Abstract
The objective of the study was to associate soil moisture, aerial biometric characteristics, and root distribution patterns in the yield of sugarcane genotypes in the fourth ratoon and the relationships between these characteristics. We used five sugarcane genotypes (CTC9002, RB044311, RB044313, RB044336, and RB867515) and five soil depths (0–20, 20–40, 40–60, 60–80 and 80–100 cm deep). The evaluations were carried out in December 2019. We evaluated the root systems, the soil water content at the five depths, the aerial biometric characteristics, and the productive yield. In the roots, the genotypes RB044311, RB044313, RB044336, and RB867515 showed a higher yield. On average, genotypes showed 50% of dry weight and total root length at a depth of 0–20 cm, 73% between 0 and 40 cm, and 86% between 0 and 60 cm. The RB044336 genotype showed maximum height and number of buds. The RB867515 genotype had the largest average stem diameter and CTC9002, the largest number of tillers. The genotypes CTC9002 and RB867515 presented the highest soluble solids' content, apparent sucrose in the juice, and total reducing sugars. These genotypes showed a 26% and 57% higher root density in soil layers at depths of 60–80 cm, respectively, 79% and 86% in the 80–100 cm layer, about the layer with the highest concentration of 0–20 cm roots. These layers remained with the highest soil moisture levels during the period of establishment and intensive cultivation growth. Thus, there was a relationship between root distribution dynamics and soil moisture in different areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Brazil is the world's largest producer of sugarcane, extending the cultivation to more than 10 million hectares, resulting in 30 million tons of sugar and 34 billion liters of ethanol, with emphasis on the Midwest and Southeast regions with a total production of 589 million tons, representing almost 92% of the national total (Companhia Nacional de Abastecimento—Conab 2019). In the Midwest region, sugarcane is cultivated between April and September, a period in which evapotranspiration overcomes precipitation, with the existence of a water deficit (Alvares et al. 2013).
The growth and development of plants depend on the absorption of nutrients and the water available in the soil and, therefore, are closely associated with the roots' morphology and physiology (Bashir et al. 2012; Ju et al. 2015). The distribution of roots controls the time that crops have access to water and nutrients and, therefore, decisively contribute to productive yield, the susceptibility of nutrients to leaching, and, in sugarcane, the longevity of the cane field (Cardozo and Sentelhas 2013; Tron et al. 2015; Nichols et al. 2019). Furthermore, the water deficit reduces root growth in the soil's superficial layers (Battie Laclau and Laclau 2009).
Sugarcane genotypes respond differently to water deficit and present variations in the root system, aerial characteristics, and productive quality (Bashir et al. 2012). In sugarcane ratooning, reducing these characteristics is evident due to the low capacity of the sugarcane varieties and the crop's inadequate management. However, the ratoon production has a lower production cost of plant canes, around 25 to 30% (Bashir 2013).
In this way, the development of water-efficient genotypes can contribute to sustainable agricultural production's intensification (Raza et al. 2012). Genetic improvement, with a focus on root development, is crucial for efficiency in the use of water by the crop, this factor being little explored by genetic improvement programs (Tron et al. 2015). The natural diversity of root systems may indicate dehydration by efficient absorption compatible with high yields (Gewin 2010; Kell 2011; Palta et al. 2011).
There is little information avialble about water interactions in the soil and its effects on root characteristics and their production yield in sugarcane (Tron et al. 2015). The present study hypothesizes the positive correlation between the root system's distribution and water availability as a soil depth function. Thus, this study aimed to determine the root distribution dynamics of five sugarcane genotypes, their relationship with soil moisture at five soil depths, and correlate it with the aerial biometric characteristics and yield.
