Abstract
We report a rare case of adenosquamous carcinoma of the gallbladder which simultaneously produces granulocyte-colony-stimulating factor (G-CSF) and parathyroid hormone-related protein (PTHrP), confirmed serologically and histologically. A 71-year-old man was examined for a gallbladder tumor with multiple lymph nodes and liver metastases. Histopathological evaluation by endoscopic ultrasound fine-needle aspiration revealed adenosquamous carcinoma of the gallbladder. Laboratory data showed markedly elevated white blood cell (WBC) count of 34,700 µL and corrected serum calcium level of 14.9 mg/dL. Serum G-CSF (191 pg/mL) and PTHrP (23.1 pmol/L) levels were high. Zoledronic acid and calcitonin were administered to treat hypercalcemia, which normalized serum calcium levels. Gemcitabine–cisplatin chemotherapy was started for cStage IVB gallbladder cancer. After chemotherapy initiation, WBCs showed a rapid downward trend; however, the patient suddenly developed acute respiratory distress syndrome; thus, chemotherapy was discontinued. Subsequently, WBC count increased again, and the patient’s overall condition deteriorated. The patient died on day 27. Immunohistochemistry using autopsy specimens demonstrated patchy staining for G-CSF in the squamous cell carcinoma portion and diffuse and weak positive staining for PTHrP in the squamous cell carcinoma and poorly differentiated adenocarcinoma portions of the tumor, suggesting simultaneous G-CSF and PTHrP production by the tumor. This is the first report of a patient with gallbladder cancer with serological and histological evidence for G-CSF and PTHrP production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The histological type of gallbladder cancer is mostly adenocarcinoma. Adenosquamous carcinoma makes up only 3.3–3.6% of all gallbladder cancers, according to Henson et al. [1], and as reported in the 1997 National Biliary Cancer Registry Survey from Japan [2]. Leukocytosis and hypercalcemia are paraneoplastic syndromes, which are mainly caused by the granulocyte-colony-stimulating factor (G-CSF) and parathyroid hormone-related protein (PTHrP) that are produced by the tumor. These paraneoplastic syndromes have often been associated with cancers of squamous cell origin, such as head, neck, and lung cancer [3,4,5,6]. A few cases of gallbladder cancer with elevated G-CSF and PTHrP have been reported [2, 7]; however, to the best of our knowledge, there are no reports of serum and histological evidence of both G-CSF and PTHrP production by the tumor. Therefore, this report aimed to describe a rare case of adenosquamous carcinoma of the gallbladder with a rapid clinical course, wherein simultaneous production of G-CSF and PTHrP was confirmed both serologically and histologically.
Case report
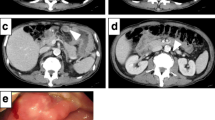
A 71-year-old man visited our hospital with a chief complaint of right-sided abdominal pain. On physical examination, his abdomen was flat and soft, with a palpable mass in the right costal region and tenderness in the same area. Laboratory data revealed a markedly elevated white blood cell (WBC) count of 34,700 µL (neutrophil, 94%) and C-reactive protein (CRP) level of 9.3 mg/dL. Additionally, interleukin (IL)-6 was elevated at 30.0 pg/mL (normal range, ≤ 7.0 pg/mL). Elevated hepatobiliary enzyme levels, including that of total bilirubin (0.6 mg/dL), aspartate aminotransferase (43 U/L), alanine aminotransferase (50 U/L), alkaline phosphatase (731 U/L), and γ-glutamyl transferase (297 U/L), were observed. In addition, corrected serum calcium was markedly high at 14.9 mg/dL. The tumor markers, carbohydrate antigen 19–9 (122.0U/mL), squamous cell carcinoma antigen (10.4 ng/mL), and cytokeratin 19 fragment (9.8 ng/mL) were elevated (Table 1). Contrast-enhanced abdominal computed tomography scan revealed a gallbladder tumor (Fig. 1a), as well as multiple enlarged intra-abdominal lymph nodes around the pancreatic head, and multiple liver tumors (Fig. 1b).
