletal muscle tissue begins when myoblasts align and fuse to make longer multinucleated myotubes. Myotubes continue differentiating and synthesize proteins to form functional myofilaments, where the nuclei are displaced against the sarcolemma. Part of the myoblast population does not fuse and differentiate but remains as a satellite cell located on an external surface of muscle fibers. Satellite cells proliferate and produce new muscle fibers following muscle injury. b Example of mouse-tissue derived myotubes [1]
Satellite cells have been until now successfully isolated and characterized from the skeletal muscle tissue of cattle [31], chickens [147], fish [107], lambs [33], pigs [13, 143], and turkeys [84]. Cells from respective animal species’ have their benefits and limitations. Thus, satellite cells isolated from different muscles have different capabilities to proliferate, differentiate, or be regulated by growth modifiers that mimic the extracellular matrix (ECM) environment encircling muscle cells (e.g., proteoglycans, growth factors, and steroid hormones) [20, 29].
Adult Stem Cells
Myosatellite or satellite cells are one of the representatives of an adult stem cell type with multilineage potential. Adult stem cells, also known as progenitor cells, are preferred sources for cultured meat production, independent of their in vivo origin. They have been obtained from pigs [68, 149] and cattle [67]. Alas, at present, the proliferative ability of adult stem cells does not match that of embryonic stem cells, mainly because they tend to differentiate spontaneously in vitro [6, 70].
Adipose Tissue-Derived Adult Stem Cells
Another cell type, relevant to in vitro meat engineering, are adipose tissue-derived adult stem cells. As the name suggests, this unique population of multipotent cells is located in adipose tissue. These highly expandable cells can be relatively non-invasively obtained from subcutaneous fat and further transdifferentiated to myogenic, osteogenic, chondrogenic, or adipogenic cell lineages [65]. The most significant worry regarding their usage remains their proneness to the malignant transformation in long-term cultures. Adipose tissue-derived adult stem cells can be immortalized at high frequency and can undergo a rapid transformation in long-term culturing; however, to date, researchers have been unable to reproduce some of the reported spontaneous transformation events [121]. For in vitro meat production, re-harvesting of adult stem cells might be necessary to minimize the risk of spontaneous transformation. Thus, harvesting adipose tissue-derived adult stem cells from subcutaneous fat is far less invasive than collecting satellite cells from muscle tissue via biopsy. More importantly, from the ethical point of view, these samples can be obtained from certain animals without causing considerable harm [29].
Mesenchymal Stem Cells
Another cell type being deemed ever more usable for skeletal muscle tissue engineering is mesenchymal stem cells (MSCs). These are multipotent cells, which possess the ability to differentiate into osteogenic, chondrogenic, and adipogenic lineages. Also, MSCs can undergo myogenic differentiation, provoking the formation of myotubes that can contribute to muscle growth. It has been reported that this cell source can be differentiated towards the myogenic lineage by expressing muscle-specific markers, regardless of their limited myogenic potential. However, it is still unclear how reproducible the transformation of MSCs into skeletal myoblasts is, especially having in mind their multipotency combined with the uncertainty of collecting them from different sources such as bone marrow, adipose tissue, synovial membrane, and umbilical cord blood [109, 144]. One of the main disadvantages of using MSCs is the decline in their regenerative properties over time, which is further compromised with in vitro expansion [109]. Although myogenic differentiation of MSCs alone might not be satisfactory, they can still represent a promising cell source for co-cultivation with myoblasts; they can fuse myoblasts and therefore play a part in the muscle regeneration process. Moreover, it is known that MSCs secrete several growth factors involved in the muscle regeneration process, as well as stimulate myoblast migration, proliferation, differentiation, and cell survival upon co-cultivation [144].
Co-cultures
The simplest approach for the production of a cultured meat system is to use a single myogenic cell line from an animal or co-culture it with other cells that are beneficial for the whole system. Once primary cells are differentiated into myoblasts, these cells, among producing some components of the extracellular matrix (ECM), are specialized in synthesizing contractile proteins. The ECM is the non-cellular part present within all tissues and organs which fills the space around the cells, and it is arranged in a unique 3D organization. Its precise composition and structure vary from tissue to tissue, depending on its particular functional need. Therefore, the ECM not only provides a mere structural scaffold for cells, but it is also one of the key regulators of cellular activities. Its active role involves modulation of many cellular functions in different ways. Moreover, through varying the degree of stiffness of the matrix components, it acts as a mechanical stimulator and thus directly influences cell differentiation [42].
To engineer functional skeletal muscle, it is necessary to combine different cell types. This is experimentally and technically challenging because the number of culture parameters that need to be carefully considered exponentially increases with an increasing number of different cell types. Furthermore, cell types are very specific in their nutritional and stimuli needs [102]. Co-culturing myoblasts with another (lone) cell population (e.g., fibroblasts, mesenchymal stromal or endothelial cells) has been shown to influence myoblast differentiation and proliferation capability, as well as alignment. Within a muscle, the main “factory” of EMC is fibroblasts. One of the concerns regarding co-culturing fibroblasts and myoblasts involves the risk of fibroblasts overgrowing the myoblasts, due to the difference in growth rate [11, 23, 70]. Previous studies in monolayer cultures revealed that stromal cells positively impact the expansion of myoblasts; an enhanced proliferation of myoblasts was present in the first 24 h of contact co-culture and then gradually decreased and became negligible after 72 h [123]. These findings in two-dimensional (2D) co-cultures have been partially reproduced in 3D systems. Co-culture with stromal vascular fraction (SVF) cells (i.e., non-expanded MSCs from adipose tissue) promoted proliferation and differentiation of myoblasts, resulting in muscle-like constructs. Besides, SVF cells deposited an increased amount of ECM and formed organized endothelial cell-structures [23].
The skeletal muscle has an abundant blood vessel supply, so one of the major challenges in engineering thick, complex tissues like muscle, is to vascularize the construct in vitro. The introduction of vascular networks in vitro could aid in maintaining cell viability during tissue growth and induce structural organization. Levenberg et al. hypothesized that embryonic endothelial cells in the appropriate 3D environment could be employed to promote the formation of endothelial vessel networks in in vitro engineered skeletal muscle tissue [74]. When both myoblasts and endothelial cells were cultured on scaffolds, the endothelial cells organized into tubular structures amid myoblasts and throughout the structure, assembling vessel networks within the cultured muscle tissue in vitro. The inclusion of embryonic fibroblasts under appropriate culture conditions into the mentioned co-cultures strongly advanced vascularization of the engineered muscle; their addition promoted stabilization of vessel organization over time. Their study highlights the importance of multi-cell cultures in providing relevant signals for vascular structure development in skeletal muscle tissue. Furthermore, co-cultures with endothelial cells may also be important to prompt differentiation of engineered tissues, because embryonic endothelial cells are pivotal for the earliest stages of organogenesis of muscle tissue [74]. Namely, the formation of microvascular networks is essential to generate suitable conditions for adipogenesis and to affect the maturation of nearby ECM secreting cells [10]. Recent studies have demonstrated that bovine stromal vascular cells (SVC) can promote both angiogenesis and adipogenesis in vitro, owing to their heterogeneity [10, 80]. Albeit vascularization is one of the crucial factors in functional muscle tissue development, production of the cultured meat of lower cell plurality can be achieved without them, as blood vessels may be a negligible component of meat taste and texture [10].
Scaffolds
Myoblasts are attachment-dependent cells, capable of spontaneous contraction. For proliferation and differentiation of myoblasts to take place, they require an appropriate substratum or scaffolds. Scaffolds made from biocompatible materials present an effective tool to control and guide tissue development locally. Scaffolds can play an essential role in providing a suitable environment also for myogenesis; they can act as a mimicked ECM for cells and can provide desired mechanical, as well as biochemical stimuli to the cells [109, 119]. An ideal scaffold would have a large surface area for attachment and growth, be flexible to enable contractions, maximize media diffusion, and be easily removed from the meat culture. Besides, scaffold and its by-products must be non-toxic or even edible, if they are not removed after cell culturing and may be of non-animal origin [29, 36]. The best choice would be a scaffold that closely mimics in vivo conditions in the skeletal muscle. It has been reported that myotubes best differentiate on the substrates with tissue-like stiffness [38]. Moreover, to achieve the optimal in vivo cell niche, a 3D muscle construct that leads to uniform cell alignment and reproducible architecture is needed. The advantage of 3D matrices with tubular pores over patterned 2D substrates is that large quantities of cells can be concentrated into a small scaffold volume and can be simultaneously stimulated to form myotubes. Furthermore, physiologically relevant 3D models permit longer culture times and more significant developmental maturation. In a 3D setting, the scaffolds’ material mechanical properties, forces generated by cells and constructs mechanical behavior (e.g., deformation), also seem to have an impact on cell survival, organization, and differentiation [63, 70, 109]. Cutting-edge micro- and nanofabrication techniques have facilitated the development of novel biomaterial substrates and 3D scaffolds that can, through their unique architecture, promote alignment and fusion of myoblasts in vitro. An example of such a model that is being developed in our laboratories is shown in Fig. 4.
Myofiber Alignment
Myofiber alignment is one of the specific requirements of muscle cells and thus should be investigated in scaffold design. Native muscle fibers are either aligned along the longitudinal axis or are oriented at an angle to the axis (i.e., pennation angle). The texturized surface of scaffolds can contribute to myotube alignment. Despite numerous strategies to recreate skeletal muscle tissue, the reality in the majority of cases is that myoblasts were embedded in bulk hydrogels where they assembled in a “chaotic-like” 3D lattice [91]. Without surface markers or patterns, myotubes cannot align. Therefore, they create a branched network. As a result, misaligned networks may generate contractile forces in opposing directions, contrary to those in native muscle fibers, reducing the overall contractility of the construct, and hence hinder the desired continuous protein production. In the absence of surface stimulation, external electrical and mechanical cues (including applied passive tension) can boost alignment. Furthermore, it has been shown that increased fiber alignment is associated with enhanced differentiation as well as upregulation of contractile proteins and the presence of advanced sarcomeric structures. Myoblast elongation and alignment are generally encouraged by utilizing a micropatterned surface [119]. Findings from various studies have shown that scaffold topology has a significant impact on the organization of aligned myofibers. By slight modifications of parameters, the degree of alignment can be altered [24]. Myofiber organization dictates the functional characteristics of muscle and therefore also the texture properties of meat [29].