Material and Methods
We carried out this study in the Interuniversity Network's experimental area for the Development of the Sugar-Energy Sector (RIDESA), located at Jalles Machado S. A. (15º 08′S, 48º 53′W, 570 m of altitude), in the city of Goianésia, Goiás, Brazil. According to Köppen, the region has a tropical savanna climate with dry winter and rainy summer (Aw) (Alvares et al. 2013). Average temperatures vary between 16 and 29 °C and annual rainfall is approximately 1,500 mm. After the third cut, the analyses started in the cane period, ending the evaluations in the harvest (fourth ratoon) in December 2019, without water supplementation by irrigation.
The experimental design was in randomized blocks, with four replications, in subdivided plots, with five sugarcane genotypes (CTC9002, RB044311, RB044313, RB044336 and RB867515) established in November 2012, and in the subplots, five layers of soil (0–20, 20–40, 40–60, 60–80 and 80–100 cm). The genotypes CTC9002 and RB867515 were used, because these genotypes are established in the region and more tolerant of water deficit (Bressan et al. 2020). The rest are new experimental genotypes developed by RIDESA in partnership with the Federal University of Goiás, we have no conclusive information about these genetic materials.
The experimental units contained 27 m2, four cane rows 10 m long and 0.9 m spacing between lines. The soil was classified as Oxisol, clay texture, following physical attributes (Table 1), and chemical attributes (Table 2). It should be noted that there was no mineral supplementation in the soil due to the ideal levels of nutrients for the development of the crop, as described by Malavolta et al. (1997).
Meteorological conditions: temperature and relative humidity, wind speed, vapor pressure deficit, precipitation, and reference evapotranspiration (ETo), were monitored by an automatic weather station (Metos®, Pessl Instruments, Weiz, Austria), located at 25 m from the experimental area.
Capacitive soil moisture sensors (ECH2O EC-5, Decagon Devices, Inc., Pullman, United States) were installed at five depths and four experimental blocks. We calibrated the sensors following the methods proposed by Antunes Júnior et al. (2018), and the acquired data were recorded in data loggers (Em50, Decagon Devices, Inc., Pullman, United States).
Biometric and root analyzes occurred at 290 days after the 3rd cut, when the sugarcane plants matured. The biometric variables evaluated were as follows: number of leaves, number of tillers, number of gems, stem diameter (mm) and height (m), measured with the aid of a digital caliper, tape measure, and leaf area according to the methodology proposed by Teixeira et al. (2011).
We carried out the root system's characterization by evaluating soil monoliths collected and evaluated according to the methodology described by Silva-Olaya et al. (2017). The monoliths contained 0.45 m in width, 0.3 m in height and, 0.2 m in depth for each of the depth profiles and genotypes in the four blocks. The soil samples, including the roots, were packed in plastic bags, individualized, and transported to the laboratory, subsequently washed and sieved.
After, we placed the roots in an acrylic box (20 cm wide and 30 cm long), with a layer of approximately 1 cm of water on the scanner. We used the WinRHIZO 2012b software (Regent Instruments, Quebec, Canada) coupled with a digital image scanner to record the total length, volume, and average diameter of the roots. We used a relationship between length and volume to estimate the root length density (Chumphu et al. 2019). We divided the samples into subsamples, and the final length was defined as the sum of all the subsamples' lengths. The root samples were then stored in an oven for 10 days at 65 °C for drying. After stabilizing its weight, the root dry matter was determined.
The sugarcane's industrial quality was determined at the time of harvest by evaluating the harvested stalks, determining the fiber content, soluble solids content, apparent sucrose in the juice, and total reducing sugars procedures proposed by (CONSECANA 2015).
The analysis of the results obtained was performed utilizing variance (ANOVA) to verify the difference in root measurements in depths. When the results were significant (p < 0.05), the treatment means were compared by the LSD test (p < 0.05). ANOVA and the LSD test were performed using the R software (R Core Team, 2016).
Results
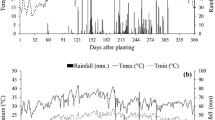
The variation in air temperature, precipitation, and relative humidity was observed during the experimental period (Fig. 1a, b). The average temperature during the experimental period was 24.2 °C, with a maximum of 36.5 °C at 72 days after cutting (DAC) and 9.9 °C at 238 DAC. The average annual relative humidity (RH) was 73.8%, ranging from 41.9 to 95.6%.