For histopathological evaluation, endoscopic ultrasound fine-needle aspiration was performed on the enlarged lymph node around the pancreatic head (Fig. 2a). H–E staining showed solid proliferation of large, poorly differentiated cells with high-grade nuclear atypia. Some of the cells formed glandular ducts, suggesting poorly differentiated adenocarcinoma (Fig. 2b). There was a mixture of squamous cell carcinoma components with keratinization in some areas (Fig. 2c), which led to the diagnosis of adenosquamous carcinoma. Based on imaging findings, clinical stage IVB adenosquamous carcinoma of the gallbladder with multiple lymph nodes and liver metastases was diagnosed according to the 8th edition of the Union for the International Cancer Control TNM staging system.
Endoscopic ultrasound and histopathological findings of the gallbladder cancer. a EUS-FNA is performed on the enlarged lymph node around the pancreatic head. b The EUS-FNA specimen shows solid proliferation of large, poorly differentiated cells with high-grade nuclear atypia. Some cells form glandular ducts, suggesting a poorly differentiated adenocarcinoma (H-E 40x). c A mix of squamous cell carcinoma components with keratinization (dotted circle) in some areas is noted (H-E 40x). EUS-FNA, endoscopic ultrasound fine-needle aspiration; H–E, hematoxylin–eosin
After admission, his WBC count rose to the 50,000/µL range. There were no clinical signs of infection, no foci of infection were found on imaging studies, and all culture tests were negative. It was hypothesized that the tumor produced G-CSF; serum G-CSF levels were measured and found to be as high as 191 pg/mL (normal range, ≤ 39.0 pg/mL). In addition, blood tests on admission showed marked hypercalcemia. Since there were no evident bone metastases on imaging and parathyroid hormone was suppressed below the lower limit of normal, PTHrP production from the tumor was suspected. Serum PTHrP levels were found to be high at 23.1 pmol/L (normal range, ≤ 1.0 pmol/L). It was suggested that the tumor was simultaneously producing G-CSF and PTHrP.
To treat hypercalcemia, zoledronic acid and calcitonin preparations were initiated on day 8 with infusion loading, which normalized serum calcium levels. Although the patient’s performance status (PS) declined (ECOG PS 2), he was willing to undergo chemotherapy. Thus, gemcitabine–cisplatin chemotherapy was initiated for cStage IVB gallbladder cancer on day 9 after admission. After chemotherapy was started, WBCs showed a rapid downward trend. However, shortly after the initiation of chemotherapy, he developed acute respiratory distress syndrome (ARDS), and his respiratory condition rapidly worsened. Chemotherapy was discontinued, WBCs increased, and the patient’s overall condition deteriorated. The patient died on day 27 (Fig. 3).
Clinical course of the patient. After admission, his WBC count increased to a maximum of 50,000/µL. The administration of zoledronic acid and calcitonin, initiated on day 8 as a hypercalcemia treatment, normalized serum calcium levels. Gemcitabine-cisplatin chemotherapy, which was started on day 9 for cStage IVB gallbladder cancer, caused a rapid decline in the WBC count. However, soon after the initiation of chemotherapy, ARDS developed and the chemotherapy was discontinued, the WBC count increased again, and the patient’s general condition worsened. The patient died on day 27. WBC white blood cell, Ca calcium, ARDS acute respiratory distress syndrome
An autopsy was performed with the approval of the patient’s family. Autopsy revealed a gallbladder tumor involving the common bile duct, pancreas, and transverse colon as a single mass, with multiple liver and lymph-node metastases. Histopathologic findings showed poorly differentiated adenosquamous carcinoma, with extensive necrosis and neutrophilic infiltration into the tumor. Immunohistochemistry demonstrated patchy staining for G-CSF in the squamous cell carcinoma portion (Fig. 4a) and diffuse and weak positive staining for PTHrP in the squamous cell carcinoma and poorly differentiated adenocarcinoma portions of the tumor (Fig. 4b), suggesting that the tumor was simultaneously producing G-CSF and PTHrP. There was no evidence the tumor had metastasized into bone. The bone marrow was highly hyperplastic, with increased granulocytic cells in each maturation stage, which accounted for > 95% of the bone marrow hematopoietic cells. This suggested leukocytosis due to G-CSF producing tumor. The lungs were filled with infantile fibrous tissue and macrophages in the alveolar space, with fibrin precipitation consistent with the findings of the organic stage of diffuse alveolar damage caused by ARDS.