Mechanical Properties
To imitate native muscle tissue mechanical characteristics, an ideal scaffold should possess similar elasticity and mechanical stiffness as its native counterpart. The muscle is an elastic tissue (Young’s modulus of ~ 12 kPa) [38, 46]. Therefore, a suitable scaffold elasticity is a prerequisite to resemble the contraction and relaxation abilities of native muscle fascicles. An improper elasticity can reduce force transmission, which eventually leads to motion hindering. In tissue engineering, unsuitable stiffness of a construct can cause a mechanical mismatch between the construct and native tissue, resulting in the formation of stress concentrations [119]. Mechanical loading of the tissue construct has a beneficial influence on cell alignment, elongation, proliferation, and fusion [136]. Additional cyclic mechanical stretching can even increase protein synthesis and hypertrophy of in vitro engineered muscle [135]. Two main characteristics govern cell phenotyping, namely, elasticity and stiffness. Cells receive mechanical cues from their surrounding that can either promote or inhibit both proliferation and differentiation. Under proper conditions, such cues can be applied in vitro as a means to amend and accelerate myogenic differentiation [5, 46, 76]. The most straightforward and scalable format to obtain “self-anchored” muscle fiber is letting them grow in a ring around a central column of elastic material. Using this strategy, the maturation period of a muscle fiber takes about 3 weeks [101]. As described above, Gilbert et al. showed that when cultured on soft polyethylene glycol hydrogels with an elasticity of 12 kPa, skeletal muscle stem cells exhibit in vivo-like self-regenerative properties [46]. Another research group reported that alginate hydrogels with an elasticity between 10 and 16 kPa displayed the highest potential for myogenic differentiation as evidenced by upregulation of myogenic genes (myogenin, MyoD, and Myf5) [5]. While static mechanical stimulation is largely associated with induction of myogenic differentiation, there have been inconsistencies concerning the role of cyclic mechanical strain on signaling and myogenic marker expression [50]. In early stages of myogenesis, uniaxial passive tension is superior to cyclic strain as it more closely reflects the natural situation in muscle development and growth [50, 136]. However, cyclic tension might play a role as an upregulatory stimulus for muscle hypertrophy and maturation of myotubes at later stages of myogenesis [88, 106]. The continuous static strain on skeletal muscle, caused by bone growth and elongation during embryogenesis and neonatal development, affects muscle weight, muscle length, and myofilament organization. Bone growth can be simulated by using ramp stretch. The mixed outcomes with beneficial [137] and negative effects [15] of combined stretch protocols from various studies are likely linked to the amount of stretch that the myotubes undergo [63].
Electrical Stimulation (Contraction)
Neural stimulation (i.e., regular contraction) presents an essential environmental signalization during embryogenesis in adult muscle since it promotes differentiation and healthy myofiber morphology. In a prolonged absence of it, muscle is subjected to atrophy [29, 45]. Muscles in vivo are innervated, which enables regular and controlled contraction. It was found that subjecting scaffolds to mechanical stretching can fulfill the requirement of providing contraction. However, this approach is less effective than electrical stimulation for optimal skeletal muscle development [29]. Incorporation of external electrical cues induces contraction internally and, under in vitro culture conditions, can be used to simulate part of the in vivo niche in the muscle [17]. Living cells create electrical forces in the form of membrane (i.e., action) potentials. Studies have shown that aside from the impact on muscle cell phenotype [35], myosin expression [94, 142], and contractile sarcomere assembly [41], electrical stimulation can also modulate fiber-type switch [98], as well as induce contractility in differentiated myotubes [58]. By incorporating electroactive materials directly into scaffolds, an alternative strategy can be used to imitate the natural cellular microenvironment. Inclusion of electrically conductive polymers (e.g., polyaniline, polypyrrole, polythiophene) [18, 47, 116], coupled with externally applied electrical stimuli, can promote tissue development (as evident by increased alignment and differentiation) on account of the delivery of electrical signals to cells via conductive polymers. According to literature, external electrical cues can trigger the action potential in in vitro cultures due to the difference in electrical resistance between culture media and intracellular fluid [148]. Consequently, calcium ions are released from the sarcoplasmic reticulum, and myotube contraction is triggered. On the contrary, when the applied electrical forces are too strong, they can cause membrane damage, decrease force generation, and prevent the increase in force generation after electrical induced contraction [63]. Boonen et al. have observed a complex interaction between electrical stimulation, surface protein coating, and substrate stiffness [16]. The response of muscle progenitor cells to electrical stimulation was most efficient on substrates with an elasticity of 21 kPa, which is close to the physiological stiffness of differentiated myoblasts [16, 27]. In another study, they showed that electrical induced contractions of cells on different coatings could activate different pathways of maturation [17]. These effects were also reproduced in a 3D culture system, in which myotube maturation was even faster [69]. The authors confirmed the advantages of cell cultures grown in a 3D construct through histological analysis, which showed that the myotubes were organized in the direction of stress. Uniform alignment of myotubes is important for the final construction of engineered muscle tissue, as it supports a maximal force generation upon contraction. However, the strength and duration of the electric stimulus require careful fine-tuning to result in active contractions of the myotubes [69]. Electrical stimulation has been successfully applied for the expansion of myogenic progenitor cells in 3D scaffolds without loss of myogenic potential. This is a useful method, especially for expanding satellite cells, which are known for their loss of regenerative potential after expansion in “standard” cell cultures [124].
Culture Conditions and Growth Media Composition
Despite recent advances in tissue engineering and consequent rapid development of novel-engineered muscle models, with each new model utilizing a diverse range of culture conditions, there is still no established “gold standard” for growing muscle cells in vitro [63]. In the process of building engineered muscle tissue, which could be used in regenerative medicine and cultured meat production, it is necessary to develop a native-like tissue architecture that possesses the ability of active force generation. Regardless of the efforts and breakthroughs in this field, it remains a challenge to overcome muscle cell’s inability to fully mature within engineered muscle constructs. Biochemical cues have the main role in the initial differentiation process, whereas (bio)physical stimuli have been proven to be pivotal in maturation towards fully functional and mature engineered tissue [70]. Moreover, to replicate the biological processes of the muscle tissue formation at an industrial scale raises several technical issues [54, 141]. Due to the complexity of the development of a mature muscle fiber, the step-wise production of a meat tissue will encompass the critical decision points [141].
“Base” Growth Media
To compete with the conventional meat industry, large-scale production of cultured meat would need to employ a low-cost media system. Aside from affordability, such media should contain essential nutrients, which are readily available (mostly through diffusion) for the cultured myoblasts and associated cells. Refinements in the composition of commercially available cell culture media have improved the cultivation of various types of animal cells [36]. For culturing mammalian cells in vitro on an industrial scale, serum-free media is a more realistic option, because it reduces both operating costs and process variability while lessening the potential source of infectious agents. Serum-free media have been developed for culturing satellite cells from turkey, sheep, and pig. Variations among different serum-free media outline the fact that satellite cells, derived from different animal origins, have different requirements and also respond variously to certain additives [32]. The animal serum is usually added to primary media formulations to supply an undefined assortment of growth factors, hormones, and other additives that promote cell survival, proliferation, and differentiation. The proportion of serum supplementation is one of the key parameters to induce myogenesis [48] as it guides skeletal myoblasts either towards proliferation or differentiation. In general, fetal bovine serum (FBS) is added in high levels (10–20% of final concentration) into media to encourage proliferation [6, 23, 63]. Based on previous studies, myogenic differentiation can be achieved in vitro by using lower levels (2–10%) of serum content in culture media [23, 25, 63, 71, 72]. Reduced concentrations of serum are needed for cell cycle withdrawal, which is a key factor for the onset of differentiation [71]. When using animal-derived serums, lot-to-lot variations and serum origin need to be considered as both were shown to significantly affect spontaneous contractility and force production (up to 3-fold), as well as influence growing muscle cell phenotype [61]. To develop commercially available serum substitutes that will replace FBS, some research groups have already designed animal-free serums. In their attempt to create an in vitro and edible muscle protein production system, Benjaminson et al. noticed that mushroom extracts were comparable with animal-derived serum in promoting explant surface area expansion [11]. Aside from chitin, cellulose, and melanin, mushrooms yield high-quality amino acids that can be applied as a rich serum supplement for an in vitro meat production. Plant-derived serum surrogates seem to be the ideal solution. However, their potential allergenicity should be considered [29].
Antibiotics
Extensive use of antibiotics in traditional livestock farming is an important risk factor for cultivating resistant bacteria (e.g., Salmonella, Campylobacter, Listeria, and Escherichia coli), which could be eliminated in cell culture conditions in a strictly controlled industrial environment. In this context, it is expected that cultured meat production will reduce the incidence of animal-borne infections (i.e., zoonosis) and other emerging diseases [29]. In particular, the high degree of environmental control will likely prevent problems associated with foodborne diseases by reducing the risk of foodborne pathogens and consequently improve food safety [10, 141]. In general, antibiotics are added into growth media as prophylaxis to prevent microbial contamination of cultured cells. Typically, used antibiotics in cell culture are penicillin and streptomycin. Streptomycin has a negative impact on skeletal muscle culture; it decreases protein synthesis and developmental maturation of myotubes due to inhibition of spontaneous contractility. In static cultured muscle tissue, the addition of streptomycin resulted in a 3- to a 4-fold reduction of force generation and a decline in frequency and intensity of spontaneous contractions [62]. Based on these trends, it has been advised to eliminate streptomycin from culture media to minimize variability and maximize maturation and functionality of in vitro skeletal muscle and rather increase the concentration of penicillin instead (to 100 IU/ml) [63]. As an alternative, sodium benzoate could be added to growth media to protect cells from yeast and fungus infection [141]. Sodium benzoate does not pose an additional risk to consumer health since it is generally used as a preservative in processed meat products [53]. Even though cultured meat is produced under sterile culture conditions with the possible use of antibiotic-free media, it is mandatory to investigate the magnitude of their use in-depth. Promoters of cultured meat production advocate that the process can be achieved with a notable lower level of antibiotics compared with the current use in the conventional meat industry [10, 130] or even without their use [134, 141]. At this point, providing the required sterile environment during production represents a great financial burden and is very difficult on a larger scale. Since the sterile environment can be difficult to conserve long term at lab level, there is a liability for the addition of chemicals and antimicrobials in the production process. However, it remains unknown whether antibiotics, antimicrobials, or chemicals will be routinely or occasionally requisite during muscle cell culture. On the contrary, the benefits of establishing the cultured meat production will reduce the exposure to noxious chemicals (e.g., pesticides and fungicides) in a traditional animal-agriculture industry [141].