Figure 2 shows the variation and distribution profile of the soil's volumetric moisture in the soil layers analyzed at different depths. Figure 3 shows the water balance of the soil for the cultivation of sugarcane.
During the experimental period, the total precipitation was 1986 mm, and the water demand of the culture was 1097 mm. The total precipitation would supply all the sugarcane needs, were it not for the heterogeneous rainfall distribution over the seasonal period that occurs in the region. Thus, there were 44 days of surplus and 228 days of water deficit. The largest surplus observed was 108.9 mm at 71 DAC and the lowest 5.6 mm at 1 DAC.
The distribution of the amount of water stored in the soil showed a gradual distribution between the depths, with greater humidity in the soil's deeper layers, excluding the depth of 60–80 cm, which up to 176 DAC was more significant than the depth of 80–100 cm. Up to 181 DAC, the moisture in the analyzed soil depths was higher than the permanent wilt (θpwp). The 0–20 cm layer of soil exceeded θpwp at 181 DAC. This limit's crossing occurred at the other depths at 191, 200, 201, and 214 DAC, for the 60–80, 20–40, 40–60 layers and 80–100 cm.
Table 3 shows the results for the analysis of variance (ANOVA) of the five sugarcane genotypes' root parameters as a function of the evaluated soil layer.
There was a significant effect on the genotypes (G), depth (D), and interaction between them (G × D) for the variables of total length, length of roots with a diameter greater than 1.5 mm, average diameter, dry root mass, and density of length.
Figures 4, 5, 6, 7, and 8 showed the averages of the total length of the roots, average diameter of the roots, length of the roots with a diameter greater than 1.5 mm, the average volume of the roots, and dry weight of the roots of the five genotypes of sugarcane in the five depths studied.
Total length of the roots of the five sugarcane genotypes as a function of the five depths studied. *Means followed by different capital letters that show the effect of the sugarcane genotypes on the depths and averages followed by different lower letters that show the effect of the depths on the sugarcane genotypes differ by the LSD test (p < 0.05)
Mean root diameter of the five sugarcane genotypes at the five depths studied. *Means followed by different capital letters that show the effect of the sugarcane genotypes on the depths and averages followed by different lower letters that show the effect of the depths on the sugarcane genotypes differ by the LSD test (p < 0.05)
Length of roots with diameters greater than 1.5 mm from the five sugarcane genotypes at the five depths studied. *Means followed by different capital letters that show the effect of the sugarcane genotypes on the depths and averages followed by different lower letters that show the effect of the depths on the sugarcane genotypes differ by the LSD test (p < 0.05)
Root length density of the five sugarcane genotypes at the five depths studied. * Means followed by different capital letters that show the effect of the sugarcane genotypes on the depths and averages followed by different lower letters that show the effect of the depths on the sugarcane genotypes differ by the LSD test (p < 0.05)
Five sugarcane genotypes at the five depths studied. *Means followed by different capital letters that show the effect of the sugarcane genotypes on the depths and averages followed by different lower letters that show the effect of the depths on the sugarcane genotypes differ by the LSD test (p < 0.05)
The genotype RB0444336 showed the longest roots in the layer 0–20 cm deep (732.5 cm), followed by RB044313 with 624.8 cm long (Fig. 3b and d). Average, 55% of the total length of the roots for the genotypes was established in the 0–20 cm layer, with a tendency to decrease with increasing depth (Fig. 4). However, the CTC9002 genotype did not show a significant difference between the 20–40 and 60–80 cm deep layers and the RB044311 genotype, which showed different behavior, with the roots of the 80–100 cm layer being significantly larger, than the roots in the 60–80 cm layer (Fig. 4a and b).
