Abstract
Thirty-eight isolates of Trichoderma spp. established from the rhizosphere of sugarcane were characterized using a multiplex PCR assay and further assessed for the production of hydrolytic enzymes chitinase and cellulase. The results of multiplex PCR assay successfully identified 29 isolates and revealed T. harzianum to be the predominant species (21 isolates) in sugarcane rhizosphere followed by T. longibrachiatum (8 isolates). In enzymatic assays, chitinase production was recorded in 18 isolates and cellulase production observed in 17 isolates. However, there was a considerable variability in both chitinase and cellulase production potential across the isolates. Three T. longibrachiatum isolates (STr-52, STr-83 and STr-108) exhibited high production of both chitinase and cellulase. Talc formulation of two promising isolates (STr-83 and STr-108) was prepared and evaluated in the field conditions for their potential to suppress red rot under three different delivery systems, viz. sett treatment, soil application and their combination. All Trichoderma treatments resulted in considerable reduction in red rot (29.5–56.3%) over untreated control. However, the level of reduction accorded was much higher when the Trichoderma isolates were applied as a combination of sett and soil treatment (> 53% reduction) as compared to soil application (43–49% reduction) or sett treatment alone (< 35% reduction). Delivery of Trichoderma isolates as a combination of sett and soil treatment also exhibited highest NMCs and yield over untreated control. The application methods of talc formulations of these two isolates offer a feasible and effective option for large-scale application of these isolates for the management of red rot disease in sugarcane growing regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum sp. hybrids) is an important commercial crop, cultivated in 5 m ha area in India. Due to its commercial importance, it holds a strategic position in world agriculture and is now considered as the major renewable energy crop. At global level, 189 million tonnes of sugarcane was produced in the year 2016 with major shares from Brazil and India (FAOSTAT 2014 ). The crop is affected by several biotic and abiotic factors with red rot caused by the fungus Colletotrichum falcatum Went (perfect stage = Glomerella tucumanensis [Speg] von Arx & Muller) being a major constraint to sugarcane production in India (Duttamajumder 2008). Red rot causes considerable losses mainly in terms of cane yield and sugar recovery, and under severe conditions, it may cause total failure of the crop (Duttamajumder 2008). Management of this disease has been tried using multiple approaches including use of disease-resistant varieties, fungicides and biological control agents.
Amongst the various biocontrol agents evaluated for disease management in crops, members of the genus Trichoderma have been the most extensively explored. In addition to disease control, these fungi also provide benefits like plant growth promotion, solubilization of plant nutrients as well as induction of systemic resistance against biotic and abiotic stresses (Harman 2011). Studies on the mechanisms employed by Trichoderma spp. for biocontrol of pathogens have established the integral role of various lytic enzymes and secondary metabolites in disease control. These enzymes/metabolites can act by direct inhibition of the target pathogen or indirectly by inducing systemic resistance, and quite often the biocontrol potential of Trichoderma strains is correlated with their ability to produce specific metabolites/enzymes (Howell 2003; Vinale et al. 2008). Moreover, it has also been observed that application of enzymes in combination with secondary metabolites may result in enhanced antifungal activity against various plant pathogens (Lorito et al. 1994, 1996). Since the ability of Trichoderma strains to act as effective biocontrol agents is strongly influenced by the ecological conditions of the region where they are applied, the use of indigenous strains may provide more consistent and higher disease control under field conditions since such strains have a better adaptability to the prevailing conditions in which it is to be used as a biocontrol agent (Howell 2003).
In our earlier studies with Trichoderma isolates established from sugarcane agro-ecosystem, it was observed that direct application of secondary metabolites of selected Trichoderma isolates as sett treatment can effectively suppress red rot in vitro and in field conditions (Joshi and Misra 2013; Joshi et al. 2016). Since various hydrolytic enzymes also play an important role in disease suppression by Trichoderma in several crops (Howell 2003; Vinale et al. 2008), there is a need to explore Trichoderma isolates from sugarcane agro-ecosystem for the production of antifungal enzymes as well. The production of chitinase, in particular, is attributed to be a major factor determining the mycoparasitic activity and biocontrol potential of Trichoderma strains against various pathogens (Howell 2003; Vinale et al. 2008). Also in case of sugarcane, the involvement of Trichoderma chitinolytic enzymes in the suppression of C. falcatum has been previously reported (Viswanathan et al. 2003). Trichoderma strains also produce cellulase, which plays a major role in biomass recycling (Kubicek et al. 2009; Benoliel et al. 2013). Keeping this in view, the present study was undertaken to characterize Trichoderma isolates and assess their ability to produce the hydrolytic enzymes chitinase and cellulose. Since direct application of purified enzymes or secondary metabolites is not a feasible option for mass production and large-scale field applications of Trichoderma, talc-based formulations of the promising isolates identified in this study were prepared and evaluated for their potential to suppress red rot under different delivery systems.