Immunostaining findings of autopsy specimen. a Granulocyte-colony-stimulating factor immunostaining shows patchy positive staining only in the squamous cell carcinoma component (red frame) of the tumor but is negative in the adenocarcinoma component (green frame) (20x). b Parathyroid hormone-related protein immunostaining shows diffuse and weak positive staining in both squamous cell carcinoma (red frame) and poorly differentiated adenocarcinoma (green frame) portions of the tumor (20x)
Discussion
Hyperleukocytosis and hypercalcemia are paraneoplastic syndromes, mainly caused by G-CSF and PTHrP that are produced by the tumor. G-CSF producing tumors were first reported in 1977 [8]. Four diagnostic criteria have thus far been proposed for G-CSF producing tumors: (1) a marked increase in leukocytes, (2) an increase in serum G-CSF activity, (3) a decrease in leukocytes and serum G-CSF activity after tumor resection/treatment, and (4) immunohistological evidence of G-CSF production in the tumor [8]. In this case, the following were observed: (1) persistently elevated WBC level, (2) elevated serum G-CSF (191 pg/mL) level, and (3) a decrease in WBC level as a result of chemotherapy. In addition, (4) tumor cells were positive for G-CSF immunostaining and met all diagnostic criteria for G-CSF-producing tumors. G-CSF-producing tumors are most common in undifferentiated carcinoma, squamous cell carcinoma, and adenocarcinoma, in that order, and are primarily located in the lung (28.9%), bladder (9.4%), and stomach (6.8%) [9]. Reported cases of gallbladder cancer are few, and the histologic types of the cases are frequently adenosquamous or squamous cell carcinoma (43.5%), which are rare histologic types for gallbladder cancer [10].
G-CSF-producing adenosquamous carcinoma has been reported in the pancreas and stomach in addition to the gallbladder [11, 12]. It has been reported that immunostaining for G-CSF was positive only for the squamous cell carcinoma component of the tumor and negative for the adenocarcinoma component [10,11,12]. Similar immunostaining findings were observed in the present case, suggesting that G-CSF was produced mainly by the squamous cell carcinoma portion of the tumor.
G-CSF-producing tumors have been found to progress rapidly and with a very poor prognosis and a mean survival time of 4.7 ± 3.1 months [10]. This poor prognosis can be attributed to the following mechanisms: (1) autocrine tumor growth by the appearance of G-CSF receptors on tumor cells, (2) neutrophils proliferated by G-CSF promote tumor growth and metastasis, and (3) G-CSF suppresses cellular immunity by suppressing macrophages and killer cells, suggesting that G-CSF may be involved in the growth and promotion of tumors [13]. Many cases of leukocytosis by G-CSF-producing tumors have shown fever and elevated CRP levels [14], which have been reported to be caused by inflammatory cytokines, such as IL-1 and IL-6, that are simultaneously produced by G-CSF-producing tumors [15]. Elevated levels of IL-6 and CRP were further found in our patient.
Recently, ARDS and interstitial pneumonia have been reported with G-CSF administration [16, 17] and ARDS has also been reported in G-CSF-producing tumors [18]. Although the mechanism by which G-CSF induces ARDS is unclear, it has been reported that when G-CSF administration causes ARDS, there is frequently some inflammatory episode preceding the onset of ARDS, such as anticancer drug administration or pneumonia, and inflammatory cytokines, including TNF-α and IL-6, have been reported to be elevated at the onset of ARDS [19]. A possible mechanism is that hypercytokinemia and G-CSF cause a rapid increase in neutrophils with enhanced adhesion to the vascular endothelium, resulting in an accumulation of neutrophils in the lungs and the development of ARDS [17]. In the present case, ARDS developed immediately after the initiation of chemotherapy for gallbladder cancer, suggesting a similar mechanism.