The genotype RB044336 had the highest average diameter of the roots at depths of 0–20, 20–40, and 40–60, together with the genotype RB867515, which presented diameters greater than the other genotypes in the soil layer 0–20 cm deep (Fig. 5a and e). The genotypes CTC9002, RB044311, and RB867515 presented the largest diameters in the depth 60–80 cm (Fig. 5a, b, and e). In the 80–100 cm layer, the largest mean diameters were observed in genotype RB044311 (Fig. 5b). There was no standard distribution or trend in mean root diameters at the depths evaluated between genotypes (Fig. 5).
As with the total length of the roots, the sum of the roots with a diameter greater than 1.5 mm (L > 1.5 mm) occurred, for the most part, at a depth of 0–20 cm (Fig. 6). It was possible to observe that there was a tendency for the length to decrease between the depths, except in the genotype RB044311, which presented lower values in the soil layer of 60–80 cm about the 80–100 cm (Fig. 6b). Among the genotypes, RB867515 presented L > 1.5 mm greater at depths of 0–20 and 20–40 cm (Fig. 6e). At a depth of 40–60, genotypes RB044313 and RB044336 were superior (Fig. 6c and d). There was no significant difference between the genotypes in the 60–80 cm layer. The roots' sum with a diameter greater than 1.5 mm represented 8%, 8%, 4%, 11%, and 10% of the roots' total length for the genotypes, CTC9002, RB044311, RB044313, RB044336, and RB867515, respectively.
The RB044313 genotype showed higher root length density at depths of 0–20, 20–40, 40–60 and 60–80 cm and 80–100 cm, respectively (Fig. 7). The depths of 60–80 and 80–100 showed a higher root density (Fig. 7). In the RB867515 genotype, it was possible to observe an increasing trend in root density values with increasing depth. It was impossible to observe the same pattern of distribution of the average volumes between genotypes CTC9002, RB044311, RB044313, and RB044336.
The root dry mass decreased about the depth of the studied soil layer (Fig. 8). The highest dry mass of roots at depths of 0–20 and 20–40 cm was observed in genotype RB867515. At a depth of 40–60 cm, the largest dry mass of roots was genotype RB044313 (Fig. 8c). For depths of 60–80 and 80–100 cm, it was not possible to observe a significant difference between the mean dry mass of roots by the LSD test (p < 0.05).
Table 4 shows the results of the analysis of variance (ANOVA) of the biometric parameters of the aerial part of the five sugarcane genotypes.
There was a significant effect between the genotypes for the variables plant height, stem diameter, number of buds, number of tillers, and leaf area (Table 4). Table 5 presents the average values of the aerial biometric variables for the five sugarcane genotypes studied.
The genotype RB044336 showed greater height and number of buds, standing out in the number of tillers. The RB867515 genotype had the largest average stem diameter and the largest leaf area and CTC9002, the largest tillers.
Table 6 presents the results of the analysis of variance (ANOVA) for the industrial quality variables of the five sugarcane genotypes. There was a significant effect among the genotypes for fiber content, soluble solids content, apparent sucrose in juice, and total reducing sugars. There was no significant effect on productivity.
Table 7 shows the average values of the industrial quality variables for the five sugarcane genotypes studied.
The genotypes CTC9002 and RB867515 presented the highest soluble solids' content, apparent sucrose in the juice, and total reducing sugars. The CTC9002 genotype had lower fiber content, with the other genotypes showing no significant differences according to the LSD test (p < 0.05). There was no significant difference between the production values for the five genotypes.
Discussion
Sugarcane is a deep-rooted crop due to its long growth cycle and the root system's longevity through multiple rotations compared to other crops. The root systems reach depths that can vary between 1.5 and 6.0 m (Smith et al. 2005). Studies report that the root system of sugarcane can increase, seeking water in greater depths of the soil (Battie Laclau and Laclau 2009; Jongrungklang et al. 2013).