Regulatory Biological/Growth Factors
Stimulation with different supplements, such as growth factors, potentiates myoblast proliferation and differentiation capacity. To ensure high rates of cell growth, the formulation of the growth media needs to contain not only the appropriate level of nutrients but also the appropriate level of the myogenic regulatory growth factors and hormones [141]. In addition to promoting myoblast proliferation and differentiation, different members of the transforming growth factor-β (TGF-β) superfamily, basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), and insulin-like growth factor 1 (IGF-1) also stimulate migration and cell survival upon co-cultivation [81, 144]. Both TGF-β and bFGF reduce myoblast recruitment and differentiation (under independent mechanisms) by increasing myoblast proliferation, with the difference being that bFGF is more stimulatory in its action [49, 70]. Myostatin, a member of the TGF-β superfamily that is highly expressed in skeletal muscle, is a negative regulator of skeletal muscle growth. Loss of myostatin function is associated with an increase in muscle mass in mice, cows, and humans [10, 37, 55, 64]. Similar action is prescribed to TGF-β1, another member of the TGF-β superfamily, which inhibits the proliferation of myogenic cells [59]. The isoform TGF-β1 promotes ECM preservation by enhancing matrix protein synthesis and, at the same time, suppressing the activity of ECM degradation proteins, resulting in an increased overall quantity of the ECM (i.e., connective tissue) in muscle cross-section [55]. In the case of the bFGF role, Guthridge et al. [49] proposed a model whereby intracellularly produced bFGFs regulate myogenesis by autocrine and paracrine action. The complex mechanisms involving both paracrine and autocrine regulation dictate the proliferation capability of myoblasts. A bFGF positive feedback loop, which is initiated by an exogenous factor, maintains myoblast proliferation. Disruption of the auto-stimulatory loop by inhibition of FGF synthesis or by inhibition of FGF export leads to the terminal differentiation of myoblasts [49]. In other words, when a sufficient level of bFGF is present in growth media surrounding cells, most myoblast will proliferate. When the growth factor in growth media is depleted, the myoblasts cease division and fuse into multinucleated myotubes. It is well known that HGF primarily induces the proliferation of satellite cells by binding to the c-met tyrosine receptor and stimulates different downstream targets. The majority of circulating IGF-1 is bound to specific IGF-binding proteins (IGFBPs), and its function, among many others, is to activate proliferation as well as differentiation of satellite cells. Although stimulation with HGF and IGF-1 induces upregulation of different myogenic markers, it was discovered by Witt and colleagues that it is probably not essential for myogenic differentiation. IGFBPs participate during myogenic differentiation, differing among culture and stimulation conditions [144]. It is important to note that the specific selection of suitable extrinsic regulatory factors must be based according to the chosen cell type and animal species, as they respond differently to the same regulatory factors [20]. Besides, increasing the number of cells (i.e., proliferation) is feasible only at the satellite cell stage and the myoblasts’ stage [141]. Regarding a serum supplement ratio in growth media, it is very likely that the combination of growth factors should be changed over the course of culturing; one particular combination of growth factors may be beneficial for the proliferation period, whereas the differentiation and maturation period may require a different mixture [29].
Bioreactors
Animal cell culture has its roots in cell-based therapy, which has become viable on a commercial level and thus provides important guidelines for the transfer of cultured meat technologies to the industrial scale [130]. It is generally believed that a sufficiently advanced tissue bioreactor system will enable the development, growth, and maintenance of mature living muscle [30]. For the most part, some basic functions are common to many tissue engineering bioreactors: (1) maintaining an aseptic environment; (2) controlling environmental parameters such as temperature and pH; (3) controlling nutrient delivery; and (4) offering controlled experimental interventions, including online sensors. In addition, a system intended for developing skeletal muscle tissue generally requires mechanical and/or electrical stimuli that mimic the in vivo environment. However, the complete process from single-cell proliferation and differentiation to the maturation of large and well-defined structured cuts of meat probably requires several stages taking place under different conditions. Thus, the construction of a technological blueprint for a muscle bioreactor system is a complex engineering endeavor [4, 30], let alone the scaling up to an industrial level, which will require a substantial effort to achieve sustainable and cost-effective large-scale production of cultured meat [14, 141, 150]. Also, decisions related to the type, size, and the number of bioreactor units are influenced by several factors, such as seeding density, final cell number or density, and passaging steps. The obtained cell density will differ for suspension systems that use microcarriers for anchorage-dependent cells compared with single-cell suspension systems. Importantly, when designing a bioreactor, engineers should note the fact that the “working volume” of a bioreactor does not dictate the process of media requirements. Due to cell metabolic requirements over the entire duration of the culture period, the media requirements will be much greater than the bioreactor working volumes [4].
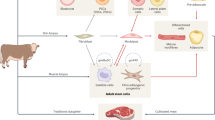
Bioreactor System
Companies currently working on scalable cultured meat products do not disclose information about their bioprocess design. However, a theoretical model of a bioreactor system, which could fulfill all of the criteria mentioned above, was presented in the work of [130, 29], as shown in Fig. 5. In essence, tissue development would take place into two stages: (a) for cell proliferation (e.g., in a stirred tank bioreactor) to provide a sufficient amount and density of cells and (b) for tissue development (e.g., on a porous scaffold in a perfusion bioreactor) where a structured and large-scale cut of meat would grow and mature [103, 130, 29]. Ideally, both stages should allow precise control over the bio-physicochemical parameters of the culture, continuous production, and efficient medium recycling. Although the production of cultured meat from self-replicating satellite cells derived from a biopsy is a simple concept, one of the main problems to overcome is its scalability [14, 141]. Satellite cells are anchorage-dependent, meaning that they need a surface for attachment, as well as have to be expanded in vitro, to obtain large cell numbers. When the required cell density is reached, the differentiation process is initiated, resulting in the formation of myotubes and the expression of proteins characteristic to functional myocytes [14].
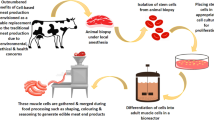
Overview of a possible scaffold-based preparation of cultured meat. It starts with the isolation of myosatellite cells from muscle and their proliferation. The sequence of the following steps includes: 1) A formulated media is kept in the feed tank before being deposited 2) into a bioreactor with a scaffolding system. Media perfusion enables expanded cells to be seeded 3) onto a scaffold within a bioreactor. During the culturing process, media is being constantly replenished and oxygenated in an external fluid loop 4). After the culturing process is completed, media can be recycled 5), while the scaffold and cultured tissue are removed from the bioreactor system 6). Further processing includes tissue removal from the scaffold [29]
Proliferation and Differentiation Stage
Present conventional planar cell culture systems have notable limitations related to their low surface-to-volume ratio, the lack of pH, gas, and metabolite concentration control, offering to produce up to 1011 cells [14, 120]. To meet the demands for large-scale production, generation of a significantly higher amount of cells (in comparison 1012–1013 cells, correspond approximately to 10–100 kg of meat) is favored while using limited space, time, and amount of resources and requiring minimal handling [14, 89]. Stirred tank bioreactors remain the most prevalent type of bioreactors in the biotechnology industry. However, possible cell damage due to the shear forces caused by the impeller, alternatives such as airlift bioreactors, is approaching the market [95]. Due to the increased production efficiency and consequently lower requirements of volume per output, the biopharmaceutical sector has recently also begun adopting single-use bioreactors (SUBs), which eliminate the need for cleaning and validation while preventing cross-contamination [95]. With the advent of smaller culture vessels, new approaches to agitation can be deployed. Thus, SUBs can be adapted to several stirring mechanisms, such as orbitally shaken or wave bioreactors [95]. The described approaches provide new possibilities for process optimization in the experimental phase of cellular agriculture. For continuous production on an industrial level, perpetual supply of nutrients, and biological regulators (e.g., growth factors, hormones), as well as a supply of oxygen and removal of waste products, is integral in the culture media [141]. Therefore, the development of cultured meat can be seen as a finite-state machine (Fig. 5), meaning that every development stage is a stable condition that depends on its previous condition and the present values of its inputs. At each development stage, the growing muscle tissue (i.e., meat) will require a variety of inputs to reach the next level of development. Important development stages encompass (1) terminal differentiation of myoblasts to myocytes, (2) fusion of myoblasts to form primary (weak and low excitability) myotubes, (3) growth of secondary (stronger, more excitable) myotubes, (4) expression of adult muscle proteins, and (5) formation of the target product [30]. Alongside the units for proliferation and differentiation, a muscle bioreactor system should incorporate additional components such as media storage tanks, media heat exchangers, and suitable controllers to maintain a constant temperature within the system. In addition, an advanced bioreactor should also include units for automatic and frequent monitoring of muscle tissue development without affecting it, thus aiding in assessing suitable quantity and duration of applied stimuli to the tissue in real-time [4, 30].
Maturation Stage—Final Product Development
At the development stage of the final product, the bioreactor system should provide conditions for maturation and maintain the engineered muscle tissue. To facilitate the development of a large and structured “cut of meat,” scaffolds are likely to be included in the process to provide the cells with a well-defined 3D growth substrate, along with which the tissue will develop. Successful nutrient supply and removal of waste products inside large 3D structures is currently a major limiting factor in the scalability of cultured meat. Within the scope of realizing a highly structured product that mimics large cuts of meat (e.g., steak, chops, tenderloin), several research groups endorse the use of a perfusion-based bioreactor system with a 3D engineered tissue construct as the most suitable technique for the production of cultured meat [150]. To enable continued growth and long-term sustainability of viable 3D engineered tissue constructs, homogeneous and adequate oxygen supply is paramount to avoid a necrotic nucleus. Albeit, owing to the microporous structure of the scaffold material, media flow can only be laminar, restricting the heat and mass transfer efficiency. To maintain the optimal, replenishing rate across scales, it is vital that the flow rate increases linearly with the scale of the structure. This, in turn, contributes to high shear stress and a considerable pressure drop. To subdue such problems, a typical approach encompasses enlarging the pore size of 3D engineered construct to improve permeability [150]. However, this could limit control over the texture and consistency of the final product. Various approaches to sustaining continuous medium perfusion through the scaffold have been proposed, including stirred tank, rotating vessel, or direct perfusion bioreactors (Fig. 6). While a high mass transfer of medium flow is required, it is accompanied by a significant increase in shear stress, therefore determining that a suitable flow rate is critical for successful and effective growth [29, 82]. Direct perfusion bioreactors provide this type of bioreactor which is mainly intended for scaffold-based myocyte cultivation and high-density, uniform myocyte seeding [110]. Cerino et al. [23] demonstrated a one-step (proliferation and maturation) platform by the use of SVF co-culture and a 3D perfusion bioreactor, creating a complex biological model for engineering functional 3D uniform muscle tissue that resembles its native multi-cellular environment [23]. However, at this point, the proposed system is only suitable for small sample sizes with limited scalability. Despite the accomplishments in tissue engineering, in its current form, such a perfusion-based bioprocess system is unsuitable for upscaling the cultured meat production [150]. To ensure controlled tissue production, monitoring of its development is crucial. Biomarkers that can be used to evaluate the developmental stage of the muscle harmlessly are contractility (force and rate of contraction and relaxation), excitability (energy required to achieve stimulation), and metabolism, which can be, among other methods, determined by oxygen consumption [30]. Besides, several technologies for in-line, real-time monitoring of systems performance, culture conditions, and cell viability have been validated [151], alongside with Raman-based strategies for in situ monitoring of chemical composition [85]. For all bioprocessing systems, three main sensor parameters, in particular, pH, dissolved oxygen, and temperature, are prerequisites. Cell culture media normally contains buffer agents and sodium bicarbonate to maintain pH values within optimal working conditions. The optimal pH of cell media alters throughout the bioprocess; therefore, it must be carefully monitored at each stage of the process. Namely, even a (rather) small change of 0.1 pH units from the optimum can greatly affect cell viability and concentration [95]. Dissolved oxygen is another critical parameter that must be closely monitored and optimized. This is crucial for the survival of aerobic cell types. However, high levels of dissolved oxygen levels can lead to the production of super-oxides or peroxides, which have a damaging effect on the cell membranes or even cause DNA breakdown. To ensure optimal cell viabilities and high product yields during bioprocessing, the temperature inside a bioreactor must be accurately measured. For the production of mammalian cells, the optimal temperature has been widely established for many years to be around 37 °C (like body temperature). Though recent studies demonstrated that at lower temperatures, in the range of 30–35 °C, the high production of some proteins can be achieved. Thus, depending on the desired product, the temperature sensors must operate accurately in the range of 30–40 °C as the process temperature will vary over time. Moreover, they must be highly sensitive in temperature variations to prevent loss in cell viability. The bioreactor’s monitoring system can be further implemented for continuous measurements of the substrate and product concentrations (viable), cell density, and metabolites [95].