Materials and Methods
Trichoderma Strains
Thirty-eight Trichoderma strains native to sugarcane rhizosphere were used in the present study (Table 1). These strains were previously isolated from sugarcane rhizosphere soils collected from different geographical regions and farming systems (conventional/organic) of subtropical India and selected strains characterized for their colony characters and growth rates (Joshi and Misra 2013). The 38 Trichoderma isolates were selected based on distinct colony and morphological characters and representing different regions, and were maintained on potato dextrose agar (PDA) slants at 4 °C for further studies.
Multiplex Polymerase Chain Reaction (PCR) of Trichoderma Isolates
For extraction of genomic DNA, Trichoderma isolates were grown on 150 ml potato dextrose broth (HiMedia) in stationary state at 27 ± 2 °C for 4 days. Mycelium of individual isolates was collected on a filter paper (Whatman 40) in a Bucher funnel, washed with sterile distiled water, frozen in liquid nitrogen and then ground using a mortar and pestle to a fine powder. The genomic DNA was extracted from the fungal mat using a DNA extraction kit (Genetix Biotech, New Delhi). DNA was quantified using a spectrophotometer and diluted to a final concentration of 10 ng/µl for further use. Multiplex PCR assay was performed using four species-specific primers (for species T. harzianum, T. virens, T. asperellum and T. longibrachiatum) as per the protocol of Prabhakaran et al. (2015) (Table 2). The multiplex PCR was performed in 25 µl reaction volume containing 2.5 µl of 10× PCR buffer, 1.75 µl of 1.5 mM MgCl2, 0.75 µl of 0.2 mM of each dNTP, 0.5 µl of each forward and reverse primer (10 pmol) and 0.25 µl of Taq DNA polymerase and 16 µl of sterile distiled water. The PCR was conducted in a Thermal Cycler (T100; Bio-Rad Laboratories, USA) with the following temperature profiles: 1 min hot start at 94 °C followed by 30 cycles of denaturation at 94 °C for 45 s, annealing at 60 °C for 45 s and primer extension at 72 °C for 1 min with a final extension at 72 °C for 7 min. PCR products were analysed by electrophoresis in a 1.2% agarose gel in 1× TAE buffer (40 mM Tris, 20 mM acetic acid, 1 mM EDTA [pH 8]) and stained with ethidium bromide.
Enzymatic Assay of Trichoderma Isolates
Chitinase
The chitinolytic activity of the Trichoderma isolates was assessed using a chitinase detection medium (Agrawal and Kotasthane 2012). The 1000 ml basal medium contained 20 g moist colloidal chitin, 0.3 gm MgSO4·7H2O, 3.0 g (NH4)2SO4, 2.0 g KH2PO4, 1 g citric acid monohydrate, 15 g agar, 0.15 g bromocresol purple and 200 μl tween-80. The prepared medium was sterilized and poured into 90 mm Petri plates (Borosil, India). Solidified plates were inoculated in the centre with a 5-mm-diameter plug of Trichoderma isolates cut from the edge of a 3–4 days old culture of Trichoderma and incubated at 28 ± 1 °C with five replications for each isolate. Inoculated plates were observed at 24 h intervals for formation of purple zone. After 5 days, the diameter and intensity of the purple zone were recorded and the isolates were categorized as (1) no chitinase activity (no colour change) (2) low chitinase activity (light purple zone of < 50 mm diameter after 5 days) (3) high chitinase activity (dark purple colour zone of > 50 mm diameter).
