Abstract
Fever, abdominal pain, and liver dysfunction are almost inevitable complications of transcatheter arterial chemo embolization (TACE) for hepatocellular carcinoma, but these symptoms may also be due to bile duct obstruction caused by shedding of necrotic tumor material into the bile duct. A 68-year-old man presented with persistent fever, liver dysfunction, and abdominal pain after TACE. Computed tomography revealed stone-like hyperdensities in the bile duct. Endoscopic retrograde cholangiopancreatography revealed these structures to be necrotic material from hepatocellular carcinoma. We believe this is an instructive case of an often overlooked situation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcatheter arterial chemo embolization (TACE) is a widely performed treatment option for hepatocellular carcinoma (HCC) [1,2,3,4]. Due to the nature of the treatment, which involves embolizing the hepatic artery, damage to normal hepatocytes as well as tumor cells is unavoidable, and fever, abdominal pain, and liver dysfunction are almost inevitable complications of TACE. However, these symptoms can in fact be caused by obstruction of the bile duct by a necrotic tumor fragment. We report the instructive case of a patient who presented with fever, abdominal pain, and liver dysfunction after TACE, in whom these complications were due to bile duct obstruction caused by shedding of necrotic tumor material into the bile duct.
Case report
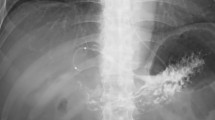
A 68-year-old man had undergone TACE for HCC due to chronic hepatitis B a total of 6 times over the past 3 years. Follow-up computed tomography (CT) identified HCC recurrence in liver segment S2 and S6, and the patient was admitted to the hospital for TACE. He had a history of hypertension and no history of drinking alcohol. Relevant laboratory results on admission were as follows: white blood cell count, 4.6 × 109/L; hemoglobin, 15.3 g/dL; platelet count, 14.6 × 104/μL; AST, 46 U/L; ALT, 49 U/L; ALP, 410 U/L; GGT, 225 U/L; total bilirubin, 1.00 mg/dL; CRP, 0.25 mg/dL. CT during arterial portography (CTAP) showed perfusion defects in S2 and S6 (Fig. 1a, b), hepatic arterial-phase CT arteriography (CTA) showed hyperenhancement in S2 and S6 (Fig. 1c, d), and delayed-phase CTA showed washout, which are considered characteristic findings of HCC recurrence. Digital subtraction angiography (DSA) showed tumor staining consistent with these lesions (Fig. 1e). The lesion was embolized with lipiodol, myriplatin hydrate, and gelatin sponge. Post-embolization CT showed good accumulation of lipiodol in the lesion (Fig. 2a, b). After TACE, the patient suffered continuous fever and liver dysfunction, and on day 6 in addition complained of abdominal pain. CT revealed an area of high density in the common bile duct (Fig. 3a, b). The patient was considered to have obstructive jaundice and cholangitis, and endoscopic retrograde cholangiopancreatography (ERCP) was performed. Cholangiography showed shadow defects in the lower bile duct (Fig. 4a) and a soft tissue-like object was recovered by basket catheter after endoscopic sphincterotomy (Fig. 4b, c). Pathologic examination revealed that histologically, the object was a tissue mass that had undergone coagulation necrosis. The nuclei displayed polygonal spicules and nucleoli were observed as shadows, consistent with the appearance of tumor cells undergoing necrosis (Fig. 5). After ERCP, the fever, abdominal pain, and hepatobiliary enzyme level showed a trend toward improvement, and the patient was discharged at 15 days after TACE.
Computed tomography during arterial portography (CTAP) shows perfusion defects (arrows) in liver segments S2 (a) and S6 (b). Arterial-phase hepatic CT arteriography (CTA) shows hyperenhancement (arrows) in S2 (c) and S6 (d). CTAP (a) and CTA (c) show intrahepatic bile duct dilatation (arrowhead) due to bile duct invasion by hepatocellular carcinoma. Digital subtraction angiography (DSA) (e) shows tumor staining (arrows) consistent with S2 and S6
Histologic slides. Necrosis is seen with almost complete loss of nuclear staining (a: hematoxylin and eosin (H&E) staining × 100). Nuclei of atypical cells with slight-nuclear staining are seen (b: H&E × 400). Necrotic material that could be bile duct epithelium and liver tissue is seen (c: H&E × 400)
Discussion
We consider that in the present case, fragments of a necrotic tumor became detached from the bile duct wall after TACE and migrated into the common bile duct, causing obstructive jaundice. Migration of intraductal-tumor invasion in hepatocellular carcinoma after TACE is very rare [5,6,7], but has been noted to occur more often than might have been reported [8]. It has also been reported that some patients remain asymptomatic even when necrotic tissue drains into the bile duct [8]. In patients with hepatocellular carcinoma, who are more likely to have reduced hepatic reserve, there is a risk of liver failure due to worsening liver function. Therefore, if fever, abdominal pain, or liver dysfunction persist or if new symptoms are observed after several days, it is advisable to perform appropriate imaging studies and closely examine the patient for other causes, rather than simply attempting to reduce the fever. In addition, as excretory tumor tissue is difficult to detect by plain CT in the absence of iodine oil accumulation, some such cases may have been missed after being judged as postembolic syndrome due to TACE, and no appropriate treatment given [9]. From this perspective, the present case is very instructive. We believe it is very important to raise awareness of the existence of such cases. Endoscopic ultrasound (EUS) has a much higher detection rate of common bile duct stones than other imaging tests and is a very useful test for identifying structures in the bile duct [10]. EUS is also a low-risk test and should be considered in patients with no specific abnormalities on CT scan.