Commonly used bioreactors for the production of 3D tissue-engineered constructs: a spinner flask, b rotating wall vessel, and c perfusion bioreactor [100]
Microcarriers
Owing to a large surface-to-volume ratio, microcarriers (MCs) are the most promising candidates for large-scale implementation. The suspended MCs provide a 3D environment, but the cells still grow on a 2D surface, although the strong curvature of the bead surface affects cell attachment and proliferation. Since the microenvironment of the cells remains unchanged, the translation from the traditional monolayer culture to a suspension one is smoother. Moreover, depending on their buoyancy and density, they can be used in various types of vessels (e.g., stirred tank, fluidized bed, packed bed, bubble column bioreactor) that are generally employed for scaling up chemical and bioprocesses. As opposed to fixed-bed bioreactor cultures, such as hollow fiber or microplate, MC-based bioprocesses are easier to control and monitor, resulting in the quality and consistency of the products. A significant advantage of MCs is that the cell growth surface can be increased simply by adding new MCs to the culture. This is feasible due to a phenomenon referred as “bead to bead transfer,” which describes the cell migration from bead to bead, and the population of newly added MCs [14, 52, 66, 97, 112, 113, 138, 150]. Introducing a new surface area into satellite cell culture also is beneficial to avoid aggregation of MCs [14, 138]. The use of MCs has long been instituted in the cell-based therapy industry and vaccine production for expansion of cells producing a molecule of interest (e.g., monoclonal antibodies, proteins, vaccines) [99, 150]. Verbruggen et al. [138] provided a proof-of-concept experimental work of using MCs-based bioprocess for cultured beef production. The materials for such MCs should comply with food regulations (following good manufacturing practice, GMP, hazard and critical points, HACCP, and standards). MCs can be designed to serve as a temporary substrate for cell attachment and proliferation, or they can be embedded in the final product, and ergo need to be edible. Using edible materials can obviate the need for dissociation/degradation/separation steps and limit the risk of non-edible residues. Besides, they can be even tailored to enhance the sensorial and nutritional qualities if embedded in the final product. Towards that end, abundant, edible polymers, such as alginates, pectins, and chitosan, that are prevalent in the food industry, such as stabilizers, thickeners, coatings, and emulsifiers [3, 14, 127], seem to be promising candidates for upscaling the process.
Oxygen Carriers
Cell viability and density positively correlate with oxygen gradient in statically grown tissue cultures [111]. To overcome limitations in oxygen diffusion in tissue cultures and to maintain the high oxygen concentration in the bioreactor, oxygen carriers can be added into growth media. They can be divided into two varieties: hemoglobin-based oxygen carriers (HBOCs), which are mostly modified hemoglobin versions, and chemically inert, artificially produced perfluorocarbons (PFCs). Even though PFCs dissolve large amounts of oxygen and can perform the same functions as hemoglobin, they are extremely potent greenhouse gases on a per molecule basis [29].
Recycling
Technical experiences from the established biotechnology industries (e.g., brewery, pharmaceutical, and recombinant/therapeutic protein industries) pose good parallels for upscaling the bioprocess of cultured meat manufacture. Downstream units require recycling operation with cell debris removal, media refinement, cell harvesting, and product formulation [4]. The management of metabolic waste by recycling (as well as by disposal or upgrading) must satisfy the requirements of the HACCAP procedure [141]. If recycling is put into operation, the emissions from excess nutrients in the wastewater could be comparable with, or lower than, a poultry operation [83, 141]. One approach for media refinement is through the replenishment of utilized nutrients (e.g., glucose and glutamine) and removal of waste by-products such as lactate and ammonia, which, even in small quantities, are known to inhibit cell growth. One strategy for media replenishment is by perfusion mode, in which fresh media is continuously pumped at a given perfusion rate together with the removal of used media at the same rate. At the same time, the cells are retained in the bioreactor. This implantation of continuous perfusion processes has gained prevalence in the biopharmaceutical industry for large-scale production, as constant media refinement and removal of toxic by-products ensure high productivity and product quality. Moreover, for industry related to cell therapy, maintaining the concentration of essential nutrients and metabolic products at optimal levels may be crucial in the regulation of cell growth, differentiation, and other vital attributes [2]. It might be favorable to reuse a part of supplements in growth media (e.g., growth factors, cytokines) that were either added or produced by cells and therefore stimulate further cell growth [89]. Nath et al. [93] established a culture media refinement method using a dialysis for the expansion of human-induced pluripotent stem cells (hiPSCs) in suspension culture. By exchanging fresh media only once, following the refinement of media with the proposed dialysis system, they removed toxic metabolites. At the same time, essential macromolecules of media (e.g., growth and autocrine factors) were recycled efficiently [93]. Recovery of purified water is also feasible by using downstream separation units that comprise one or more of the following processes: membrane filtration, (electro)dialysis, precipitation, solvent extraction, and absorption systems [4].
Production and Supply at the Regional Level
As cultured meat production is generally believed to be most feasible at an industrial level, some companies like FM Technologies propose two conceivable scenarios where cultured meat production could proceed at (1) a small business or (2) an individual consumer scale [141]. FM Technologies (https://www.future-meat.com/) portrays this as a “distributive approach to sustainable manufacturing.” Their proposed model system centralizes stores of stem cells, which are periodically delivered to local communities. The latter would have small-scale bioreactors with the capacity to feed small villages or regions, and a central point will give the technological know-how. Models of the distributive approach present are purely theoretical and remain to translate into practice [141].
Nutritional and Sensorial Aspects
The reasoning behind cultured meat is that “traditional” consumers expect meat, which is as readily accessible and comparable (if not superior in nutritional value) with native meat and not a meat substitute with perceived lower quality (from the start). The resemblance should be guided not only by sensory factors (e.g., color, flavor, tenderness) but also by nutritional and health values. In other words, the biochemical and structural composition of engineered meat needs to be like the natural counterpart. The key factors that govern cell and tissue cultures on the way to becoming a desired product are culture conditions, as well as the external dynamic environment (mechanical and electrical cues). With existing technology, all these conditions can be controlled and further optimized, with a focus on flavor (taste and aroma), texture, nutritional value, and food safety [10, 101, 102]. However, some differences will still be present; some nutrients that are not produced by myoblasts nor adipocytes, such as vitamin B12, are absorbed from the environment (in vivo from blood). The requirements of these “environmental” nutrients can be easily fulfilled by supplementing the growth media. For efficiency and safety reasons, future studies will guide the selection of nutrients, which are essential for the development of cultured meat and at the same time harmless for consumers [4]. Although alternatives to farm-grown meat (i.e., plant-based protein and cultured meat) have received considerable attention within academia and the popular press, the consumers’ willingness to adopt cultured meat is unclear even if it tastes the same. Nevertheless, the current market is less optimistic about a lab-grown version. It is important to note that consumers’ preferences are amenable and can be changed by new information govern by marketing campaigns or social norms [9, 129]. The abundance of vegetarian meat substitutes may “prepare the ground” for acceptances of cultured meat in the form of burgers and sausages, as well as the possibility to prepare meat variations from animals that are considered a delicacy [129].
Protein Composition
The highly complex meat composition, which includes a wide variety of proteins, carbohydrates, fatty acids, and aromatic compounds, is the main contributor to its flavor [90]. Muscle tissue is rich in proteins as it contains up to 6500 types of proteins with an extremely wide expression range of several orders of magnitude [96]. The most abundant proteins are myosin, actin, and titin, which together make up 75% of cytoskeletal proteins of myoblast cells and represent between 40 and 60% of the total amount of protein in muscle fibers (total percentage depends on the type of muscle fiber) [92]. It is hypothesized that highly aligned and tightly co-expressed myosin and actin molecules have the most significant contribution to the meat’s texture. Nutritional value, taste, and mouthfeel are co-determined by the amount and composition of the most abundant proteins (e.g., actin and myosin, respectively) in myoblasts. Therefore, the muscle proteome presents the foundation for the creation of meat tissue that consumers “perceive as meat.” Optimizing protein composition focuses primarily on the proteins actin and myosin. However, it is also likely that scarcer proteins, such as heme-containing proteins (e.g., hemoglobin and myoglobin) possess specific components contributing disproportionally to taste and appearance [101].
The culture conditions that will lead to these improvements are well-known in medical tissue engineering but will need to be implemented in a scalable process for meat production. Current culturing methods are suitable for creating fully mature muscle tissue with typical cross-striation that indicates the development of sarcomeres [16, 17, 69]. The overall protein content is 20%, which is comparable with native muscle fibers [101]. On the other hand, the previously mentioned electrical stimulation has not yet proven to be a resource-efficient method, and alternative ways for stimulating the muscles to produce higher amounts of relevant proteins will need to be developed (explored) to achieve the same result. Similarly, stimulation of myoglobin expression has been described and confirmed in many other muscle cells of vertebrates. It can be easily used in ways that are compatible with scalable production methods. For example, by reducing oxygen concentration during cell culturing, myoblasts started to show a 5-fold increase in myoglobin expression [51, 60]. Sufficient myoglobin is important for color, nutritional value (heme iron), and taste of meat (serum-like taste and metallic mouthfeel of red meat) [87].