Cellulase
Cellulase-producing potential of the Trichoderma isolates was assessed using the carboxymethyl cellulose plate assay (Bradner et al. 1999). For this, 1000 ml basal medium containing carboxymethyl cellulose (10 g), NaNO3 (6.5 g), K2HPO4 (6.5 g), yeast extract (0.3 g), KCl (6.5 g), MgSO4·7H2O (3.0 g) and agar (17.5 g) was prepared, sterilized and poured into 90 mm Petri plates after sterilization. After solidification, the plates were inoculated in the centre with a 5-mm disc of the Trichoderma isolates cut from the edge of a 3–4-day-old culture. The inoculated plates were incubated for 7 days at 28 ± 1 °C, and the growth of the isolates was measured as the diameter of the colony. Thereafter, 10 ml Congo red dye (1% solution) was added to each plate. After 30–45 min, the solution was discarded and the cultures were de-stained by washing with 10 ml of 1 M NaCl for 15–20 min. Cellulase production was indicated by the appearance of a pale halo with orange edges, indicative of areas of hydrolysis. The diameter of the halo zone was measured, and the enzymatic index (EI) was calculated as follows:
Talc-Based Formulation of Promising Trichoderma Isolates and Their Field Evaluation for Red Rot Management
Two most promising Trichoderma isolates (STr-83 and STr-108) were selected, and a field experiment was conducted during 2016–17 crop season to study their efficacy. The experiment was conducted at the research farm of ICAR-Indian Institute of Sugarcane Research, Lucknow, India, located at 26°56′N, 80°52′E and 111 m above mean sea level. The climate of the experimental site is semi-arid subtropical, with dry hot summer and cold winter. For field application, talc-based formulations of the two isolates were prepared. Since previous studies by Joshi et al. (2016) indicated that malt extract broth (MEB) is a suitable medium for higher production of diffusible inhibitory metabolites by Trichoderma, the isolates were inoculated on MEB and incubated for 30 days at 28 ± 1 °C. After 30 days, the MEB along with Trichoderma biomass was mixed with sterilized talc in the ratio of 1:2. Five grams of carboxymethyl cellulose was added as a sticker to the powder, and formulation was air dried to a moisture level of < 8% in the final formulation. The spore count of Trichoderma in the talc formulation was estimated and was stored at 4 °C till further use.
The field experiment was planted in March 2016 in a randomized block design with eight treatments, three replications and plot size of 32.4 m2 (5.4 m × 6 m). The treatment details are as follows: T1: sett treatment of pathogen pre-inoculated setts with Trichoderma isolate STr-83, T2: sett treatment of pathogen pre-inoculated setts with Trichoderma isolate STr-108, T3: soil application of Trichoderma isolate STr-83, T4: soil application of Trichoderma isolate STr-108, T5: sett treatment of pathogen pre-inoculated setts with Trichoderma isolate STr-83 + soil application of Trichoderma isolate STr-83, T6: sett treatment of pathogen pre-inoculated setts with Trichoderma isolate STr-108 + soil application of Trichoderma isolate STr-108, T7: untreated control, T8: healthy uninoculated setts. Three bud setts of variety Co 1148 were used in the experiment with 540 setts planted in each treatment (180 per replication). For all treatments except T8, setts were pre-inoculated with C. falcatum pathotype CF01 before treatment with Trichoderma. The setts were inoculated following the methodology of Duttamajumder and Misra (2014). Briefly, a ten-day-old sporulating culture of CF01 was washed with sterile water, spores were harvested and the conidial concentration was estimated using a haemocytometer. The three bud setts were dipped in the inoculum (104 conidia/ml) for 5 min and then incubated under a polythene cover for 16 h at ambient temperature (~ 28 °C). Prepared talc formulation of the two Trichoderma isolates was applied as sett treatment, soil application in furrows and their combination to assess its impact on red rot suppression. For sett treatment, the talc formulation was mixed with water @50 g l−1 and Cf 01 pre-inoculated setts were soaked in the suspension for 30 min and then planted. For soil application, the talc formulation was applied @20 kg ha−1. Briefly, 20 kg of formulation was mixed in 200 kg of well-decomposed moist FYM and allowed to incubate for 20 days to allow further colonization by Trichoderma and the colonized FYM was then applied in furrows at time of planting. Data on germination, number of millable canes (NMC) and yield were recorded in all treatments. Incidence of red rot-induced settling, tiller and cane mortality was recorded at regular intervals in treatments T1–T7. Data on NMC and yield were subjected to statistical analysis using the Statistical Package for Social Scientists (SPSS) software (ver. 10.0). Least significant difference test (LSD) was applied to compare the means.