In this study, up to 181 DAC at all depths studied, the soil moisture was higher than the humidity at the permanent wilt point (θpwp) (Fig. 1). It is believed that the availability of water in the soil has limited the growth of the root system in the depth range analyzed. Even if the θpwp is exceeded at 214 DAC, the need for water from the cane is greater during the first periods of the growth cycle; that is, stages of emergence, establishment, and intensive growth, periods in which the water balance is generally favorable. During the dry season, the need for water decreases and is almost negligible during maturation (Scarpare et al. 2016).
Most of the sugarcane root biomass is found close to the soil surface and decreases exponentially with increasing depth. Usually, 50% of the root biomass is found in the top 20 cm of the soil and 85% in the first 60 cm (Smith et al. 2005). In this study, on average, genotypes showed 50% of dry weight and total length of roots at a depth of 0–20 cm, 73% between 0 and 40 cm, and 86% between 0 and 60 cm, as reported in the literature. The high concentration of roots in the upper layers of the soil occurs due to the reduction in the root's branches due to the soil's high resistance in lower layers. Otto et al.(2011) analyzed the reduction in the development of the root system of sugarcane and found that restrictions to root growth in Oxisols can occur when the soil density is greater than or equal to 1.78 g cm−3. In the layers below 20 cm, the soil's density in the study varied between 1.74 and 1.77 g cm−3 (Table 1), values close to the one mentioned above, thus corroborating the above.
The genotype RB044336 had the highest height, the number of buds, and the second-largest tillers. Tilling is highly variable among the different sugarcane genotypes, depending on each one's genetic characteristics (Vasantha et al. 2012). For Terauchi et al. (1999), the high tillering is an inadequate characteristic for obtaining improved cultivars, as it would promote an energy expenditure for the production of these tillers and the aerial part, not representing a positive correlation with the increase in crop productivity producing tillers with more stems thin. Proof of this is that the genotype RB867515 had the largest average stem diameter and the aerial lowest biometric characteristics about the other genotypes.
The genotypes CTC9002 and RB867515 presented the highest soluble solids' content, apparent sucrose in the juice, and total reducing sugars. In Brazil, the industry uses sugarcane as a raw material for ethanol production, and technological quality becomes essential for culture, given the ability to convert it into sugar or alcohol through the transformation coefficients of each unit productive (Mesa et al. 2020). Sucrose and glucose are the leading low molecular weight carbohydrates for organic synthesis in sugarcane. In the ethanol production process, there is a radical transformation of these carbohydrates' chemical structure through fermentative processes (Ferreira et al. 2009).
Little is known about the precise partitioning of carbohydrates in the root system of sugarcane and its contribution to crop yield (Smith et al., 2005). Scarpare et al. (2019) con clude that the increase in dry matter below the ground may not affect the cane yield aboveground under moderate water stress conditions. However, if we observe the distribution of root length densities, genotypes CTC9002, RB044336, RB867515 showed an increase of 26%, 63%, and 57% in the 60–80 cm layers, respectively, and 79%, 39%, and 86% in the 80–100 cm layer, about the layer with the highest root concentration of 0–20 cm (Fig. S1). These soil layers (60–80 and 80–100 cm) remained with the highest soil moisture levels during the period of the crop's establishment and intensive growth (Fig. 2). We also observed that the higher the soil moisture, the greater the root length density (Fig. S2). Therefore, it is believed that greater access to water and consequently to nutrients facilitate the synthesis of carbohydrates that determine productivity, thus explaining the results of the technological qualities found in Table 7, in addition to the correlations shown in Fig. 9.
When evaluating the behavior of the diameter of the roots, it is observed in the depth of 60–80 cm (wetter layer of the soil, Fig. 2) that there was no statistical difference between the genotypes. However, genotypes CTC9002 and RB86715 showed root length values with a diameter greater than 1.5 mm greater than the average among all genotypes in 41% and 2%, respectively (Fig. 7). Reichardt and Timm (2019) describe the SWAP (Soil Water Atmosphere and Plant) model, which is based on the Darcy–Buckingham equation to explain the flow of water and nutrients from soil to roots, through the model, which realized that the flow is characterized in three directions, in an orthogonal system (horizontal, axial and radial), with the diameter being one of the main factors for determining the hydraulic conductivity of root tissues (Judd et al. 2016). The RB044336 genotype was the third with the highest soluble solids' highest levels and apparent sucrose in the juice. It is believed that this genotype was not statistically equal to the others due to the lower percentage of roots at a depth of 80–100 cm, compared to 0–20 cm. Besides, this variety showed the best results for the length of roots with a diameter greater than 1.5 mm.
