Abstract
Intensive cropping and exhaustive nature sugarcane crop in tropical India have led to depletion of inherent soil fertility resulting in serious threat to sustainable sugarcane production. Improving soil organic matter and soil fertility are important factors for sustainable sugarcane production. Microbial consortia comprising of Trichoderma viride, Humicola spp, Paecilomyces lilacinus, Gluconacetobater diazotropicus, Azospiriillum brasilense, and Bacillus subtilis have great potential to recycle crop residue and restore soil fertility which eventually promote sugarcane growth. Field experiments with in situ trash management in plant crop and bio-intensive modulation of sugarcane ratoon rhizosphere in ratoon crops were conducted during 2014, 2015 and 2016 at ICAR—Sugarcane Breeding Institute, Coimbatore, Tamil Nadu, India. The results revealed that green manuring with sunnhemp, in situ trash management and application of 280:62.5:120 kg NPK ha−1 in plant crop sustained the soil health by way of reducing soil bulk density (1.26 kg dm−3), lower soil penetration resistance in the ratoon rhizosphere (1.79, 1.82 and 1.75 MPa) and higher soil organic carbon (0.52%) which in turn increased ratoon cane yield 12.24% (99.02 t ha−1) and sugar yield by 8.64% (12.83 t ha−1) over the control. Further, bio-intensive modulation of ratoon rhizosphere by off barring, trash shredding plus soil incorporation with microbial consortia and application of 350:62.5:120 kg NPK ha−1 in ratoon crop recorded significantly higher cane yield (100.95 t ha−1) and sugar yield (13.19 t ha−1) over the control. The improvement in cane yield and sugar yield to the tune of 16.35 and 14.10% due to bio-intensive modulation of ratoon rhizosphere over control have clearly established that productivity of sugarcane ratoon can be significantly improved with balanced use of fertilizers, green manuring, crop residue recycling and microbial consortia application. Similarly, bio-intensive modulation of sugarcane ratoon rhizosphere (S3) with lowest soil bulk density (1.26 kg dm−3) and soil penetration resistance values (1.69, 1.81, and 1.75 MPa) was found effective in minimising the soil compaction and improving nutrient availability (NPK) with build-up of soil microbial population than control (Trash removal). Based on the results of 3-year field experiments, it is concluded that sunnhemp green manuring and in situ sugarcane trash management coupled with application of 280:62.5:120 kg NPK ha−1 in plant crop followed by bio-intensive modulation of ratoon rhizosphere including off barring, trash shredding and soil incorporation with microbial consortia and application of 350:62.5:120 kg NPK ha−1 in ratoon crop can be recommended for sustaining soil health and sugarcane productivity under wide-row sugarcane planting systems of tropical Indian condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum species hybrids) is an important cash crop of India which is cultivated in an area of 4.5 million hectare with an average productivity of 68 t ha−1. Intensive cropping and exhaustive nature of sugarcane crop in the tropical India have led to depletion of inherent soil fertility resulting in serious threat to sustainability of sugarcane production. It was observed in recent years that yield of sugarcane has reached a plateau due to decline in factor productivity. The loss of organic matter and soil degradation are the root cause of decline in factor productivity. Intensive sugarcane crop management with use of machineries during soil preparation, planting, intercultural operations and harvesting add traffic of machines and vehicle, causing changes both to physiochemical attributes viz. soil compaction, soil density, total porosity, water-holding capacity, and aggregate stability (Torres et al. 2015). However, soil compaction has been identified as the primary cause of soil degradation because it negatively influences all other physical attributes (Materechera 2009; Gorucu et al. 2006). Soil compaction refers to the arrangement of soil matrix caused applied forces, reducing the pore volume and increasing the soil density. Soil density and penetration resistance (PR) are soil compaction indices (Abu-Hamdeh 2003) which determine the root growth in the rhizosphere, as soil compaction creates a less favourable environment for the development of root system of sugarcane (Otto et al. 2011; Kingwell and Fuchsbichler 2011) restricting its root growth (Souza et al. 2012). According to Alameda et al. 2012 plant responds to soil compaction with changes in the development and operation of roots. Those changes can affect productivity and product quality. Sugarcane root system consists of rhizomes and fasciculated roots, 85% of which are in the 0.0–0.50 m layer, and 60% in the 0.20–0.30 m layer (Oliveira Filho et al. 2015). Souza et al. (2014) emphasises that the physical changes in soil structure caused by compaction mainly occur in the top 0.0–0.40 m layer. Without chemical or physical obstruction, cane roots can reach to a depth greater than 2.00 m in the rhizosphere. Per se the rhizosphere includes not only roots but also root exudates, soil microbes, and fungi. The rhizosphere offers a complex micro-habitat where root exudates provide a diverse mixture of organic compounds that are used as nutrients or signals by the soil microbial population (Jones et al. 2009) which results in a high degree of interaction between microbes, plant and soil. This necessitates the bio-intensive (microbial consortia mediated) modulation of ratoon rhizosphere with agronomic ratooning operations such as off barring (loosening of soil in the 0–0.30 m