Results and Discussion
Multiplex PCR of Trichoderma Isolates
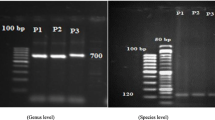
Four primers, specific for species T. harzianum, T. virens, T. asperellum and T. longibrachiatum, were used for identification of four common species of Trichoderma through multiplex PCR assay. Clear amplification of specific size products was observed in 29 of the 38 isolates (Fig. 1, Table 2). The results revealed the amplification of ~ 824 bp size amplicon, corresponding to T. harzianum, in 21 isolates (Fig. 1). Similarly, an amplification product of ~ 452 bp size amplicon, specific to T. longibrachiatum, was observed in eight isolates (Fig. 1). No clear amplification product was observed in five isolates (Fig. 1) while multiple non-specific amplification was observed in four isolates (Fig. 1). Thus, multiplex PCR assay successfully identified 29 of the 38 isolates. T. harzianum was observed to be the most abundant species (> 50% isolates) of Trichoderma in sugarcane rhizosphere soils as more than half (21 out of 29 isolates) of the isolates belonged to this species. Rest of the eight isolates belonged to T. longibrachiatum. Both these species are reported to be common soil residents and have been frequently identified from agricultural soils (Zhang et al. 2005; Hoyos-Carvajal et al. 2009; Mulaw et al. 2010; Lopes et al. 2012). A number of studies have also reported T. harzianum to be the predominant Trichoderma species present in soils and/or rhizosphere of various crops (Kubicek et al. 2003; Hermosa et al. 2004; Mulaw et al. 2010; Lopez-Quintero et al. 2013). In a recent study on Trichoderma populations in sugarcane rhizosphere by Romao-Dumaresq et al. (2016), they observed Trichoderma to be the fourth most abundant fungus associated with sugarcane. However, specific information on species diversity of Trichoderma in sugarcane is lacking.
Multiplex PCR assay of 38 endophytic Trichoderma isolates using four species specific primers. Lanes: M: 100 bp DNA size marker, 1: STr-1, 2: STr-3, 3: STr-8, 4: STr-12, 5: STr-15, 6: STr-23, 7: STr-26, 8: STr-28, 9: STr-29, 10: STr-30, 11: STr-35, 12: STr-44, 13: STr-47, 14: STr-52, 15: STr-64, 16: STr-72, 17: STr-74, 18: STr-79, 19: STr-80, 20: STr-83, 21: STr-85, 22: STr-88, 23: STr-90, 24: STr-91, 25: STr-93, 26: STr-94, 27: STr-96, 28: STr-108, 29: STr-114, 30: STr-116, 31: STr-117, 32: STr-118, 33: STr-119, 34: STr-120, 35: STr-122, 36: STr-123, 37: STr-125, 38: STr-126. The arrows indicate 824 and 452 bp amplification products specific to T. harzianum and T. longibrachiatum, respectively
Enzymatic Assay of Trichoderma Isolates
Eighteen out of 38 isolates recorded chitinase production, and based on the diameter and intensity of the purple zone developed during assay, the isolates were categorized as having (1) no chitinase activity, (2) low chitinase activity and (3) high chitinase activity (Table 3). Of the 18 isolates that showed chitinase production, only six isolates were grouped under high activity, while 12 isolates showed low chitinase production. In the cellulase production assay, only 17 out of the 38 isolates evaluated were observed to exhibit cellulase production in vitro (Table 3). The EI of the isolates ranged between 1.00 (STR-12) to 1.91 (STr-108). Amongst the 17 cellulase-producing isolates, three isolates (STr-52, STr-83, and STr-108) exhibited EI of > 1.5 indicating a high cellulase-producing potential, while for the remaining 14 isolates the EI ranged from 1.0 to < 1.5. Overall, we observed that there was a considerable variability in both chitinase and cellulase production potential of the isolates. These results are in accordance with previous reports that the enzyme-producing potential of different Trichoderma strains may vary considerably (Gajeria and Vakharia 2010; Agrawal and Kotasthane 2012; Lopes et al. 2012). When the chitinase- and cellulase-producing potential of isolates was correlated with the Trichoderma species, it was observed that amongst the 18 chitinase-producing isolates, nine isolates were T. harzianum, and six were T. longibrachiatum while three could not be identified through multiplex PCR assay. Out of the six isolates that exhibited high chitinase activity, four (STr-52, 83, 108 and 123) belonged to T. longibrachiatum while rest two were