Fat Composition
To achieve complete meat flavor, ensure its expected nutritional value, and mimic its natural texture, it is mandatory to add adipose tissue to the final meat product. It appears that a large number of small peptides are jointly responsible for taste [26], while an equal amount of aromatics (originating mostly from adipose tissue) co-determine the flavor [125]. Intramuscular fat (IMF) accounts for about 80% of the muscle fat, while the remaining 20% is stored as lipid droplets within myofibers. Its amount and fatty acid composition are accounted for meat quality (e.g., juiciness, flavor, tenderness, and nutritional value). IMF is created via adipogenesis, in which stem cells differentiate into adipocytes, and lipogenesis, in which triglycerides are accumulated inside the adipocytes [10, 77]. Between muscle types, the phospholipids coverage is relatively constant, while the muscle triglyceride content is highly variable among the species [77]. Adipose tissue can be cultured from MSCs, from adipose-derived stem cells, or from myosatellite cells that entered an alternative differentiation pathway (resulting in the formation of intermuscular adipose tissue (IMAT)) [73, 139]. Adipogenic differentiation involves activation of several transcriptional factors including a peroxisome proliferator-activated receptor-γ (PPAR-γ) that is a dominant regulator of white-adipocyte differentiation [21], and for which free fatty acids (FFAs) present natural ligands. In addition to their function as an energy source, some of those FFAs have been proven to induce adipogenesis in stem cells with mild and natural stimulation (e.g., cultivation in FFA-supplemented media) [86]. Maturation of adipose tissue into characteristic white adipose tissue takes approximately 2–3 months, whereas maturation of muscle tissue takes around 3 weeks. Despite the longer maturation period, the formation of fat tissue presents a less challenging process compared with the preparation of mature muscle fibers in vitro. The interactions between differentiating adipocytes, which are the basis to formation of a similar structure to the native tissue, are not as significant and complex as in the case of muscle fibers. Although the fat fraction is one of the main components of meats taste and texture, it may also be associated with cardiovascular diseases due to high content of saturated fatty acids and low levels of polyunsaturated fatty acids (PUFAs). In cultured meat, PUFA levels can be controlled with adjusting the composition of fat tissue culture media [145, 146]. In the interest of “not compromising” the taste of the engineered meat, the optimal amount of PUFAs will need to be derived from a combination of biochemical and sensory evaluations [101].
Tenderness
To mimic livestock derived meat in the majority of sensory aspects, the procedure of recreating conventional meat in vitro should also involve the usual aging process after native tissue’s “death.” Many studies have shown the complexity of meat tenderization. It is based on the extent of proteolysis of vital target cytoskeleton proteins within muscle fibers and the alteration of muscle structure due to the sequential actions of enzymes. After slaughtering the farm animals, muscle fibers undergo postmortem rigidity (rigor mortis) due to protein contractions, resulting in muscle toughness. The extent and mechanism behind rigor mortis are still rather poorly understood and likely vary for different harvesting conditions of the muscle. However, it is expected that mimicking only ischemic conditions in engineered meat cannot produce the same comparable effects on muscles as a sequence of death-related events in vivo (i.e., preslaughter stress and postmortem muscle metabolism), such as the release of catecholamines, increase in blood lactate, and cortisol concentration [28, 34, 102]. In the case of farm animals, the aging process is accompanied by a decline in aerobic metabolism due to decreasing oxygen supply. Subsequently, glycogen is converted into lactic acid, which induces a decrease in pH to 5.4–5.8 [102], activating enzymes responsible for tenderization and formation of aroma precursors [40]. The aging period depends on the meat; for example, in beef that has a low amount of proteases, aging takes approximately 14 days. Aside from pH, temperature also has an impact on the palatability of meat. Hence, both factors should decrease within a precise timeframe [132]. The involvement of different groups of muscle peptidases that are responsible for the postmortem protein breakdown during cadaver storage is still a controversial topic. Among these peptidases, the calpain system accompanied by its inhibitors (e.g., calpastatin) was considered to be the predominant system accountable for meat tenderization [75]. The extent to which proteolytic enzymes act is strongly influenced by the microenvironment conditions (e.g., pH, ionic strength, cellular oxidative, and nitrosylation status) [78]. Hence, mechanisms that are responsible for postmortem degradation of structural proteins are under the scope of various studies, which aim to elucidate the underlying processes. Likely, intracellular conditions in cultured meat might overall differ from conventional meat, which would affect the rate and extent of tenderization and flavor development [40]. Considering this, further insights into the phenomena of postmortem degradation would enable a better prediction of the quality and resemblance to the conventional meat. Isoforms of actin and myosin in cultured muscle tissue were found to be rather neonatal or embryonic than adult [40, 133]. This could affect the postmortem protein breakdown, resulting in altered postmortem transformations. In the case of their absence, the engineered muscle tissue is not transformed into the meat and, consequently, biochemically dissimilar [29].
Moreover, particularly in cattle, connective tissue influences meat tenderness by its composition and structure, whereby collagen is seen as a major determinant of the shear force. However, there exist substantial differences between raw and cooked meat that are, in the case of raw meat, highly correlated with the collagen content. On the other hand, in cooked meat, the level of the relationship between the content, thermal solubility, or cross-linked level of collagen and meat shear force varies according to muscle type and cooking conditions [77]. All mentioned will aid the newly emerging industry of cultured meat to create a product that is as close an imitation of livestock meat as possible [4].
Color
As mentioned above, the myoglobin, which contains heme, is “the main culprit” for the red color of meat, specifically its chemical state [40, 77, 102]. In deep muscles and meat stored under vacuum, myoglobin is in a reduced state, which gives the meat the purple red color. In an oxygenated form (i.e., when exposed to oxygen conditions) such as oxymyoglobin, it exhibits an attractive red color. During the meat storage and cooking process, oxymyoglobin is further oxidized into metmyoglobin that is displayed as a darker brown-red color. Muscles that are rich in myoglobin (e.g., from cattle, sheep, horses) are apt to metmyoglobin formation and decreased color stability [77, 128]. Due to the suppressed myoglobin expression at ambient aerobic conditions and, therefore, the absence of myoglobin, the engineered muscle tissues have a pale color [40, 44, 102]. Thus, several strategies have been proposed to improve the myoglobin load of cultured meat. Briefly mentioned in the previous subsection (“Protein Composition”), the first approach involves culturing myofibers under hypoxic conditions [51, 60, 102]. However, deeper insight is needed to determine whether a low amount of oxygen is sufficient enough, as well as any potential impact on culturing efficiency [89]. Namely, this can lead to media acidification, which is harmful to cell viability [29, 40, 57]. Supplementation of culture media with additives, such as lipids and acetic acid, could also stimulate myoglobin expression [89]. Only myoglobin protein synthesis is not enough for color development; the presence of a sufficient amount of iron in the cell is also of great importance. In general, cell culture media contain no iron (e.g., IMDM) or only low amounts of it in the form of ferric nitrate nonahydrate (e.g., DMEM 0.1 mg/L) or ferrous sulfate heptahydrate (Ham’s media 0.8 mg/L). The addition of extra iron into culture media increases the iron content in the cultured cells. However, only part of the iron is uptaken. This indicates that while media can be formulated to affect nutritional coverage, there is a limit to the amount of this microelement the cells can incorporate [122]. Since the iron uptake is dependent on transferrin, a protein that binds iron and mediates transport in the cell [57, 89], further investigations are necessary to determine the extent to which iron is then incorporate into heme (for good iron bio-accessibility) and myoglobin (for red color development) [40, 102].
Recently, Simsa et al. [128] proposed a second approach to implement the myoglobin content in cultured cells by direct addition of myoglobin into media, which could be of value for large-scale cell expansion. Authors discovered that the proliferation and metabolic activity of bovine muscle satellite cells were significantly increased when cultured in myoglobin-supplemented media, resulting in a more meat-like coloration of cultured muscle tissue. However, given the limitations of the proposed cultivation system, the myoglobin proportion in the cultured cells was much lower compared with fresh beef meat, and the resulting color had a more similar tone to cooked meat [40, 128].
Flavor
More than 750 compounds are accounted for the flavor of the meat. Upon the heating (i.e., cooking), complex thermally induced reactions ensuing in the formation of an enormous range of the volatiles contribute to the aroma and odor [40, 141]. Meat derived from a whole muscle is a unique mixture of muscle cells, fat cells, and connective tissue that all together provide the whole experience of the taste of the meat. In cattle and lambs, increased content of myoglobin attributes not only to the color of the meat but also to the finest juiciness and flavor. This favorable effect can be assigned to the high phospholipid content, which are decisive factors of the flavor of cooked meat [77]. Other compounds involving in the taste development are lactate (sour taste) and inosine-5′-monophosphate (umami taste), both formed during postmortem metabolism [40]. Furthermore, upon prolonged heating in suitable conditions, collagen (from connective tissue) breaks down to gelatin, which has a distinctive and attractive flavor. Also, it contains essential amino acids for human muscle function and health (e.g., glutamate) [141].
Intracellular lipids, in particular phospholipids from membranes, are accountable for lipid degradation during the cooking process even in lean meat and meat products [40, 90]. Phospholipids generally have a higher proportion of PUFAs that are more susceptible to oxidation. The higher amounts of fat in meat tissue and, consequently, the contribution of these volatiles give the additional kick to the overall flavor [90]. Albeit products formed upon oxidation promote the desirable aroma, they can also cause the unpleasant taste of meat and are often the causative agent of meat spoilage [40]. The fat presence in cultured meat should be viewed from dissimilarity between whole cut and processed meat. In the case of the former, incorporating a fat fraction to cultured meat can be achieved by co-culturing of muscle cells with adipocytes. On the contrary, in finely minced meat products, fat is often added at the end of culture process; therefore, the substitutes, such as cultured fat or plant-based fat (used in vegan diet), may replace animal-derived fat [39, 40]. If the cultivation of meat itself does not provide a satisfactory flavor in a final product, additional artificial flavor compounds similar to those at present used in plant-based meat alternatives might overcome this issue [40, 70].
Texture
The texture of meat contributes to the full sensory experience [40, 141]. In the conventional animal-derived meat, the texture depends on numerous factors, including myofibrillar structure as affected by rigor mortis and aging, the amount and structure of connective tissue in the muscle, and the proportion and composition of fat in the muscle [39, 40, 77]. The texture of meat changes during cooking because of fluid loss and proteins’ response to temperature. Cooking losses from meat seem to be driven predominantly by thermal denaturation of proteins at different temperatures, causing shrinkage in the muscle at a macro- and microlevel and alterations in the gel matrix binding proteins together [108, 141].