Furthermore, hypercalcemia of malignancy is classified into two major categories: humoral hypercalcemia of malignancy (HHM), wherein PTHrP produced by the tumor causes increased bone resorption (80%) and local osteolytic hypercalcemia, in which local bone destruction occurs by tumor metastasis (20%) [20]. HHM is found in various histologic types, but it is particularly common in squamous cell carcinoma. It is the most frequently observed paraneoplastic syndrome and is found in approximately 10% of patients with advanced lung, head and neck, renal, breast, bladder, and other cancers [21,22,23]. The prognosis of patients with HHM is extremely poor, with a survival time of 1–3 months after the onset of hypercalcemia [22, 24]. In this case, the diagnosis of HHM due to the PTHrP-producing tumor was made based on the absence of obvious bone metastases on imaging, autopsy findings, high-serum PTHrP level, and positive tumor cells on immunostaining for PTHrP.
Malignant tumors simultaneously producing G-CSF and PTHrP have been reported in cases of lung, tongue, esophageal, renal pelvis cancer, intrahepatic cholangiocarcinoma, and pancreatic cancers, among other cancers [6, 9, 25,26,27]. All of these progressed rapidly, were resistant to treatment, and had a poor prognosis.
Gallbladder cancer was poorly recorded, and a search of reports on gallbladder cancer with G-CSF and PTHrP production revealed only five cases, including the present case (Supplementary Table 1) [2, 7, 28, 29]. The average age was 71, including one man and four women. There were two cases of adenosquamous carcinoma and one each of moderately differentiated adenocarcinoma, poorly differentiated adenocarcinoma, and undifferentiated carcinoma. The mean serum calcium level was 14.6 mg/dL; the mean WBC count was 32,200/µL; and in all patients, multi-organ involvement or distant metastases were observed at the time of diagnosis. Most patients underwent chemotherapy; however, all died within 3 months, indicating a poor prognosis.
Tumor cells producing G-CSF and PTHrP have been shown to be interrelated and have been reported to harbor mutations in K-ras exon2 codon12 [7, 30]. In this case, an autopsy showed a mutation in the K-ras exon2 codon13 (G13D). The frequency of K-ras mutations varies by cancer type and race [31, 32]. K-ras mutations are oncogenes that cause activation of several downstream signaling pathways, affecting tumor growth, metastases formation, and angiogenesis. It has been reported that PTHrP production in tumors is induced by K-ras mutations [33, 34], and G-CSF production has also been linked to abnormal RAS signaling [35, 36]. Therefore, a single gene mutation may induce both G-CSF and PTHrP overexpression downstream, which may induce signals related to tumor growth, progression, and metastasis, often associated with a poor prognosis. Mutations in the K-ras gene have been identified in various cancers; mutations in the K-ras gene are found in > 95% of pancreatic cancers, as well as in colorectal cancer, lung cancer, multiple myeloma, and uterine cancer [37]. However, cases with G-CSF or PTHrP production are not common in these cancers. There may be tumors that do not produce G-CSF or PTHrP because of different downstream signaling pathways activated by KRAS mutations, or tumors that produce a small amount of G-CSF or PTHrP without causing clinical manifestations, such as leukocytosis or hypercalcemia, owing to weak signaling transduction. The present case may be called G-CSF and PTHrP-hyper-producing gallbladder cancer rather than G-CSF and PTHrP-producing gallbladder cancer.
Furthermore, the detailed mechanism of the simultaneous production of G-CSF and PTHrP is unclear. To elucidate this, further investigation is required.
In conclusion, we report a rare case of gallbladder adenosquamous carcinoma that simultaneously produced G-CSF and PTHrP. To the best of our knowledge, this is the first report of gallbladder cancer with serum and histological evidence of both G-CSF and PTHrP production and can be considered a valuable case. In patients with malignant tumors, G-CSF and PTHrP production by the tumor should be considered when unexplained leukocytosis and hypercalcemia are observed. Further, tumors that simultaneously produce G-CSF and PTHrP have a rapid clinical course and a very poor prognosis. A detailed mechanism of the simultaneous production of G-CSF and PTHrP should be investigated in the future.