unidentified. Similarly, in case of cellulase-producing potential, out of the 17 isolates, seven were identified as T. harzianum, six as T. longibrachiatum and four were unidentified. All the three isolates (STr-52, STr-83 and STr-108) that exhibited high cellulase activity were identified as T. longibrachiatum. Trichoderma strains belonging to both T. harzianum and T. longibrachiatum have been reported to be effective producers of chitinase and cellulase (de Marco et al. 2000; El-Katatny et al. 2001; Kovacs et al. 2004; Sanchez et al. 2007). In particular, species belonging to the sect. Longibrachiatum which includes T. longibrachiatum are considered highly effective producers of cellulase (Kubicek et al. 2009), as was also observed in our present study where all three isolates having EI > 1.5 were identified as strains of T. longibrachiatum. The potential of Trichoderma strains to produce chitinase has often been directly correlated with their potential as biocontrol agents and is reported to be a major mechanism employed by Trichoderma spp. for disease control (de Marco et al. 2000; El-Katatny et al. 2001; Howell 2003; Sanchez et al. 2007). In case of sugarcane too, the involvement of Trichoderma chitinolytic enzymes in the suppression of C. falcatum has been reported (Viswanathan et al. 2003). Therefore, selecting strains with established high chitinase-producing potential may provide added advantage in red rot suppression. Similarly, ability of Trichoderma isolates to produce cellulase may facilitate their survival and colonization in soils since cellulase plays a major role in decomposition and recycling of biomass. In addition, isolates with high cellulolytic activity can also be exploited for decomposition of trash in fields. Sugarcane, in particular, produces large quantities (8–10 t/ha) of dry leaves (trash) annually, the disposal of which is a major problem. Farmers often resort to burning of trash resulting in air pollution as well as massive loss of nutrients and soil organic carbon (Jain et al. 2014). As such the application of effective cellulase-producing strains of Trichoderma to trash mulch can ensure faster in situ trash decomposition and simultaneously improve soil health and ratoon yields.
Field Evaluation of Trichoderma Isolates for Red Rot Management
The results of the enzymatic assays identified three isolates, viz. STr-52, STr-83 and STr-108, which had exhibited high production of both cellulase as well as chitinase enzymes. In addition, secondary metabolites of these isolates had shown high inhibitory activity against C. falcatum in previous studies (Joshi et al. 2016). Two of these isolates, viz. STr-83 and STr-108, were selected for field evaluation. The talc formulations of the isolates had a population of > 2 × 107 cfu g−1. The results of the field studies revealed that application of talc-based formulation of the Trichoderma isolates by all the three delivery methods, i.e. sett treatment, soil application as well as combination of sett + soil treatment, was effective in suppressing red rot (Table 4). It was also observed that pre-inoculation with C. falcatum pathotype CF01 resulted in considerable reduction in germination in all treatments compared to healthy untreated setts. The highest germination (36.5%) was recorded in treatment T8 (healthy setts), while the lowest germination (21.3%) was recorded in untreated control plots. However, treatment of pathogen pre-inoculated setts with Trichoderma resulted in improved germination compared to untreated control with the germination per cent varying from 26.4 to 31.5% across the six different treatments with Trichoderma. In terms of germination failure relative to healthy uninoculated setts, highest reduction (41.7%) in germination was recorded in untreated control, while in case of the Trichoderma treatments, germination failure was much less (13.7–27.7%). Total incidence of settling, tiller and cane mortality ranged from 11.9 to 24.6% in different treatments with the highest incidence recorded in untreated control. Amongst the different Trichoderma treatments, lowest (11.9%) red rot incidence was observed in sett + soil application of isolate STr-108 (T6), while highest incidence (19.0%) was observed in sett treatment with isolate STr-108 (T2).