rhizosphere), addition of organic amendments, crop residues from green manuring crop, sugarcane trash for enhancement of physiochemical attributes of rhizosphere and soil organic matter. Organic matter is key factor in maintaining the soil fertility as it is the reservoir of nutrients and provides metabolic energy for biological processes. Soil organic matter and soil fertility are important factors in the sustainability of sugarcane (Saccharum spp.) production. Sugarcane trash is the potential source of organic matter which can be recycled in a better way with the help of microbial consortia as in situ trash application in plant crop followed by bio-intensive modulation of sugarcane rhizosphere with agronomic practices in ratoon crop. Microbial consortia comprising of Trichoderma viride, Humicola spp, Paecilomyces lilacinus, Gluconacetobater diazotropicus, Azospiriillum brasilense and Bacillus subtilis have great potential to recycle crop residue and restore soil fertility eventually promote sugarcane rhizosphere. Gluconacetobacter, Azospirillum, Azotobacter species have been observed to improve plant growth through stimulation of root development. It has potential to fix atmospheric nitrogen up to 300 kg ha−1. Besides this, it is also able to solubilise insoluble phosphate in culture broth due to acid production (Bhowmik and Konde 1997; Mowade and Bhattacharyya 2000). In this way, rhizosphere microorganisms directly and indirectly influence the composition and productivity of natural plant communities (Schnitzer et al. 2011). Hence, below ground microbial species richness has been proposed as a predicator of above ground plant diversity and productivity (De Deyn et al. 2004; Van der heijden et al. 2008; Lau and Lennon 2011). Acetobacter exhibit antagonistic potential against Colletotrichum falcatum which causes red rot of sugarcane. It also produces plant growth hormones like IAA (Indole acetic acid) and Gibberellins in culture (Fuentes-Ramirez et al. 1993). It is evident that from the previous literature that soil rhizospheric microbes plays an important role in sugarcane ecosystem and act as insurance for sugarcane ratoon productivity and soil health under different environment. Keeping these in view, to address the issues of soil compaction, depleting soil organic matter, poor soil health and stagnating sugarcane ratoon cane yield, the present study was conducted to examine the effects of bio-intensive modulation of sugarcane ratoon rhizosphere for enhanced soil health and sugarcane productivity under tropical Indian conditions.
Materials and Methods
Field experiments were conducted for one plant crop followed by two ratoon crops during 2014, 2015 and 2016 at ICAR—Sugarcane Breeding Institute, Coimbatore, India, located at 11°N latitude, 77°E longitude, and 427 m above mean sea level with tropical wet and dry climates having the wet season lasting from October to December due to north east monsoon. The mean atmospheric temperatures ranged in between 21.6 and 33.1 °C with a mean relative humidity of 56–85%. As against the normal rainfall of 674.2 mm, only 678.9 mm and 386.5 mm of rainfall was received during 2015 and 2016 crop seasons indicating the erratic behaviour of rainfall. Before planting the crop, the soil samples were collected by core sampler at 0–15 cm depth from five spots of experimental plots, air-dried and passed through 2-mm sieve. The soil samples were analysed for determination of organic carbon (Walkley and Black method), available nitrogen [Potassium permanganate (KMnO4) method], 0.5 M sodium bicarbonate (NaHCO3, pH 8.5) extractable phosphorus and 1 N ammonium acetate (NH4OAC)—extractable potassium (K), following Jackson (1973). The soil of the experimental site was sandy clay loam (32.58% clay, 13.35% silt, and 54.07% sand) moderately drained taxonomically classified as typic haplustalf. The initial soil pH (8.54) was determined by 1:2.5 soil/water suspensions by pH meter and initial soil EC (0.30 dS m−1) was determined by conductivity meter. The initial organic carbon, available nitrogen, phosphorus and potassium of the experimental soil were 0.35%, 258.40, 31.92 and 553.84 kg ha−1, respectively. Soil moisture characteristics such as field capacity (30.56%), permanent wilting point (9.82%) and available soil moisture (20.74%) were also recorded. The good quality water with pH of 6.9 and SAR 8.37 values was used for irrigation. The sugarcane trash used for in situ trash management and bio-intensive modulation of ratoon rhizosphere during plant and ratoon sugarcane crop were analysed for nutrient content and recorded 0.54, 0.15, 0.85% NPK content and C/N ratio of 69:1. After the harvest of second sugarcane ratoon crop, to evaluate the effect of bio-intensive modulation of ratoon rhizosphere on soil compaction, soil penetration resistance was used as an indicator of soil compaction due to speed and ease of measurement. Soil penetration resistance data were recorded in each treatment using cone penetrometer at three spots, i.e. centre of ridge and on both the right and left shoulder of the ridge (30 cm away from the centre of ridge where off-baring and other ratooning operations were done). For soil bulk density (BD) measurements, undisturbed samples were collected from each plot with metallic cylinders of 0.05 m diameter and 0.21 m height, in the 0–0.25 m layer. The population of bacteria, fungi and actinomycetes in soil was determined by serial dilution pour plate method as described by Wollum (1982) using Thornton’s medium for bacteria, Ken Knight and Munaier’s medium for actinomycetes and Martin’s Rose-Bengal streptomycin agar medium for fungi.