The production of ground cultured meat products, such as hamburgers or sausages, is more doable, as demonstrated by the cultured meat hamburger patty in 2013 [105]. Despite that, this prototype represents one of the milestones in biotechnology. From a culinary perspective, it did not accomplish a satisfactory level. Namely, its sensorial characteristics (e.g., taste, color, and texture) were different from conventional beef patty [43]. However, ongoing advancement in technology has enabled refinement in terms of sensorial traits of culture meat. From a textural point of view, the minced cultured meat products ease the complexity of the production process, since the scaffold materials for cell culturing can be omitted [40, 130]. Therefore, structure formation in such products strongly relies on technical approaches. Since the tissue fragments in beef patty from 2013 were significantly smaller compared with the traditional ones, to bind these fragments and to provide the final product with akin texture, addition of breadcrumbs, egg whites, and other binders is requisite. The resulting texture will be likely to resemble industrially processed burgers as opposed to fresh, high-quality burgers [40, 102, 130]. In the case of cooked, processed meat products (e.g., sausages), the texture is affected by functional properties of dissolved proteins, in particular, the gelation of the myofibrillar protein actin and myosin during pasteurization. With the addition of fat, the proteins stabilize the fat through the formation of an interfacial protein film around the fat globules. The gelling and emulsifying properties of meat proteins are vital in the production of finely minced meat products [39, 40]. Given that the cultured and conventional meat contain muscle fibers, it is presumed that the biochemical composition will be more or less the same [12, 40]. As already mentioned, at the current stage, muscle fibers formed by in vitro cell culturing methods include only a small proportion of predominantly embryonic or neonatal isoforms of actin and myosin. Therefore, electrical and/or mechanical stimulation is needed to (1) increase myofiber diameter, (2) strengthen myotube structure, and (3) increase myofibrillar protein fraction. In terms of large-scale production, it needs to be determined whether such stimulation is scalable or economically manageable and whether the dissolved protein will provide the gelling and emulsifying properties that are crucial in processed meat production [40].
Food Safety and Regulation
One of the hot topics regarding cultured meat production is food safety and regulation. From the public point of view, the safety concerns were linked to the perception of unnaturalness and a sense of scientific uncertainty [19]. If the cultured meat production proceeds, it is likely that it will supplement the current market of animal-derived meat proteins [141]. Since the high demand for meat protein and, consequently, the potential increased consumption in the developing countries, some predictions state that the animal agriculture industry will remain alongside the development of the cultured meat industry [131, 141]. The safety standards required to maintain the safety and guarantee consumer confidence would be at some level included in a standard industrial setting for cultured meat production. Albeit an extensive regulatory system is requisite to monitor and regulate the safety, there are certain benefits related to cultured meat production [12]. The use of sterile and aseptic environments will eliminate microbial infections and contaminations, and through a continuous monitoring system, any deviation from accepted standards will be reduced. Apart from that, continuous monitoring of production will also enable the detection of any contamination at each stage of the process, providing the capability to incorporate the correction measures on time [12]. All components needed for cultured meat production could also be used as food ingredients. Nevertheless that the culture media and its components will not be consumed, traceability of the components should be in place [12, 130], reducing the cost of post-processing.
To set the regulatory framework, the procedures for cultured meat production must be clearly outlined. Since this technology is still in its infancy, the regulatory framework is not yet established. Therefore, regulations will need to follow the development of document procedures. Since the final product will be regarded as a food product, the food safety authorities are most likely to supervise the cultured meat production system rather than medical authorities. Regulatory guidelines, HACCP plans, and auditing must consider some stages and aspects, encompassing governance at local, state, and federal levels [12, 141].
Conclusions and Future Remarks
Cultured meat is an alternative animal protein source that will ameliorate some of the environmental, sustainability-related, and ethical issues associated with the conventional meat industry. At this point, considering the knowledge established from cell-based therapy and tissue engineering techniques, it is currently only feasible to create in vitro minced meat with composition and taste, comparable with the “traditional farm-derived meat.” Creating whole cuts of meat requires a more sophisticated tissue engineering approach, including (1) incorporating a high-density channel system that enables the perfusion of culture media through the entire growing tissue construct; (2) fabricating a more complex and larger 3D constructs, tailored specifically (in terms of morphology, biochemical, and mechanical properties) for different cell type requirements and individual meat cuts; and (3) establishing optimized differentiation protocols for co-culturing myoblasts, adipocytes, and fibroblasts. Since these demands are shared with tissue engineering for applications in regenerative medicine, scientific advances in both areas will eventually give rise to an abundance of strategies that promise to increase the efficiency of scaling up and reduce cost expenses of culturing meat in vitro. Last but not least, an important consumer-oriented approach in culturing meat will certainly be the possible preparation of meat variations based on exotic animals (e.g., dinosaurs, panda bear) or supplemented variants with or without specific molecules (even drugs for specific therapies).
References
Abmayr SM, Pavlath GK (2012) Myoblast fusion: lessons from flies and mice. Development. 139(4):641–656
Abraham E, Gupta S, Jung S, McAfee E (2017) Chapter 6 - bioreactor for scale-up: process control. In: Viswanathan S, Hematti P (eds) Mesenchymal stromal cells: translational pathways to clinical adoption. P^pp 139–178. Elsevier
Ahmadi F, Oveisi Z, Samani SM, Amoozgar Z (2015) Chitosan based hydrogels: characteristics and pharmaceutical applications. Res Pharm Sci 10(1):1–16
Allan SJ, De Bank PA, Ellis MJ (2019) Bioprocess design considerations for cultured meat production with a focus on the expansion bioreactor. Front Sustain Food Syst 3:44
Ansari S, Chen C, Xu X, Annabi N, Zadeh HH, Wu BM, Khademhosseini A, Shi S, Moshaverinia A (2016) Muscle tissue engineering using gingival mesenchymal stem cells encapsulated in alginate hydrogels containing multiple growth factors. Ann Biomed Eng 44(6):1908–1920
Arshad MS, Javed M, Sohaib M, Saeed F, Imran A, Amjad Z (2017) Tissue engineering approaches to develop cultured meat from cells: a mini review. Cogent Food Agric 3(1):1320814
Bach AD, Beier JP, Stern-Staeter J, Horch RE (2004) Skeletal muscle tissue engineering. J Cell Mol Med 8(4):413–422
Basciano L, Nemos C, Foliguet B, de Isla N, de Carvalho M, Tran N, Dalloul A (2011) Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Mol Cell Biol 12(1):12
Bekker GA, Fischer ARH, Tobi H, van Trijp HCM (2017) Explicit and implicit attitude toward an emerging food technology: the case of cultured meat. Appetite. 108:245–254
Ben-Arye T, Levenberg S (2019) Tissue engineering for clean meat production. Front Sustain Food Syst 3:46
Benjaminson MA, Gilchriest JA, Lorenz M (2002) In vitro edible muscle protein production system (mpps): stage 1, fish. Acta Astronautica 51(12):879–889
Bhat ZF, Morton JD, Mason SL, Bekhit AEDA, Bhat HF (2019) Technological, regulatory, and ethical aspects of in vitro meat: a future slaughter-free harvest. Compr Rev Food Sci Food Saf 18(4):1192–1208
Blanton JR Jr, Grant AL, McFarland DC, Robinson JP, Bidwell CA (1999) Isolation of two populations of myoblasts from porcine skeletal muscle. Muscle Nerve 22(1):43–50
Bodiou V, Moutsatsou P, Post MJ (2020) Microcarriers for upscaling cultured meat production. Front Nutr 7:10
Boonen KJ, Langelaan ML, Polak RB, van der Schaft DW, Baaijens FP, Post MJ (2010) Effects of a combined mechanical stimulation protocol: value for skeletal muscle tissue engineering. J Biomech 43(8):1514–1521
Boonen KJ, Rosaria-Chak KY, Baaijens FP, van der Schaft DW, Post MJ (2009) Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Phys Cell Phys 296(6):C1338–C1345
Boonen KJ, van der Schaft DW, Baaijens FP, Post MJ (2011) Interaction between electrical stimulation, protein coating and matrix elasticity: a complex effect on muscle fibre maturation. J Tissue Eng Regen Med 5(1):60–68
Breukers RD, Gilmore KJ, Kita M, Wagner KK, Higgins MJ, Moulton SE, Clark GM, Officer DL, Kapsa RM, Wallace GG (2010) Creating conductive structures for cell growth: growth and alignment of myogenic cell types on polythiophenes. J Biomed Mater Res A 95(1):256–268
Bryant C, Barnett J (2018) Consumer acceptance of cultured meat: a systematic review. Meat Sci 143:8–17
Burton NM, Vierck J, Krabbenhoft L, Bryne K, Dodson MV (2000) Methods for animal satellite cell culture under a variety of conditions. Methods Cell Sci 22(1):51–61
Casteilla L, Cousin B, Carmona M (2007) PPARs and adipose cell plasticity. PPAR Res 2007:68202
Catts O, Zurr I (2002) Growing semi-living sculptures: the tissue culture and art project. Leonardo. 35(4):365–370
Cerino G, Gaudiello E, Grussenmeyer T, Melly L, Massai D, Banfi A, Martin I, Eckstein F, Grapow M, Marsano A (2016) Three dimensional multi-cellular muscle-like tissue engineering in perfusion-based bioreactors. Biotechnol Bioeng 113(1):226–236
Chen S, Nakamoto T, Kawazoe N, Chen G (2015) Engineering multi-layered skeletal muscle tissue by using 3D microgrooved collagen scaffolds. Biomaterials. 73:23–31
Cheng CS, El-Abd Y, Bui K, Hyun YE, Hughes RH, Kraus WE, Truskey GA (2014) Conditions that promote primary human skeletal myoblast culture and muscle differentiation in vitro. Am J Phys Cell Phys 306(4):C385–C395