In terms of overall reduction in red rot (total buds affected by red rot at germination + total settling, tiller and cane mortality) incidence across all crop stages, it becomes evident that all six Trichoderma treatments resulted in considerable reduction in red rot (29.5–56.3%) over untreated control. However, the level of reduction accorded was much higher when the Trichoderma isolates were applied as a combination of sett + soil treatment (53.3 and 56.3% reduction by STr-83 and STr-108, respectively) as compared to soil application (43.1 and 49.7% reduction by STr-83 and STr-108, respectively) or sett treatment alone (< 35% reduction). The highest NMCs and yield (68.5 t ha−1) was recorded in sett + soil application of isolate STr-83 (T5), and it was significantly superior to untreated control (42.3 t ha−1) and treatments T1 and T3 (Table 4).
Both the isolates STr-83 and STr-108 were identified as T. longibrachiatum in multiplex PCR assay. Strains of T. longibrachiatum have been previously reported as biocontrol agents of a number of plant pathogens (Freeman et al. 2004; Sanchez et al. 2007). However, in case of sugarcane, previous studies using Trichoderma have mainly explored the species T. harzianum for red rot suppression (Singh et al. 2008, 2009), and to our knowledge, this is the first study reporting suppression of C. falcatum by strains of T. longibrachiatum. Also, most studies to date have focussed on use of spores and/or secondary metabolites of Trichoderma for red rot management (Singh et al. 2008, 2009; Joshi et al. 2016). However, potent isolates identified in this study are not only effective producers of inhibitory secondary metabolites (Joshi et al. 2016) but also exhibit enzymatic activity (chitinase and cellulase production) which may contribute further towards their high suppressive potential, as also observed in this study. Sanchez et al. (2007) had also reported a major role of hydrolytic enzymes like chitinase and inhibitory secondary metabolites in suppression of Thievaliopsis paradoxa by strain of T. longibrachiatum. In addition to contributing towards their biocontrol potential, the ability of the isolates to produce the enzyme cellulase can also be exploited for faster trash decomposition in sugarcane.
Conclusion
Studies on species diversity of Trichoderma by multiplex PCR assay revealed T. harzianum as the predominant species in sugarcane rhizosphere. In field studies conducted to evaluate potent isolates for red rot suppression, our findings indicate that talc formulation of selected Trichoderma isolates could effectively suppress red rot of sugarcane in field conditions. Moreover, the delivery of isolates as a combination of sett as well as soil application was most effective in reducing red rot (by > 50%) over control. In our previous studies, it was observed that direct application of secondary metabolites of potent Trichoderma isolates as sett treatment could reduce red rot by 37–55% (Joshi et al. 2016). However, direct application of secondary metabolites is not a viable option for large-scale delivery of Trichoderma for red rot management. Application of talc formulations of potent isolates, as observed in this study, also gave high level of red rot suppression (up to 56%) which is comparable to direct application of secondary metabolites and offers a much more feasible alternative. This formulation was developed from liquid culture of the isolates and contains not only the inhibitory secondary metabolites produced by the respective strains but also their active spores which can establish and propagate in the soil and/or delivery medium after application and may give long-term suppression. Moreover, high cellulase production by the isolates can be further exploited for trash decomposition.
References
Agrawal, T., and A.S. Kotasthane. 2012. Chitinolytic assay of indigenous Trichoderma isolates collected from different geographical locations of Chattisgarh in central India. Springer Plus 1: 73.
Benoliel, B., F.A.G. Torres, and L.M.P. de Moraes. 2013. A novel promising Trichoderma harzianum strain for the production of a cellulolytic complex using sugarcane bagasse in natura. Springer Plus 2: 656. https://doi.org/10.1186/2193-1801-2-656.
Bradner, J.R., M. Gillings, and K.M.H. Nevalainen. 1999. Qualitative assessment of hydrolytic activities in Antarctic microfungi grown at different temperatures on solid media. World Journal of Microbiology & Biotechnology 15: 131–132.