Also, a higher root density in the variety RB044311 and RB044311, about 12% and 18%, at a depth of 60–80 cm, compared to 0–20 cm. However, this smaller percentage of the density difference indicates a more homogeneous distribution of the root system of these varieties, and although root growth is associated with an increase in the uptake of water and nutrients (Chopart et al. 2010; Marasca et al. 2015), a higher rate of root growth implies that less assimilates are partitioned into organs above the ground (stem and leaves). Also, there is a metabolic cost of maintaining the roots (Veen 1981). Thus, this distribution of assimilates due to the higher biomass of roots in low-humid layers impaired sucrose accumulation and these genotypes' technological quality.
Conclusion
There was a relationship between root distribution dynamics and soil moisture at different depths analyzed. The genotypes with the best production rates showed a higher density of length and length of roots with a diameter greater than 1.5 mm in the depths that remained for a longer time moist. The soil depths of 60–80 and 80–100 cm remained with the highest soil moisture levels during establishment and intensive cultivation. The genotypes CTC9002 and RB867515 presented the highest soluble solids' content, apparent sucrose in the juice, and total reducing sugars.
References
Alvares, Clayton A., José L. Stape, Paulo C. Sentelhas, José L.M. Gonçalves, and Gerd Sparovek. 2013. Köppen’s climate classification map for Brazil. MeteorologischeZeitschrift 22 (6): 711–728. https://doi.org/10.1127/0941-2948/2013/0507.
Bashir, Shahid. 2013. Ratooning potential of different promising sugarcane genotypes at varying harvesting dates. Journal of Agricultural and Biological Science 8: 437–440.
Bashir, Shahid, Naeem Fiaz, Abdul Ghaffar, and Farhan Khalid. 2012. Ratooning ability of sugarcane genotypes at different harvesting dates. International Sugar Journal 114: 273–276.
Bressan, Eduardo A., Igor A.S. de Carvalho, Maria T.M.R. Borges, Monalisa S. Carneiro, Edson F. da Silva, Rodrigo Gazaffi, Regina T. Shirasuna, et al. 2020. Assessment of gene flow to wild relatives and nutritional composition of sugarcane in Brazil. Frontiers in Bioengineering and Biotechnology 8: 598. https://doi.org/10.3389/fbioe.2020.00598.
Cardozo, N.P., and P.C. Sentelhas. 2013. Climatic effects on sugarcane ripening under the influence of cultivars and crop age. Scientia Agricola. 70: 449–456. https://doi.org/10.1590/S0103-90162013000600011.
Chopart, J.L., M.C.B. Azevedo, L. Le Mézo, and D. Marion. 2010. Functional relationship between sugarcane root biomass and length for cropping system applications. Sugar Technology 12: 317–321. https://doi.org/10.1007/s12355-010-0044-2.
Chumphu, Saranya, Nuntawoot Jongrungklang, and Patcharin Songsri. 2019. Association of physiological responses and root distribution patterns of ratooning ability and yield of the second ratoon cane in sugarcane elite clones. Agronomy 9: 200. https://doi.org/10.3390/agronomy9040200.
Companhia Nacional de Abastecimento (Conab). 2019. Acompanhamento da safra brasileira de cana-de-açúcar. v. 6 – Safra 2019/20. 3. Brasília: Conab.
Conselho de Produtores de Cana-de-Açúcar (CONSECANA). 2015. Manual de Instruções. 6. Piracicaba: Consecana.