Claeys E, De Smet S, Balcaen A, Raes K, Demeyer D (2004) Quantification of fresh meat peptides by SDS–PAGE in relation to ageing time and taste intensity. Meat Sci 67(2):281–288
Collinsworth AM, Zhang S, Kraus WE, Truskey GA (2002) Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am J Phys Cell Phys 283(4):C1219–C1227
Daskalova A (2019) Farmed fish welfare: stress, post-mortem muscle metabolism, and stress-related meat quality changes. Int Aquat Res 11(2):113–124
Datar I, Betti M (2010) Possibilities for an in vitro meat production system. Innovative Food Sci Emerg Technol 11(1):13–22
Dennis RG, Smith B, Philp A, Donnelly K, Baar K (2009) Bioreactors for guiding muscle tissue growth and development. Adv Biochem Eng Biotechnol 112:39–79
Dodson MV, Martin EL, Brannon MA, Mathison BA, McFarland DC (1987) Optimization of bovine satellite cell-derived myotube formation in vitro. Tissue Cell 19(2):159–166
Dodson MV, McFarland DC, Grant AL, Doumit ME, Velleman SG (1996) Extrinsic regulation of domestic animal-derived satellite cells. Domest Anim Endocrinol 13(2):107–126
Dodson MV, McFarland DC, Martin EL, Brannon MA (1986) Isolation of satellite cells from ovine skeletal muscles. J Tissue Cult Methods 10(4):233–237
Dokmanovic M, Baltic ZM, Markovic R, Boskovic M, Loncina J, Glamoclija N, Dordevic M (2014) Relationships among pre-slaughter stress, rigor mortis, blood lactate, and meat and carcass quality in pigs. Acta Veterinaria 64(1):124–137
Dusterhoft S, Pette D (1990) Effects of electrically induced contractile activity on cultured embryonic chick breast muscle cells. Differentiation. 44(3):178–184
Edelman PD, McFarland DC, Mironov VA, Matheny JG (2005) Commentary: in vitro-cultured meat production. J Tissue Eng 11(5–6):659–662
Elkasrawy MN, Hamrick MW (2010) Myostatin (GDF-8) as a key factor linking muscle mass and skeletal form. J Musculoskelet Neuronal Interact 10(1):56–63
Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE (2004) Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166(6):877–887
Feiner G (2006) Meat products handbook: practical science and technology. Elsevier
Fraeye I, Kratka M, Vandenburgh H, Thorrez L (2020) Sensorial and nutritional aspects of cultured meat in comparison to traditional meat: much to be inferred. Front Nutr 7:35
Fujita H, Nedachi T, Kanzaki M (2007) Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res 313(9):1853–1865
Fuoco C, Petrilli LL, Cannata S, Gargioli C (2016) Matrix scaffolding for stem cell guidance toward skeletal muscle tissue engineering. J Orthop Surg Res 11(1):86
Gaydhane MK, Mahanta U, Sharma CS, Khandelwal M, Ramakrishna S (2018) Cultured meat: state of the art and future. Biomanufacturing Rev 3(1):1
Gholobova D, Gerard M, Decroix L, Desender L, Callewaert N, Annaert P, Thorrez L (2018) Human tissue-engineered skeletal muscle: a novel 3D in vitro model for drug disposition and toxicity after intramuscular injection. Sci Rep 8(1):12206
Gielen FL, Wallinga-de Jonge W, Boon KL (1984) Electrical conductivity of skeletal muscle tissue: experimental results from different muscles in vivo. Med Biol Eng Comput 22(6):569–577
Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM (2010) Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 329(5995):1078–1081
Goldspink DF, Cox VM, Smith SK, Eaves LA, Osbaldeston NJ, Lee DM, Mantle D (1995) Muscle growth in response to mechanical stimuli. Am J Phys 268(2 Pt 1):E288–E297
Goto S, Miyazaki K, Funabiki T, Yasumitsu H (1999) Serum-free culture conditions for analysis of secretory proteinases during myogenic differentiation of mouse C2C12 myoblasts. Anal Biochem 272(2):135–142
Guthridge M, Wilson M, Cowling J, Bertolini J, Hearn MT (1992) The role of basic fibroblast growth factor in skeletal muscle regeneration. Growth Factors 6(1):53–63
Heher P, Maleiner B, Pruller J, Teuschl AH, Kollmitzer J, Monforte X, Wolbank S, Redl H, Runzler D, Fuchs C (2015) A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain. Acta Biomater 24:251–265
Helbo S, Weber RE, Fago A (2013) Expression patterns and adaptive functional diversity of vertebrate myoglobins. Biochim Biophys Acta 1834(9):1832–1839
Hervy M, Weber JL, Pecheul M, Dolley-Sonneville P, Henry D, Zhou Y, Melkoumian Z (2014) Long term expansion of bone marrow-derived hMSCs on novel synthetic microcarriers in xeno-free, defined conditions. PLoS One 9(3):e92120
Hoang YT, Vu AT (2016) Sodium benzoate and potassium sorbate in processed meat products collected in Ho Chi Minh City, Vietnam. Int J Adv Sci Eng Inf Technol 6(4):477–482
Hocquette J-F (2016) Is in vitro meat the solution for the future? Meat Sci 120:167–176
Ismaeel A, Kim JS, Kirk JS, Smith RS, Bohannon WT, Koutakis P (2019) Role of transforming growth factor-beta in skeletal muscle fibrosis: a review. Int J Mol Sci 20(10):2446
Gordon Betts JKAY, James A. Wise, Eddie Johnson, Brandon Poe, Dean H. Kruse, Oksana Korol, Jody E. Johnson, Mark Womble, Peter DeSaix (2013) Chapter 10 - muscle tissue. In: Anatomy and Physiology p^pp 10–10.10. OpenStax
Kadim IT, Mahgoub O, Baqir S, Faye B, Purchas R (2015) Cultured meat from muscle stem cells: a review of challenges and prospects. J Integr Agric 14(2):222–233
Kaji H, Ishibashi T, Nagamine K, Kanzaki M, Nishizawa M (2010) Electrically induced contraction of C2C12 myotubes cultured on a porous membrane-based substrate with muscle tissue-like stiffness. Biomaterials. 31(27):6981–6986
Kamanga-Sollo E, Pampusch MS, White ME, Hathaway M, Dayton WR (2005) Insulin-like growth factor binding protein (IGFBP)-3 and IGFBP-5 mediate TGF-β-and myostatin-induced suppression of proliferation in porcine embryonic myogenic cell cultures. Exp Cell Res 311(1):167–176
Kanatous SB, Mammen PP (2010) Regulation of myoglobin expression. J Exp Biol 213(Pt 16):2741–2747
Khodabukus A, Baar K (2014) The effect of serum origin on tissue engineered skeletal muscle function. J Cell Biochem 115(12):2198–2207
Khodabukus A, Baar K (2015) Streptomycin decreases the functional shift to a slow phenotype induced by electrical stimulation in engineered muscle. Tissue Eng A 21(5–6):1003–1012
Khodabukus A, Baar K (2016) Factors that affect tissue-engineered skeletal muscle function and physiology. Cells Tissues Organs 202(3–4):159–168
Kim J, Lee J (2017) Role of transforming growth factor-beta in muscle damage and regeneration: focused on eccentric muscle contraction. J Exerc Rehabil 13(6):621–626
Kim M, Choi YS, Yang SH, Hong HN, Cho SW, Cha SM, Pak JH, Kim CW, Kwon SW, Park CJ (2006) Muscle regeneration by adipose tissue-derived adult stem cells attached to injectable PLGA spheres. Biochem Biophys Res Commun 348(2):386–392
Kong D, Gentz R, Zhang J (1998) Long-term stable production of monocyte-colony inhibition factor (M-CIF) from CHO microcarrier perfusion cultures. Cytotechnology. 26(2):131–138
Kook SH, Choi KC, Son YO, Lee KY, Hwang IH, Lee HJ, Chang JS, Choi IH, Lee JC (2006) Satellite cells isolated from adult Hanwoo muscle can proliferate and differentiate into myoblasts and adipose-like cells. Mol Cell 22(2):239–245
Kues WA, Petersen B, Mysegades W, Carnwath JW, Niemann H (2005) Isolation of murine and porcine fetal stem cells from somatic tissue. Biol Reprod 72(4):1020–1028
Langelaan ML, Boonen KJ, Rosaria-Chak KY, van der Schaft DW, Post MJ, Baaijens FP (2011) Advanced maturation by electrical stimulation: differences in response between C2C12 and primary muscle progenitor cells. J Tissue Eng Regen Med 5(7):529–539
Langelaan MLP, Boonen KJM, Polak RB, Baaijens FPT, Post MJ, van der Schaft DWJ (2010) Meet the new meat: tissue engineered skeletal muscle. Trends Food Sci Technol 21(2):59–66
Lassar AB, Skapek SX, Novitch B (1994) Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr Opin Cell Biol 6(6):788–794
Lawson MA, Purslow PP (2000) Differentiation of myoblasts in serum-free media: effects of modified media are cell line-specific. Cells Tissues Organs 167(2–3):130–137
Lepper C, Fan CM (2010) Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 48(7):424–436
Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D'Amore PA, Langer R (2005) Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23(7):879–884
Lian T, Wang L, Liu Y (2013) A new insight into the role of calpains in post-mortem meat tenderization in domestic animals: a review. Asian Australas J Anim Sci 26(3):443–454
Liao IC, Liu JB, Bursac N, Leong KW (2008) Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Cell Mol Bioeng 1(2–3):133–145
Listrat A, Lebret B, Louveau I, Astruc T, Bonnet M, Lefaucheur L, Picard B, Bugeon J (2016) How muscle structure and composition influence meat and flesh quality. Sci World J 2016:3182746
Lonergan EH, Zhang W, Lonergan SM (2010) Biochemistry of postmortem muscle—lessons on mechanisms of meat tenderization. Meat Sci 86(1):184–195
Lu D, Luo C, Zhang C, Li Z, Long M (2014) Differential regulation of morphology and stemness of mouse embryonic stem cells by substrate stiffness and topography. Biomaterials. 35(13):3945–3955
Ma YN, Wang B, Wang ZX, Gomez NA, Zhu MJ, Du M (2018) Three-dimensional spheroid culture of adipose stromal vascular cells for studying adipogenesis in beef cattle. Animal 12(10):1–7
Maley MA, Davies MJ, Grounds MD (1995) Extracellular matrix, growth factors, genetics: their influence on cell proliferation and myotube formation in primary cultures of adult mouse skeletal muscle. Exp Cell Res 219(1):169–179
Martin I, Wendt D, Heberer M (2004) The role of bioreactors in tissue engineering. Trends Biotechnol 22(2):80–86
Mattick CS, Landis AE, Allenby BR (2015) A case for systemic environmental analysis of cultured meat. J Integr Agric 14(2):249–254
McFarland DC, Pesall JE, Norberg JM, Dvoracek MA (1991) Proliferation of the Turkey myogenic satellite cell in a serum-free medium. Comp Biochem Physiol A Physiol 99(1–2):163–167
Mehdizadeh H, Lauri D, Karry KM, Moshgbar M, Procopio-Melino R, Drapeau D (2015) Generic Raman-based calibration models enabling real-time monitoring of cell culture bioreactors. Biotechnol Prog 31(4):1004–1013
Mehta F, Theunissen R, Post MJ (2019) Adipogenesis from bovine precursors. Methods Mol Biol 1889:111–125
Miller RK (2012) Sensory evaluation of beef flavor. In: Nollet LM (ed) handbook of meat, poultry and seafood quality. P^pp 173-191
du Moon G, Christ G, Stitzel JD, Atala A, Yoo JJ (2008) Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng A 14(4):473–482
Moritz MSM, Verbruggen SEL, Post MJ (2015) Alternatives for large-scale production of cultured beef: a review. J Integr Agric 14(2):208–216
Mottram DS (1998) Flavour formation in meat and meat products: a review. Food Chem 62(4):415–424