De Marco, J.L., L.H.C. Lima, M.V. de Sousa, and C.R. Felix. 2000. A Trichoderma harzianum chitinase destroys the cell wall of the phytopathogen Crinipellis perniciosa, the causal agent of witches’ broom disease of cocoa. World Journal of Microbiology & Biotechnology 16: 383–386. https://doi.org/10.1023/A:1008964324425.
Duttamajumder, S.K., and S.C. Misra. 2014. Dynamics of red rot (Colletotrichum falcatum) development and spread in relation to sett-borne inoculum. In: Proceedings of international conclave on sugar crops: sweeteners and green energy from sugar crops: emerging technology, IISR, Lucknow, Feb. 15–17, pp. 128.
Duttamajumder, S.K. 2008. Red rot of Sugarcane. Lucknow: Army Printing Press.
El-Katatny, M., M. Gudelj, K.H. Robra, M.A. Elnaghy, and G.M. Gubitz. 2001. Characterization of a chitinase and an endo-β-1,3-glucanase from Trichoderma harzianum Rifai T24 involved in control of the phytopathogen Sclerotium rolfsii. Applied Microbiology and Biotechnology 56: 137. https://doi.org/10.1007/s002530100646.
FAOSTAT. 2014. Food and Agriculture Organization of the United Nations. http://faostat.fao.org/site/567/default.aspx#ancor.
Freeman, S., D. Miz, I. Kolesnik, O. Barbul, A. Zveibil, M. Maymon, Y. Nitzani, B. Krihshner, D. Rav-David, A. Bilu, A. Dag, S. Shafir, and Y. Elad. 2004. Trichoderma biocontrol of Colletotrichum acutatum and Botrytis cinerea and survival in strawberry. European Journal of Plant Pathology 110: 361–370.
Gajera, H.P., and D.N. Vakharia. 2010. Molecular and biochemical characterization of Trichoderma isolates inhibiting a phytopathogenic fungi Aspergillus niger Van Tieghem. Physiological and Molecular Plant Pathology 74: 274–282.
Harman, G.E. 2011. Multifunctional fungal plant symbionts: new tools to enhance plant growth and productivity. New Phytologist 189: 647–649.
Hermosa, M.R., E. Keck, I. Chamorro, B. Rubio, L. Sanz, J.A. Vizcaino, I. Grondona, and E. Monte. 2004. Genetic diversity shown in Trichoderma biocontrol isolates. Mycological Research 108: 897–906.
Howell, C.R. 2003. Mechanisms employed by Trichoderma species in biological control of plant diseases: The history and evolution of current concepts. Plant Disease 87: 4–10.
Hoyos-Carvajal, L., S. Orduz, and J. Bissett. 2009. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal Genetics and Biology 46: 615–631.
Jain, N., A. Bhatia, and H. Pathak. 2014. Emission of air pollutants from crop residue burning in India. Aerosol Air Quality Research 14: 422–430.
Joshi, Deeksha, P. Singh, A.K. Singh, R.J. Lal, and Nidhi Tripathi. 2016. Antifungal potential of metabolites from Trichoderma sp. against Colletotrichum falcatum Went causing red rot of Sugarcane. Sugar Tech 18: 529–536. https://doi.org/10.10007/s12355-015-0421-y.
Joshi, Deeksha, and S.C. Misra. 2013. Characterization of Trichoderma isolates from sugarcane agro-ecosystem and their efficacy against Colletotrichum falcatum causing red rot of sugarcane. Sugar Tech 15: 192–196.
Kovacs, K., G. Szakacs, T. Pusztahelyi, and A. Pandey. 2004. Production of chitinolytic enzymes with Trichoderma longibrachiatum IMI 92027 in solid substrate fermentation. Applied Biochemistry and Biotechnology 118: 189. https://doi.org/10.1385/ABAB:118:1-3:189.
Kubicek, C.P., J. Bisset, I. Druzhinia, C.M. Kullnig-Gradinger, and G. Szakacs. 2003. Genetic and metabolic diversity of Trichoderma: a case study on South East Asian isolates. Fungal Genetics and Biology 38: 310–319.
Kubicek, C.P., M. Mikus, A. Schuster, M. Schmoll, and B. Seiboth. 2009. Metabolic engineering strategies for the improvement of cellulase production by Hypocrea jecorina. Biotechnology Biofuels 2: 19.