The experiment with sugarcane (var. Co 86032) under wide row (150 cm) was planted during the last week of January 2014. The plot size was 9 m × 6 m (6 rows of 6 m length spaced 1.5 m apart). Ten days after planting, four rows of sunnhemp (Crotalaria juncea L.) green manuring crop were grown in between two rows of sugarcane. Sunnhemp was harvested at 45th day and mulched between sugarcane rows. At full earthing up, i.e. 90 days after planting, sunnhemp mulches were incorporated in sugarcane planted furrows which contributed an additional biomass of 3.55 t ha−1. In plant crop, 280:62.5:120 kg ha−1 NPK was applied wherein, phosphorous of 62.5 kg ha−1 was given as basal dressing before planting whereas nitrogen and potassium were applied in two equal splits at 45 and 90 days after planting. De-trashing was done 5, 7 and 10 months after planting and sugarcane trash was kept between the furrows as in situ trash mulch. In a randomized block design with three replications, in situ trash management treatments viz. control (trash removal), in situ trash mulching (ISTM), ISTM + use of microbial consortium and ISTM + green manure crop as mulch were imposed in plant crop. Microbial consortia comprising T. viride (5 × 106 colony-forming units (cfu) g−1 culture), Humicola spp. (2 × 106 cfu g−1 culture), P. lilacinus (2 × 106 cfu g−1 culture), Gluconacetobacter diazotrophicus (1.4 × 107 cfu g−1 culture), Azospirillum brasilense (2.1 × 108 cfu g−1 culture) and B. subtilis (1 × 107 cfu g−1 culture) at 10 kg ha−1 each were mixed with composted coir pith and applied twice in plant crop at 240 and 300 DAP. The plant crop was harvested manually during February 2015.
With four sub-plot treatments (S1, S2, S3 and S4) experiment was initiated in February 2015, after the harvest of the plant crop, the ratooning operation (“stubble shaving”) was done in all the treatments. In S1 treatment (control) trash was removed off the field and after off barring 100% of RDF (350:62.5:120 kg NPK ha−1) was applied in both the ratoon crops. In case of S2 treatment, after off barring, shredded trash was retained as mulch over the soil and 100% RDF was applied to the ratoon crops. With slight modification of nutrient scheduling in S3 (100% of RDF, i.e. 350:62.5:120 kg NPK ha−1) and S4 (75% of RDF, i.e. 262.5:46.88:90 kg NPK ha−1), bio-intensive modulation of ratoon rhizosphere treatments (S3 and S4) comprising sugarcane green tops and trash available after harvest of plant crop was shredded into small pieces and, with microbial consortia, it was incorporated to the soil. The bio-intensive modulation of ratoon rhizosphere treatments in sub-plot (S3 and S4) also involves off barring/shoulder breaking for loosening of rhizosphere (0–0.30 m deep) wherein small trenches of 0.30 wide and 0.30 m deep were opened manually with spade on both side of sugarcane stool. In open soil trenches, shredded sugarcane trash mixed with composted coir pith and microbial consortia was applied followed by irrigation. In both ratoon crops, microbial consortia application was done during off barring (at 10 days after ratoon initiation) and at earthing up, i.e. 30 and 60 days after ratoon initiation. The details of nutrient scheduling followed in the plant and ratoon are given in Table 1. The experiment aimed at studying the residual effects of plant crop in situ trash management and green manuring treatments on succeeding ratoon crops and soil health. The in situ trash management (four treatments) imposed in preceding plant crop was considered as main plot. Each main plot was further subdivided into four sub-plots with bio-intensive rhizosphere modulation treatments for two successive ratoons. Thus with 16 treatments combinations (Table 1), an experiment in split-plot design with three replications was conducted for two successive sugarcane ratoons. The biometric observations of cane growth and yield parameters such as cane height (cm), number of millable cane (NMC), single cane weight (kg), cane diameter (mm) and cane yield (t ha−1) were recorded. First and second ratoon crops were harvested after 10th month in 2015 and 2016, respectively. Juice quality parameters such as Brix, pol and purity (Meade and Chen 1977) were estimated in randomly selected five canes from each treatment. The percentage purity was calculated by dividing pol per cent over corrected Brix and multiplied by 100. Commercial cane sugar % was worked out using the formula [(Sucrose % × 1.022)–(Brix × 0.292)]. CCS (Commercial Cane Sugar t ha−1) yield was calculated by following the formula [(CCS % × cane yield t ha−1)/100]. Analysis of variance was performed for bulk density, penetration resistance values, soil chemical properties, cane yield, sucrose and sugar yield, following a split-plot design combined across years (Gomez and Gomez 1984). Differences between mean values were separated out using least significant differences (LSD) at P < 0.05.