Mozetic P, Giannitelli SM, Gori M, Trombetta M, Rainer A (2017) Engineering muscle cell alignment through 3D bioprinting. J Biomed Mater Res A 105(9):2582–2588
Murgia M, Nagaraj N, Deshmukh AS, Zeiler M, Cancellara P, Moretti I, Reggiani C, Schiaffino S, Mann M (2015) Single muscle fiber proteomics reveals unexpected mitochondrial specialization. EMBO Rep 16(3):387–395
Nath SC, Nagamori E, Horie M, Kino-Oka M (2017) Culture medium refinement by dialysis for the expansion of human induced pluripotent stem cells in suspension culture. Bioprocess Biosyst Eng 40(1):123–131
Naumann K, Pette D (1994) Effects of chronic stimulation with different impulse patterns on the expression of myosin isoforms in rat myotube cultures. Differentiation. 55(3):203–211
O'Mara P, Farrell A, Bones J, Twomey K (2018) Staying alive! Sensors used for monitoring cell health in bioreactors. Talanta. 176:130–139
Ohlendieck K (2011) Skeletal muscle proteomics: current approaches, technical challenges and emerging techniques. Skelet Muscle 1(1):6
Ohlson S, Branscomb J, Nilsson K (1994) Bead-to-bead transfer of Chinese-hamster ovary cells using macroporous microcarriers. Cytotechnology. 14(1):67–80
Pette D, Sketelj J, Skorjanc D, Leisner E, Traub I, Bajrovic F (2002) Partial fast-to-slow conversion of regenerating rat fast-twitch muscle by chronic low-frequency stimulation. J Muscle Res Cell Motil 23(3):215–221
Phillips BW, Horne R, Lay TS, Rust WL, Teck TT, Crook JM (2008) Attachment and growth of human embryonic stem cells on microcarriers. J Biotechnol 138(1–2):24–32
Pirosa A, Gottardi R, Alexander PG, Tuan RS (2018) Engineering in-vitro stem cell-based vascularized bone models for drug screening and predictive toxicology. Stem Cell Res Ther 9(1):112
Post M (2018) Chapter 11 - proteins in cultured beef. In: proteins in food processing. P^pp 289-298. Elsevier
Post M & Hocquette J-F (2017) Chapter 16 - new sources of animal proteins: cultured meat. In: new aspects of meat quality. P^pp 425-441. Elsevier
Post MJ (2012) Cultured meat from stem cells: challenges and prospects. Meat Sci 92(3):297–301
Post MJ (2014a) An alternative animal protein source: cultured beef. Ann N Y Acad Sci 1328:29–33
Post MJ (2014b) Cultured beef: medical technology to produce food. J Sci Food Agric 94(6):1039–1041
Powell CA, Smiley BL, Mills J, Vandenburgh HH (2002) Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Phys Cell Phys 283(5):C1557–C1565
Powell RL, Dodson MV, Cloud JG (1989) Cultivation and differentiation of satellite cells from skeletal muscle of the rainbow trout Salmo gairdneri. J Exp Zool 250(3):333–338
Purslow PP, Oiseth S, Hughes J, Warner RD (2016) The structural basis of cooking loss in beef: variations with temperature and ageing. Food Res Int 89(Pt 1):739–748
Qazi TH, Mooney DJ, Pumberger M, Geissler S, Duda GN (2015) Biomaterials based strategies for skeletal muscle tissue engineering: existing technologies and future trends. Biomaterials. 53:502–521
Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G (2003) High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng 82(4):403–414
Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G (2008) Cardiac tissue engineering using perfusion bioreactor systems. Nature protocoIs 3(4):719–738
Rafiq QA, Coopman K, Hewitt CJ (2013) Scale-up of human mesenchymal stem cell culture: current technologies and future challenges. Curr Opin Chem Eng 2(1):8–16
Rafiq QA, Ruck S, Hanga MP, Heathman TRJ, Coopman K, Nienow AW, Williams DJ, Hewitt CJ (2018) Qualitative and quantitative demonstration of bead-to-bead transfer with bone marrow-derived human mesenchymal stem cells on microcarriers: utilising the phenomenon to improve culture performance. Biochem Eng J 135:11–21
Ramboer E, De Craene B, De Kock J, Vanhaecke T, Berx G, Rogiers V, Vinken M (2014) Strategies for immortalization of primary hepatocytes. J Hepatol 61(4):925–943
Ramboer E, Vanhaecke T, Rogiers V, Vinken M (2015) Immortalized human hepatic cell lines for in vitro testing and research purposes. Methods Mol Biol 1250:53–76
Rangarajan S, Madden L, Bursac N (2014) Use of flow, electrical, and mechanical stimulation to promote engineering of striated muscles. Ann Biomed Eng 42(7):1391–1405
Renault V, Thornell LE, Butler-Browne G, Mouly V (2002) Human skeletal muscle satellite cells: aging, oxidative stress and the mitotic clock. Exp Gerontol 37(10–11):1229–1236
Rischer H, Szilvay GR, Oksman-Caldentey KM (2020) Cellular agriculture - industrial biotechnology for food and materials. Curr Opin Biotechnol 61:128–134
Rodriguez BL, Larkin LM (2018) Chapter 12 - functional three-dimensional scaffolds for skeletal muscle tissue engineering. In: Deng Y, Kuiper J (eds) Functional 3D tissue engineering scaffolds. P^pp 279–304. Woodhead Publishing
Rowley J, Abraham E, Campbell A, Brandwein H, Oh S (2012) Meeting lot-size challenges of manufacturing adherent cells for therapy. BioProcess Int 10(3):16–22
Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A (2005) Spontaneous human adult stem cell transformation. Cancer Res 65(8):3035–3039
Rubio NR, Fish KD, Trimmer BA, Kaplan DL (2019) In vitro insect muscle for tissue engineering applications. ACS Biomater Sci Eng 5(2):1071–1082
Sassoli C, Pini A, Chellini F, Mazzanti B, Nistri S, Nosi D, Saccardi R, Quercioli F, Zecchi-Orlandini S, Formigli L (2012) Bone marrow mesenchymal stromal cells stimulate skeletal myoblast proliferation through the paracrine release of VEGF. PLoS One 7(7):e37512
Serena E, Flaibani M, Carnio S, Boldrin L, Vitiello L, De Coppi P, Elvassore N (2008) Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurol Res 30(2):207–214
Shahidi F, Rubin LJ, D'Souza LA, Teranishi R, Buttery RG (1986) Meat flavor volatiles: a review of the composition, techniques of analysis, and sensory evaluation. Crit Rev Food Sci Nutr 24(2):141–243
Shepon A, Eshel G, Noor E, Milo R (2018) The opportunity cost of animal based diets exceeds all food losses. Proc Natl Acad Sci U S A 115(15):3804–3809
Shit SC, Shah PM (2014) Edible polymers: challenges and opportunities. J Polymers 2014:1–13
Simsa R, Yuen J, Stout A, Rubio N, Fogelstrand P, Kaplan DL (2019) Extracellular heme proteins influence bovine myosatellite cell proliferation and the color of cell-based meat. Foods. 8(10):521
Slade P (2018) If you build it, will they eat it? Consumer preferences for plant-based and cultured meat burgers. Appetite. 125:428–437
Specht EA, Welch DR, Clayton EMR, Lagally CD (2018) Opportunities for applying biomedical production and manufacturing methods to the development of the clean meat industry. Biochem Eng J 132:161–168
Stephens N, Di Silvio L, Dunsford I, Ellis M, Glencross A, Sexton A (2018) Bringing cultured meat to market: technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci Technol 78:155–166
Thompson J (2002) Managing meat tenderness. Meat Sci 62(3):295–308
Thorrez L, Vandenburgh H (2019) Challenges in the quest for ‘clean meat’. Nat Biotechnol 37(3):215–216
Van Eelen WF (2007) Industrial production of meat using cell culture methods, US7270829B2. USA Patent
Vandenburgh H, Kaufman S (1979) In vitro model for stretch-induced hypertrophy of skeletal muscle. Science. 203(4377):265–268
Vandenburgh HH, Karlisch P (1989) Longitudinal growth of skeletal myotubes in vitro in a new horizontal mechanical cell stimulator. Vitro Cell Dev Biol 25(7):607–616
Vandenburgh HH, Swasdison S, Karlisch P (1991) Computer-aided mechanogenesis of skeletal muscle organs from single cells in vitro. FASEB J 5(13):2860–2867
Verbruggen S, Luining D, van Essen A, Post MJ (2018) Bovine myoblast cell production in a microcarriers-based system. Cytotechnology. 70(2):503–512
Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, Federspil G (2009) The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab 297(5):E987–E998
Wang L, Wu Y, Guo B, Ma PX (2015) Nanofiber yarn/hydrogel core–shell scaffolds mimicking native skeletal muscle tissue for guiding 3D myoblast alignment, elongation, and differentiation. ACS Nano 9(9):9167–9179
Warner RD (2019) Review: analysis of the process and drivers for cellular meat production. Animal 13(12):3041–3058
Wehrle U, Dusterhoft S, Pette D (1994) Effects of chronic electrical stimulation on myosin heavy chain expression in satellite cell cultures derived from rat muscles of different fiber-type composition. Differentiation. 58(1):37–46
Wilschut KJ, Jaksani S, Van Den Dolder J, Haagsman HP, Roelen BA (2008) Isolation and characterization of porcine adult muscle-derived progenitor cells. J Cell Biochem 105(5):1228–1239
Witt R, Weigand A, Boos AM, Cai A, Dippold D, Boccaccini AR, Schubert DW, Hardt M, Lange C, Arkudas A, Horch RE, Beier JP (2017) Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Mol Cell Biol 18(1):15
Wood JD, Enser M, Fisher AV, Nute GR, Richardson RI, Sheard PR (1999) Manipulating meat quality and composition. Proc Nutr Soc 58(2):363–370
Wood JD, Richardson RI, Nute GR, Fisher AV, Campo MM, Kasapidou E, Sheard PR, Enser M (2004) Effects of fatty acids on meat quality: a review. Meat Sci 66(1):21–32
Yablonka-Reuveni Z, Quinn LS, Nameroff M (1987) Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev BioI 119(1):252–259
Yamasaki K, Hayashi H, Nishiyama K, Kobayashi H, Uto S, Kondo H, Hashimoto S, Fujisato T (2009) Control of myotube contraction using electrical pulse stimulation for bio-actuator. J Artificial Organs 12(2):131–137
Zeng L, Rahrmann E, Hu Q, Lund T, Sandquist L, Felten M, O'Brien TD, Zhang J, Verfaillie C (2006) Multipotent adult progenitor cells from swine bone marrow. Stem Cells 24(11):2355–2366
Zhang GQ, Zhao XR, Li XL, Du GC, Zhou JW, Chen J (2020) Challenges and possibilities for bio-manufacturing cultured meat. Trends Food Sci Technol 97:443–450
Zhao L, Fu HY, Zhou WC, Hu WS (2015) Advances in process monitoring tools for cell culture bioprocesses. Eng Life Sci 15(5):459–468
Zheng JK, Wang Y, Karandikar A, Wang Q, Gai H, Liu AL, Peng C, Sheng HZ (2006) Skeletal myogenesis by human embryonic stem cells. Cell Res 16(8):713–722
Funding
The authors acknowledge the financial support from the Slovenian Research Agency for Research (grant numbers P3-0036, J3-1762, and I0-0029); the Ministry for Education, Science and Sport (grant numbers Raziskovalci-2.1-UM-MF-952027 and Raziskovalci-2.1-UM-MF-952028); and through the Young Researcher Programme.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zidarič, T., Milojević, M., Vajda, J. et al. Cultured Meat: Meat Industry Hand in Hand with Biomedical Production Methods. Food Eng Rev 12, 498–519 (2020). https://doi.org/10.1007/s12393-020-09253-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-020-09253-w