A previous study has reported that necrotic tissue can easily be misidentified as bile duct stones because of the accumulation of highly absorbable iodine oil in the generally desquamated tumor necrotic tissue [9]. In the present case, we suspected bile duct stones on CT, but as the HCC was originally suspected to have infiltrated the intrahepatic bile duct, a definitive diagnosis was obtained after submitting the removed specimen for pathologic examination. It is important to confirm the preliminary imaging studies thoroughly and to recognize that patients with suspected bile duct invasion of HCC are particularly prone to developing obstructive jaundice due to tumor necrosis [7]. Other factors reported to be associated with obstructive jaundice due to the shedding of necrotic tumor into the bile duct include the total number of TACE procedures, the presence of bile duct dilation on CT, and tumor location [7]. However, it should be noted that TACE causes marked ischemic necrosis not only in the vascular bed of the tumor but also in the adjacent bile ducts, which can occur even in patients without bile duct invasion [9, 11]. It has been reported that in patients with an HCC greater than 5 cm in size that is in contact with the left and right hepatic ducts, even without bile duct invasion, it is not uncommon for necrotic tumors to be excreted into the biliary system after TACE [9]. It has been reported that the mechanism by which necrotic tumors are excreted into the biliary system, even in tumors without intraductal-bile duct invasion, is that bile ducts near the tumor are mainly supplied only by arterial blood, and that damage caused by TACE may result in fistula formation between necrotic tumor and the biliary system. It is important to be fully aware of the possibility of obstructive jaundice and biliary tract infection due to the shedding of necrotic tumor into the bile duct after TACE. In addition, a variety of anticancer drugs have been launched in recent years; therefore, and not only TACE but also other anticancer drugs may cause obstructive jaundice due to similar tumor necrosis, which requires caution.
References
Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9.
Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71.
Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9.
Takaki S, Sakaguchi H, Anai H, et al. Long-term outcome of transcatheter subsegmental and segmental arterial chemoemobolization using lipiodol for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2012;35:544–54.
Hiraki T, Sakurai J, Gobara H, et al. Sloughing of intraductal tumor thrombus of hepatocellular carcinoma after transcatheter chemoembolization causing obstructive jaundice and acute pancreatitis. J Vasc Interv Radiol. 2006;17:583–5.
Okuda M, Miyayama S, Yamashiro M, et al. Sloughing of intraductal tumor thrombus of hepatocellular carcinoma after transcatheter arterial chemoembolization. Cardiovasc Intervent Radiol. 2010;33:619–23.
Park HC, Park HB, Chung CY, et al. Acute obstructive cholangitis complicated by tumor migration after transarterial chemoembolization: a case report and literature review. Korean J Gastroenterol Taehan Sohwagi Hakhoe Chi. 2014;63:171–5.
Kim GM, Kim HC, Hur S, Lee M, Jae HJ, Chung JW. Sloughing of biliary tumour ingrowth of hepatocellular carcinoma after chemoembolization. Eur Radiol. 2016;26:1760–5.
Miyayama S, Yamashiro M, Nagai K, et al. Excretion of necrotic hepatocellular carcinoma tissues into the biliary system after transcatheter arterial chemoembolization. Hepatol Res. 2017;47:1390–6.
Meeralam Y, Al-Shammari K, Yaghoobi M. Diagnostic accuracy of EUS compared with MRCP in detecting choledocholithiasis: a meta-analysis of diagnostic test accuracy in head-to-head studies. Gastrointest Endosc. 2017;86:986–93.
Spahr L, Frossard JL, Felley C, Brundler MA, Majno PE, Hadengue A. Biliary migration of hepatocellular carcinoma fragment after transcatheter arterial chemoembolization therapy. Eur J Gastroenterol Hepatol. 2000;12:243–4.
Author information
Authors and Affiliations
Contributions
All authors have participated in writing, revising, and editing this manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest in relation to this manuscript.
Informed consent
Informed consent was obtained from the patient for the publication of his information and imaging.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kobori, I., Masaoka, R., Maeda, H. et al. Post-embolization syndrome-like symptoms due to shedding of necrotic material of hepatocellular carcinoma into the bile duct following transcatheter arterial chemoembolization: an instructive case. Clin J Gastroenterol 17, 563–566 (2024). https://doi.org/10.1007/s12328-024-01932-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-024-01932-z