Data availability
The data that supports the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Henson DE, Albores-Saavedra J, Corle D. Carcinoma of the gallbladder histologic types, stage of disease, grade, and survival rates. Cancer. 1992;70:1493–7.
Ueda K, Kinoshita A, Akasu T, et al. A case of adenosquamous cell carcinoma of the gallbladder with markedly elevated PTHrP and G-CSF levels. Nihon Shokakibyo Gakkai Zasshi. 2016;113:1564–71.
Yoneda T, Nishimura R, Kato I, et al. Frequency of the hypercalcemia-leukocytosis syndrome in oral malignancies. Cancer. 1991;68:617–22.
Kondo Y, Sato K, Ohkawa H, et al. Association of hypercalcemia with tumors producing colony-stimulating factor(s). Cancer Res. 1983;43:2368–74.
Sato K, Fujii Y, Ono M, et al. Production of interleukin 1 alpha-like factor and colony-stimulating factor by a squamous cell carcinoma of the thyroid (T3M–5) derived from a patient with hypercalcemia and leukocytosis. Cancer Res. 1987;47:6474–80.
Sakamoto A, Katakami H, Mukae H, et al. Simultaneous production of parathyroid hormone-related protein (PTHrP) and granulocyte colony-stimulating factor (G-CSF) in lung cancer patients with hypercalcemia and leukocytosis. Nihon Kyobu Shikkan Gakkai Zasshi. 1995;33:34–8.
Kuroki M, Uto H, Ido A, et al. A case of gallbladder cancer producing granulocyte-colony stimulating factor and possible parathyroid hormone related protein. Nihon Shokakibyo Gakkai Zasshi. 2000;97:478–83.
Asano S, Urabe A, Okabe T, et al. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977;49:845–52.
Shimomatsuya T, Hashimoto K, Takeuchi G, et al. Gastric cancer producing granulocyte colony-stimulating factor and parathyroid hormone-related protein. Jpn J Gastroenterol Surg. 2021;54:173–83.
Matsudaira S, Yarita A, Fukumoto T, et al. A case of granulocyte-colony stimulating factor-producing gallbladder adenosquamous carcinoma with rapidly growing recurrence of liver metastasis in early postoperative period. Nihon Shokakibyo Gakkai Zasshi. 2020;117:626–34.
Kobayashi H, Nozaki H, Shimizu M, et al. A case of adenosquamous carcinoma of the pancreas producing granulocyte-colony stimulating factor. Nihon Rinsho Geka Gakkai Zasshi (J Jpn Surg Assoc). 2005;66:707–11.
Kudou Y, Tokura T, Nishikawa S, et al. A case of gastric adenosquamous carcinoma producing granulocyte-colony stimulating factor. Nihon Rinsho Geka Gakkai Zasshi (J Jpn Surg Assoc). 2012;73:2847–51.
Tomizawa Y, Kuroiwa H, Suda T, et al. Rapid progression of adenocarcinoma of the lung in a patient with high levels of granulocyte colony-stimulating factor. Nihon Kyobu Shikkan Gakkai Zasshi. 1996;34:1249–54.
Naruse H, Shimoyama N, Kudo T, et al. Autopsy of invasive ductal pancreatic carcinoma that transformed into a tumor producing granulocyte colony-stimulating factor. Nihon Shokakibyo Gakkai Zasshi. 2017;114:854–64.
Suzuki A, Takahashi T, Okuno Y, et al. Liver damage in patients with colony-stimulating factor-producing tumors. Am J Med. 1993;94:125–32.
Niitsu N, Iki S, Muroi K, et al. Interstitial pneumonia in patients receiving granulocyte colony-stimulating factor during chemotherapy: survey in Japan 1991–96. Br J Cancer. 1997;76:1661–6.