Results and Discussion
Soil Bulk Density (BD) and Soil Penetration Resistance (SPR)
Data on soil physical parameters viz. soil bulk density and soil penetration resistance as influenced due to in situ trash mulching and bio-intensive modulation of sugarcane ratoon rhizosphere after harvest of second ratoon crop are presented in Table 2. Marked differences in soil bulk density were apparent in the experiment wherein residual effect of in situ trash mulching + green manuring + application of 100% of RDF in plant crop significantly reduces the bulk density (1.26 kg dm−3) and soil penetration resistance (1.79, 1.82 and 1.75 MPa) than the rest of the treatments. This effect was probably derived mainly due to greater amounts of sunnhemp biomass incorporated in soil at 90 days after planting which eventually increased soil organic carbon (0.52%). This is in agreement with the results of Van Antwerpen and Mayer (1997) who reported that the addition of trash kept the soil strength low over 100 mm soil depth and reduced the soil bulk density because of higher percentage of organic matter than the treatment with no trash cover. Ekwue and Stone (1995) also reported that penetration resistance and shear strength decreased with increasing organic matter content. All the three bio-intensive modulation of sugarcane ratoon rhizosphere treatments were found very effective in reducing the soil bulk density, soil compaction and soil penetration resistance values within “low resistance class” (1–2.5 MPa) as given by Canarache (1990). Critical soil resistance value (cone penetrometer reading) for sugarcane cultivation are scarce, although Vepraskas and Miner (1986) reported resistance values of 2.8–3.2 MPa for tillage pans in coarse textured soils of North Carolina. Swinford and Bovery (1984) reported a significant decline in cane rooting density below soil depths where cone penetrometer resistances of 2.8–3.2 MPa were measures. Moreover, in case of bio-intensive modulation of ratoon rhizosphere treatments, the lowest SPR values recorded at all the three spots, i.e. on centre of sugarcane stool and 0.30 m apart on both the side from centre, is indicative of the fact that off barring operations, soil incorporation of sunnhemp biomass and shredded sugarcane trash and microbial consortia application might have loosened the ratoon rhizosphere thus reduced the soil compaction than the furrow. Higher SPR values (1.93 ± 0.23 MPa) in sugarcane furrows were also recorded by Otto et al. (2011) since this area was subjected to direct pressure from the equipment used in sugarcane harvest.