Ferreira, Vitor F., David R. da Rocha, and Fernando C. da Silva. 2009. Potencialidades e oportunidadesnaquímica da sacarose e outros açúcares. Química Nova 32: 623–638. https://doi.org/10.1590/S0100-40422009000300007.
Gewin, Virginia. 2010. Food: An underground revolution. Nature 466: 552–553. https://doi.org/10.1038/466552a.
Jongrungklang, N., B. Toomsan, N. Vorasoot, S. Jogloy, K.J. Boote, G. Hoogenboom, and A. Patanothai. 2013. Drought tolerance mechanisms for yield responses to pre-flowering drought stress of peanut genotypes with different drought tolerant levels. Field Crops Research 144: 34–42. https://doi.org/10.1016/j.fcr.2012.12.017.
Ju, Chengxin, Roland J. Buresh, Zhiqin Wang, Hao Zhang, Lijun Liu, Jianchang Yang, and Jianhua Zhang. 2015. Root and shoot traits for rice varieties with higher grain yield and higher nitrogen use efficiency at lower nitrogen rates application. Field Crops Research 175: 47–55. https://doi.org/10.1016/j.fcr.2015.02.007.
Judd, Lesley A., Brian E. Jackson, William C. Fonteno, and J.C. Domec. 2016. Measuring root hydraulic parameters of container-grown herbaceous and woody plants using the hydraulic conductance flow meter. Hort Science 51: 192–196. https://doi.org/10.21273/HORTSCI.51.2.192 .
Júnior, Antunes, J. de Elson, José A. Júnior, and Derblai Casaroli. 2018. Calibração do sensor capacitivo ec-5 em um latossoloemfunção da densidade do solo. Revista Engenhariana Agricultura 26: 80–88. https://doi.org/10.13083/reveng.v26i1.864.
Kell, Douglas B. 2011. Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Annals of Botany 108: 407–418. https://doi.org/10.1093/aob/mcr175.
Klaus, R., and L.C. Timm. 2019. Soil, Plant and Atmosphere: Concepts, Processes and Applications, vol. 1. Springer.
Laclau, P.B., and J.-P. Laclau. 2009. Growth of the whole root system for a plant crop of sugarcane under rainfed and irrigated environments in Brazil. Field Crops Research 114: 351–360. https://doi.org/10.1016/j.fcr.2009.09.004.
Malavolta, Eurípedes, Godofredo C. Vitti, and Sebastião A. Oliveira. 1997. Avaliação do estado nutricional das plantas: princípios e aplicações. 2. Piracicaba: Associação Brasileira para Pesquisa da Potassa e do Fosfato.
Marasca, Indiamara, Stella V. Lemos, Reginaldo B. Silva, Saulo P.S.. Guerra, and Kleber P. Lanças. 2015. Soil compaction curve of an oxisol under sugarcane planted after in-row deep tillage. RevistaBrasileira de Ciência do Solo 39: 1490–1497. https://doi.org/10.1590/01000683rbcs20140559.
Mesa, Leyanis, Yenisleidy Martínez, Ana C. de Armas, and Erenio González. 2020. Ethanol production from sugarcane straw using different configurations of fermentation and techno-economical evaluation of the best schemes. Renewable Energy 156: 377–388. https://doi.org/10.1016/j.renene.2020.04.091.
Nichols, Virginia A., Raziel A. Ordóñez, Emily E. Wright, Michael J. Castellano, Matt Liebman, Jerry L. Hatfield, Matt Helmers, and Sotirios V. Archontoulis. 2019. Maize root distributions strongly associated with water tables in Iowa, USA. Plant and Soil 444: 225–238. https://doi.org/10.1007/s11104-019-04269-6.
Otto, R., A.P. Silva, H.C.J. Franco, E.C.A. Oliveira, and P.C.O. Trivelin. 2011. High soil penetration resistance reduces sugarcane root system development. Soil and Tillage Research 117: 201–210. https://doi.org/10.1016/j.still.2011.10.005.