Lopez-Quintero, C.A., L. Atanasova, A.E. Franco-Molano, W. Gams, M. Komon-Zelazowska, B. Theelen, W.H. Muller, T. Boekhout, and I. Druzhinina. 2013. DNA barcoding survey of Trichoderma diversity in soil and litter of the Colombian lowland Amazonian rainforest reveals Trichoderma strigosellum sp. nov. and other species. Antonie van Leeuwenhoek 104: 657–674.
Lopes, F.A.C., A.S. Steindorff, A.M. Geraldine, R.S. Brandao, V.N. Monteiro, M.L. Junior, A.S.G. Coelho, C.J. Ulhoa, and R.N. Silva. 2012. Biochemical and metabolic profiles of Trichoderma strains isolated from common bean crops in the Brazilian Cerrado, and potential antagonism against Sclerotinia sclerotiorum. Fungal Biology 116: 815–824.
Lorito, M., C. Peterbauer, C.K. Hayes, and G.E. Harman. 1994. Synergistic interaction between fungal cell wall degrading enzymes and different antifungal compounds enhances inhibition of spore germination. Microbiology 140: 623–629.
Lorito, M., S.L. Woo, M. D’Ambrosio, G.E. Harman, C.K. Hayes, C.P. Kubicek, and F. Scala. 1996. Synergistic interaction between cell wall degrading enzymes and membrane affecting compounds. Molecular Plant-Microbe Interaction 9: 206–213.
Mulaw, T.B., C.P. Kubicek, and I.S. Druzhinina. 2010. The rhizosphere of Coffea arabica in its native highland forests of Ethiopia provides a niche for a distinguished diversity of Trichoderma. Diversity 2: 527–549. https://doi.org/10.3390/d2040527.
Prabhakaran, N., T. Prameeladevi, M. Sathiyabama, and D. Kamil. 2015. Multiplex PCR for detection and differentiation of diverse Trichoderma species. Annals of Microbiology 65: 1591–1595.
Romaro-Dumaresq, A.S., M.N. Dourado, L.C.L. Fávaro, R. Mendes, A. Ferreira, and W.L. Araújo. 2016. Diversity of cultivated fungi associated with conventional and transgenic sugarcane and the interaction between endophytic Trichoderma virens and the host plant. PLoS ONE. https://doi.org/10.1371/journal.pone.0158974.
Sánchez, V., O. Rebolledo, R.M. Picaso, E. Cárdenas, J. Córdova, O. González, and G.J. Samuels. 2007. In vitro antagonism of Thielaviopsis paradoxa by Trichoderma longibrachiatum. Mycopathologia 163: 49–58.
Singh, V., B.B. Joshi, S.K. Awasthi, and S.N. Srivastava. 2008. Eco-friendly management of red rot disease of sugarcane with Trichoderma strains. Sugar Tech 10: 158–161.
Singh, V., R.J. Lal, S.K. Awasthi, and M.R. Verma. 2009. Managing red rot of sugarcane by Trichoderma harzianum. Indian Sugar 59: 25–30.
Vinale, F., K. Sivasithamparam, E.L. Ghisalberti, R. Marra, S.L. Woo, and M. Lorito. 2008. Trichoderma-plant—pathogen interactions. Soil Biology & Biochemistry 40: 1–10.
Viswanathan, R., A. Ramesh Sundar, and S.M. Premkumari. 2003. Mycolytic effect of extracellular enzymes of antagonistic microbes to Colletotrichum falcatum, red rot pathogen of sugarcane. World Journal Microbiology and Biotechnology 19: 953–959.
Zhang, C., I.S. Druzhinina, C.P. Kubicek, and T. Xu. 2005. Trichoderma biodiversity in China: Evidence for a north to south distribution of species in east Asia. FEMS Microbiology Letters 251: 251–257.
Acknowledgements
We are grateful to the Director, ICAR-IISR, Lucknow, for providing facilities and constant encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Joshi, D., Singh, P., Holkar, S.K. et al. Trichoderma-Mediated Suppression of Red Rot of Sugarcane Under Field Conditions in Subtropical India. Sugar Tech 21, 496–504 (2019). https://doi.org/10.1007/s12355-018-0624-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-018-0624-0