Soil Microbial Population
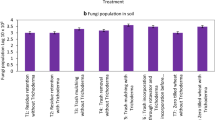
Soil bacterial population (Fig. 1) increased from ratoon initiation to 120 days after ratoon initiation (DARI) thereafter decreasing trend was observed from 240 DARI to harvest. Soil bacterial population was significantly influenced by in situ trash mulching and bio-intensive modulation of sugarcane ratoon rhizosphere treatments wherein ISTM + microbial consortia (MC) + 100% RDF applied in plant crop recorded significantly higher bacterial population at 120 DARI (13.65 × 105 cfu g−1 soil) and at harvest (10.01 × 105 cfu g−1 soil) than all other treatments. Similarly in case of sub-plot treatments, S3 (off barring + trash shredding and soil incorporation + 100% RDF + MC application) and S4 (off barring + trash shredding and soil incorporation + 75% RDF + MC application) were found statistically on par and both recorded significantly higher bacterial population than S1 and S2 treatments. Soil fungal population increased from ratoon initiation to 240 DARI thereafter decreased at harvest. Soil fungal population depicted in Fig. 2 clearly indicated that green manuring in plant crop increased fungal population (9.16 × 103 cfu g−1 soil) significantly than trash removal (4.29 × 103 cfu g−1 soil) and ISTM (4.29 × 103 cfu g−1 soil) treatments, whereas it was on par with ISTM + microbial consortia + 100% RDF application (8.70 × 103 cfu g−1 soil) at 240 DARI. In case of sub-plot treatments, the fungal population in S3 (off barring + trash shredding and soil incorporation + 100% RDF + MC application) and S4 (off barring + trash Shredding and soil incorporation + 75% RDF + MC application) was found significantly higher than S1 and S2 treatments. Similarly, in sub-plot treatments, soil actinomycetes population depicted in Fig. 3 indicated that actinomycetes population was significantly influenced by in situ trash mulching and bio-intensive modulation of sugarcane ratoon rhizosphere treatments wherein ISTM + microbial consortia + 100% RDF (M4) applied in plant crop recorded significantly higher actinomycetes population at 120 DARI (3.14 × 105 cfu g−1 soil) and at harvest (3.16 × 105 cfu g−1 soil) than rest of the treatments. The actinomycetes population in S3 (off barring + trash shredding and soil incorporation + 100% RDF + MC application) and S4 (off barring +trash shredding and soil incorporation + 75% RDF + MC application) was found significantly higher than S1 and S2 treatments. Build up of soil bacterial, fungal and actinomycetes population in S3 and S4 treatments in ratoon crops may be attributed to the better availability of carbon substrate due to addition of sugarcane trash (Tilak et al. 1999). Moreover, soil incorporation of shredded sugarcane trash and microbial consortia (MC) in both ratoon crops increased soil organic matter (evident from OC values), available nutrients and soil moisture (Tayade et al. 2016), which led to improved physical (lower bulk density and less soil compaction) and chemical properties of soil. This might have positively facilitated the structure of microbial communities with increased number of microbial colonies. This corroborates the earlier findings of Qing et al. (2014) who has reported an increase in total number of bacterial, fungal and actinomycetes by 2.38-, 1.80- and 2.74-fold, respectively, when trash was added to the soil as compared to conventional one.
Soil bacterial population (pooled data 2 year) at different growth stages of ratoon as influenced by in situ trash mulching and bio-intensive modulation of ratoon rhizosphere treatments. M1: Trash removal + 100% RDF; M2: ISTM + 100% RDF; M3: ISTM + Green Manuring + 100% RDF; M4: ISTM + MC + 100% RDF; S1: Trash removal + Off barring + 100% RDF application; S2: Off barring + 100% RDF application + Trash retention on soil surface; S3: Off barring + Trash shredding and soil incorporation + 100% RDF + MC application; S4: Off barring + Trash shredding and soil incorporation + 75% RDF + MC application
Soil fungal population (pooled data 2 year) at different growth stages of ratoon as influenced by in situ trash mulching and bio-intensive modulation of ratoon rhizosphere treatments. M1: Trash removal + 100% RDF; M2: ISTM + 100% RDF; M3: ISTM + Green Manuring + 100% RDF; M4: ISTM + MC + 100% RDF; S1: Trash removal + Off barring + 100% RDF application; S2: Off barring + 100% RDF application + Trash retention on soil surface; S3: Off barring + Trash shredding and soil incorporation + 100% RDF + MC application; S4: Off barring + Trash shredding and soil incorporation + 75% RDF + MC application
Soil actinomycetes population (pooled data 2 year) at different growth stages of ratoon as influenced by in situ trash mulching and bio-intensive modulation of ratoon rhizosphere treatments. M1: Trash removal + 100% RDF; M2: ISTM + 100% RDF; M3: ISTM + Green Manuring + 100% RDF; M4: ISTM + MC + 100% RDF; S1: Trash removal + Off barring + 100% RDF application; S2: Off barring + 100% RDF application + Trash retention on soil surface; S3: Off barring + Trash shredding and soil incorporation + 100% RDF + MC application; S4: Off barring + Trash shredding and soil incorporation + 75% RDF + MC application
Sugarcane Yield Attributes, Cane Yield, Sucrose (%) and Sugar Yield
The plant crop was harvested manually during February 2015 wherein in situ trash management (ISTM) and microbial consortia application significantly registered the highest cane yield of (106.15 t ha−1) over the rest of the ISTM treatments (Tayade et al. 2016).