Palta, Jairo A., Xing Chen, Stephen P. Milroy, Greg J. Rebetzke, M.F. Dreccer, and Michelle Watt. 2011. Large root systems: are they useful in adapting wheat to dry environments? Functional Plant Biology 38: 347–354. https://doi.org/10.1071/FP11031.
Raza, A., J.K. Friedel, and G. Bodner. 2012. Improving Water Use Efficiency for Sustainable Agriculture. In Agroecology and Strategies for Climate Change, ed. E. Lichtfouse, 167–211. Springer. https://doi.org/10.1007/978-94-007-1905-7_8.
Scarpare, F.V., T.A.D. Hernandes, S.T. Ruiz-Corrêa, M.C. AraújoPicoli, B.R. Scanlon, M.F. Chagas, D.G. Duft, and T.F. Cardoso. 2016. Sugarcane land use and water resources assessment in the expansion area in Brazil. Journal of Cleaner Production 133: 1318–1327. https://doi.org/10.1016/j.jclepro.2016.06.074.
Scarpare, F.V., Q. Jong van Lier, L. Camargo, R.C.M. Pires, S.T. Ruiz-Corrêa, A.H.F. Bezerra, G.J.C. Gava, and C.T.S. Dias. 2019. Tillage effects on soil physical condition and root growth associated with sugarcane water availability. Soil and Tillage Research 187: 110–118.
Silva-Olaya, Adriana M., Carlos E.P.. Cerri, and Carlos C. Cerri. 2017. Comparação de métodos de amostragem para avaliação do sistema radicular da cana-de-açúcar. Revista de CienciasAgrícolas 34: 7–16. https://doi.org/10.22267/rcia.173401.59.
Smith, D.M., N.G. Inman-Bamber, and P.J. Thorburn. 2005. Growth and function of the sugarcane root system. Field Crops Research 92: 169–183. https://doi.org/10.1016/j.fcr.2005.01.017.
Teixeira, Cicero, Vilma M. Ferreira, Laurício Endres, Débora T.R.G. Ferreira, and Eduardo R. Gonçalves.. 2011. Crescimento e produtividade de quatrovariedades de cana-de-açúcar no quarto ciclo de cultivo. Revista Caatinga 24: 56–63.
Terauchi, T., H. Nakagawa, M. Matsuoka, H. Nakano, and A. Sugimoto. 1999. Comparison of the early growth between sugarcane and sweet sorghum. Japanese Journal of Crop Science (Japan) 68: 414–418. https://doi.org/10.1626/jcs.68.414.
Tron, Stefania, Gernot Bodner, Francesco Laio, Luca Ridolfi, and Daniel Leitner. 2015. Can diversity in root architecture explain plant water use efficiency? A modeling study. Ecological Modelling 312: 200–210. https://doi.org/10.1016/j.ecolmodel.2015.05.028.
Vasantha, S., D. Esther Shekinah, C. Gupta, and P. Rakkiyappan. 2012. Tiller production, regulation and senescence in sugarcane (Saccharum species hybrid) genotypes. Sugar Tech 14: 156–160. https://doi.org/10.1007/s12355-011-0129-6.
Veen, B. W. 1981. Relation between root respiration and root activity. In Structure and Function of Plant Roots: Proceedings of the 2nd International Symposium, held in Bratislava, Czechoslovakia, September 1–5, 1980, ed. R. Brouwer, O. Gašparíková, J. Kolek, and B. C. Loughman, 277–280. Dordrecht: Springer Netherlands. doi: https://doi.org/10.1007/978-94-009-8314-4_53.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Costa Neto, C.A., Mesquita, M., de Moraes, D.H.M. et al. Relationship Between Distribution of the Radicular System, Soil Moisture and Yield of Sugarcane Genotypes. Sugar Tech 23, 1157–1170 (2021). https://doi.org/10.1007/s12355-021-00981-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-021-00981-9