Growth attributes and yield parameters of two ratoon crops are presented in Table 3. The effect of in situ trash management on cane height, the number of millable canes (NMC), single cane weight and cane girth was non-significant; however, ISTM + Green Manuring + 100% RDF application(M3) has improved NMC marginally and cane yield and sugar yield (99.02 and 12.83 t ha−1) significantly over the rest of in situ trash management treatments. Increase in NMC and cane yield in ISTM + Green Manuring + 100% RDF applied plot were attributed to improvement in soil micro-climate (higher soil moisture content, reduction in soil temperature, soil bulk density and soil compaction) and soil fertility build-up (higher available nitrogen, phosphorous, potassium and organic carbon). The result of present experiment corroborated the finding of Yadav et al. (2009) who reported cane yield enhancement due to Trichoderma application in all the trash management practices. Similarly, Dhanapal et al. 2018 also reported saving of 25% irrigation water and cane yield improvement over control due to application of chopped trash in furrow at the time of sugarcane planting.
In case of bio-intensive modulation of ratoon rhizosphere with off barring + trash shredding and soil incorporation + 100% RDF and microbial consortia application (S3) significantly recorded higher NMC (87.25 × 103), cane yield (100.95 t ha−1) and sugar yield (13.19 t ha−1) over control (86.76 and 11.56 t ha−1 cane and sugar yield, respectively). Improvement of 16.35% and 14.10% cane yield due to bio-intensive modulation of ratoon rhizosphere over control was attributed to higher NMC, taller and thicker cane and partly to multiple benefits in term of nitrogen fixation, phosphorus solubilisation, plant growth hormone from microbial consortia application. Moreover, basal cutting of old and decayed roots during off barring and application of shredded trash with microbial consortia have reduced the soil bulk density (1.26 kg dm−3), soil penetration resistance (1.69, 1.81 and 1.75 MPa) and increased the organic carbon (0.49%), available nutrients which eventually increased the cane yield. The results are in agreement with the result of Thakur et al. (2010) who has reported higher cane yield with application of Trichoderma inoculated trash @ 10 t ha−1 along with 150 kg N ha−1 and Azotobactor @ 4 kg ha−1. Sucrose per cent in juice did not differ significantly due to various treatments. The statistical analysis (Data pooled for 2 years) revealed that interaction effects were absent.
Post-Harvest Soil Available Nutrients
In general, data on post-harvest soil fertility status (Table 2) indicated a slight increase in available phosphorus (42.29 kg P ha−1) over initial soil phosphorus status (31.92 kg ha−1) and trash removal plots (28.83 kg P ha−1). Improved phosphorus availability could be due to less phosphorus sorption of organic compounds released by microbial consortia mediated decomposition of trash and root residues (Cong and Merckx 2005). Decreasing trends in available soil nitrogen and potassium content over initial soil status (258.40 kg N ha−1and 553.84 kg K ha−1) were observed due to nutrients uptake by one plant and two ratoon crops. Sugarcane being a nutrient exhaustive long duration crop, it requires huge amount of nutrients which could have depleted soil available nitrogen and potassium. Shukla et al. 2008 also reported decline in potassium content of soil due to its higher uptake by sugarcane. The results of present investigation corroborated the findings of Yadav et al. (2009) who reported nitrogen and potassium depletion due to ratoon cultivation. Organic carbon content in soil is a key factor for soil health and fertility, and the impact of various in situ trash managements, green manuring and bio-intensive modulation of ratoon rhizosphere is given in Table 2. The ISTM + Green Manuring + 100% RDF application resulted in increased organic carbon content of soil from initial soil organic carbon of 0.35–0.52% over a period of 3 years is attributed to incorporation of sunnhemp, sugarcane trash and subsequently its faster decomposition might have enhanced build-up of organic carbon. Jadhav et al. 2005 also reported increase in organic carbon due to continuous addition and release of nutrients during the process of trash decomposition in soil trash under multiple sugarcane ratooning.
The ISTM + green manuring + 100% RDF application (M3) has recorded significantly higher available soil nitrogen (188.88 kg ha−1) and phosphorus (42.29 kg ha−1) than control (166.03 kg N ha−1 and 28.83 kg P ha−1) and found effective in sustaining the soil fertility. Improved nitrogen and phosphorus availability was attributed to higher organic carbon content (0.52%) of the soil where in situ trash management and green manuring was practised. Shukla et al. (2008) observed a positive correlation between organic carbon and phosphorus availability. With regard to sub-plot treatments, off barring + trash shredding and soil incorporation + 100% of RDF + microbial consortia application (S3) recorded higher available nitrogen (185.80 kg ha−1), potassium (372.36 kg ha−1) and organic carbon (0.49%) than control (trash removal). Application of sugarcane trash coupled with microbial consortia application improved soil organic carbon in turn helped in sustaining soil health for longer period (Yadav et al. 2009). Residual effect of sunnhemp green manuring and in situ trash management practised in preceding plant crop was also visible on soil EC and pH, wherein lower values (0.32 dSm−1 and 8.31) was recorded than the other main plot treatments. Graham et al. (2002) reported that trash retention has substantial effect on both the soil organic matter, soil pH as well as improved soil physical and chemical qualities.
Conclusions
Based on the results of 3 years of field experiments, it is concluded that sunnhemp green manuring and in situ sugarcane trash management coupled with application of 280:62.5:120 kg NPK ha−1 in plant crop followed by bio-intensive modulation of ratoon rhizosphere including off barring, trash shredding, soil incorporation with microbial consortia and application of 350:62.5:120 kg NPK ha−1 in ratoon crops can be recommended for sustaining soil health and sugarcane productivity under wide-row sugarcane planting systems of tropical India.
References
Abu-Hamdeh, N.H. 2003. Soil compaction and root distribution for okra as affected by tillage and vehicle parameters. Soil Tillage Research 74: 25–35.
Alameda, D., N.P.R. Anten, and R. Villar. 2012. Soil compaction effects on growth and root traits of tobacco depend on light, water regime and mechanical stress. Soil Tillage Research 120: 121–129.
Bhowmik, S.N., and B.K. Konde. 1997. Isolation of efficient dinitrogen fixing Acetobacter from indigenous sugarcane genotypes. Journal of Maharashtra Agricultural Universities 22: 364–365.
Canarache, A. 1990. PENETR-A generalized semi-empirical model estimating soil resistance to penetration. Soil and Tillage Research 16: 51–70.
Cong, P.T., and R. Merckx. 2005. Improving phosphorous availability in two upland soils of Vietnam using Tihonia diversifolia. Plant and Soil 269: 11–23.
De Deyn, G., C. Raaijmakers, and W. Van der Putten. 2004. Plant community development is affected by nutrients and soil biota. Journal of Ecology 92: 824–834.
Dhanapal, R., A.S. Tayade, A. Bhaskaran, and P. Geetha. 2018. Efficient water management in sugarcane with composted coir pith and sugarcane trash under tropical Indian conditions. Sugar Tech. https://doi.org/10.1007/s12355-018-0593-3.
Ekwue, E.I., and R.J. Stone. 1995. Organic matter effects on the strength properties of compacted agricultural soils. Transactions of ASAE 38: 357–365.
Fuentes-Ramirez, L.E., T. Jimnez Salgado, I.R. Abarca-Ocampo, and J. Caballero-Mellado. 1993. A.diazotrophicus, an indole acetic acid producing bacterium isolation from sugarcane cultivars of Mexico. Plant and Soil 154: 145–150.
Gomez, K.A., and A.A. Gomez. 1984. Statistical procedures for agricultural research. Singapore: Wiley.
Gorucu, S., A. Khalilian, Y.J. Han, R.B. Dodd, and B.R. Smith. 2006. A algorithm to determine the optimum tillage from soil penetrometer data in coastal plain soils. Applied Engineering in Agriculture 22: 625–631.
Graham, M.H., R.J. Haynes, and J.H. Mayer. 2002. Changes in soil chemistry and aggregate stability induced by fertilizer applications burning and trash retention on a long term sugarcane experiment in South Africa. European Journal of Soil Science 53: 589–598.
Jackson, M.L. 1973. Soil chemical analysis. New Delhi: Prentice-Hall of India Pvt. Ltd.
Jadhav, M.B., S.M. Jagtap, R.V. Kulkarni, and A.B. Marathe. 2005. Studies on in situ trash management and fertilizer application techniques in multiple ratooning of sugarcane. Journal of Soils and Crop 15(2): 297–303.
Jones, D.L., C. Nguyen, and R.D. Finlay. 2009. Carbon flow in the rhizosphere: Carbon trading at the soil-root interface. Plant and Soil 321: 5–33.
Kingwell, R., and A. Fuchsbichler. 2011. The whole-farm benefits of controlled traffic farming: An Australian appraisal. Agricultural System 104: 513–521.
Lau, J.A., and J.T. Lennon. 2011. Evolutionary ecology of plant-microbe interactions: Soil microbial structure alters selection on plant traits. New Phytologist 192: 215–224.
Materechera, S.A. 2009. Tillage and tractor traffic effects on soil compaction in horticultural fields used for peri-urban agriculture in semi arid environment of the North West Province, South Africa. Soil and Tillage Research 103: 11–15.
Mowade, S., and P. Bhattacharyya. 2000. Resistance of P solubilizing Acetobacter diazotrophicus to antibiotics. Current Science 79: 1591–1594.
Meade, G.P., and J.C.P. Chen. 1977. Cane sugar handbook. 10th ed, 515–545. New York: Wiley.
Oliveira Filho, F.X., N.O. Miranda, J.F. Medeirors, P.C.M. Silva, F.O. Mesquita, and T.K.G. Costa. 2015. Zona de manejo para prepare do solo na cultura da cana-de-acucar. Rev Bras de Eng Agr e Amb 19: 186–193.
Otto, R., A.P. Silva, H.C.J. Franco, E.C.A. Oliveira, and P.C.O. Trivelin. 2011. High soil penetration resistance reduces sugarcane root system development. Soil and Tillage Research 117: 201–210.
Qing, L., Wei P.O. Gang, Chen Gui-Fen, L. Bin, Huang Dong Liang, and R.L. Yang. 2014. Effect of trash addition to the soil on microbial communities and physio-chemical properties of soils and growth of sugarcane plants. Sugar Tech 16: 400–404.
Schnitzer, S.A., J.N. Klironomos, J. Hillerislambers, L.L. Kinkel, P.B. Reich, K. Xiao, M.C. Rillig, B.A. Sikes, R.M. Callaaway, S.A. Mangan, E.H. Van Nes, and M. Schelffer. 2011. Soil microbes drive the classic plant diversity-productivity pattern. Ecology 92: 296–303.
Shukla, S.K., R.L. Yadav, A. Suman, and P.N. Singh. 2008. Improving rhizosphere environment and sugarcane ratoon yield through bio-agent amended farm yard manures in udic Ustochrept soil. Soil and Tillage Research 99: 158–168. https://doi.org/10.1016/j.still.2008.02.007.
Souza, H.A., A.V. Marcelo, and J.F. Centurian. 2012. Carbono organico e agregacao de um Latossolo Vermelho com colheita mecanizada de cana-de-acucar. Rev.Ci.Agr. 43: 658–663.
Souza, G.S., Z.M. Souza, R.B. Silva, R.S. Barbosa, and F.S. Araujo. 2014. Effect of traffic control on the soil physical quality and the cultivation of sugarcane. Rev Br.Ci do Sol 38: 135–146.
Swinford, J.M., and M.C. Swinford. 1984. The effect of soil compaction due to infield transport on ratoon cane yields and soil physical characteristics. Proc. S.Afr Sug Technol Ass 58: 198–203.
Tayade, A.S., P. Geetha, R. Dhanapal, and K. Hari. 2016. Effect of in situ trash management on sugarcane under wide row planting system. Journal of Sugarcane Research 6(1): 35–41.
Thakur, S.K., C.K. Jha, Geeta Kumari, and V.P. Singh. 2010. Effect of Trichoderma inoculated trash, nitrogen level and biofertilizers on performance of sugarcane (Saccharum officinarum) in calcareous soils of Bihar. Indian Journal of Agronomy 55(1): 308–311.
Tilak, K.V.B.R., A.K. Saxena, and N. Dutta. 1999. Soil microflora: Response to soil-crop management. In Management of tropical agroecosystems and the beneficial soil bioata, ed. M.V. Reddy, 137–151. New Delhi: Oxford and IBH Publishing Co. Pvt. Ltd.
Torres, J.R.L., M.G. Pereira, R.L. Assis, and Z.M. Souza. 2015. Atribtos fisicos de um Latossolo Vermelho cultivado com plantas de cobertura, em semeadura direta. Rev Br de Ci do sol 39: 428–437.
Van Antwerpen, R., and J.H. Mayer. 1997. Soil degradation -1: Effect of fertilizer use on penetrometer resistance. Proc S. Afr. Sug. Technol Ass 71: 18–21.
Van der Heijden, M.G.A., R.D. Bardgett, and N.M. Van Straalen. 2008. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystem. Ecology Letters 11: 296–310.
Vepraskas, M.J., and G.S. Miner. 1986. Effects of sub-soiling and mechanical impendence on tobacco root growth. Soil Science Society of America, Proceedings 50: 423–427.
Wollum, A.G. 1982. Cultural methods for soil microorganisms. In Methods of soil analysis. Part 2. Chemical and microbiological properties, eds. A.L. Page, R.H. Miller, D.R. Keeney, Agronomy monograph No. 9. Madison (WI): ASA and SSSA, 781–814.
Yadav, R.L., S.K. Shukla, A. Suman, and P.N. Singh. 2009. Trichoderma inoculation and trash management effects on soil microbial bio-mass, soil respiration, nutrient uptake and yield of ratoon sugarcane under subtropical conditions. Biology and Fertility of Soils 45: 461–468.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We, the authors, declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tayade, A.S., Geetha, P., Anusha, S. et al. Bio-intensive Modulation of Sugarcane Ratoon Rhizosphere for Enhanced Soil Health and Sugarcane Productivity Under Tropical Indian Condition. Sugar Tech 21, 278–288 (2019). https://doi.org/10.1007/s12355-018-0669-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-018-0669-0