Sakai C, Nakaseko C, Takagi T. Acute respiratory failure associated with G-CSF-induced leukocyte recovery in three patients with preceding infection. Kansenshogaku Zasshi. 1997;71:1080–4.
Wada H, Yoshida S, Ishibashi F, et al. Pleomorphic carcinoma of the lung producing granulocyte colony-stimulating factor: report of a case. Surg Today. 2011;41:1161–5.
Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–73.
Horwitz MJ, Stewart AF. Hypercalcemia associated with malignancy Primer on the metabolic bone diseases and disorders of mineral metabolism. In: Favus MJ, editor. American Society for Bone and Mineral Research. Washington DC USA; 2006.
Broadus AE, Nissenson RA. Parathyroid hormone related protein Primer on the metabolic bone diseases and disorders of mineral metabolism. In: Favus MJ, editor. American Society for Bone and Mineral Research. Washington DC; 2006.
Stewart AF. Clinical practice Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–9.
Gensure RC, Gardella TJ, Jüppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–78.
Ralston SH, Gallacher SJ, Patel U, et al. Cancer-associated hypercalcemia: morbidity and mortality Clinical experience in 126 treated patients. Ann Intern Med. 1990;112:499–504.
Okimoto T, Yahata H, Sugino K, et al. A case report of esophageal carcinoma that produced parathyroid hormone related protein (PTHrP) and G-CSF and manifested hypercalcemia and leukocytosis. Jpn J Gastroenterol Surg. 2002;35:35–9.
Washino S, Terauchi F, Matsuzaki A, et al. Two cases of squamous cell carcinoma of upper urinary tract with hypercalcemia. Nihon Hinyokika Gakkai Zasshi. 2008;99:703–8.
Ozawa N, Doi S, Tsujikawa T, et al. Intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor and parathyroid hormone-related protein. Nihon Shokakibyo Gakkai Zasshi. 2017;114:1285–92.
Watanabe Y, Ogino Y, Ubukata E, et al. A case of gallbladder cancer with marked hypercalcemia and leukocytosis. Jpn J Med. 1989;28:722–6.
Shizuma T, Nakayama H. A case of gallbladder carcinoma with humoral hypercalcemia of malignancy and leukocytosis (in Japanese). Naika. 2008;102:405–7.
Oshika Y, Nakamura M, Hatanaka H, et al. A human lung cancer xenograft producing granulocyte-colony stimulating factor and parathyroid hormone-related protein. Oncol Rep. 1998;5:359–62.
Deng N, Goh LK, Wang H, et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut. 2012;61:673–84.
Peng N, Zhao X. Comparison of K-ras mutations in lung, colorectal and gastric cancer. Oncol Lett. 2014;8:561–5.
Aklilu F, Park M, Goltzman D, et al. Induction of parathyroid hormone-related peptide by the Ras oncogene: role of Ras farnesylation inhibitors as potential therapeutic agents for hypercalcemia of malignancy. Cancer Res. 1997;57:4517–22.
Kamai T, Arai K, Koga F, et al. Higher expression of K-ras is associated with parathyroid hormone-related protein-induced hypercalcaemia in renal cell carcinoma. BJU Int. 2001;88:960–6.
Phan VT, Wu X, Cheng JH, et al. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proc Natl Acad Sci USA. 2013;110:6079–84.
Qeadan F, Bansal P, Hanson JA, et al. The MK2 pathway is linked to G-CSF, cytokine production and metastasis in gastric cancer: a novel intercorrelation analysis approach. J Transl Med. 2020;18:137.
Cox AD, Fesik SW, Kimmelman AC, et al. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov. 2014;13:828–51.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
Conceptualization: MY and TS; methodology: MY, TS, and MK; formal analysis and investigation: MY, TS, and MK; writing—original draft preparation: MY; writing—review and editing: TS, NO, EM, and MK; funding acquisition: none; resources: MY and MK; supervision: TS, NO, and EM.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Ethical approval
Not applicable.
Human and animal research
Not applicable. Research involving recombinant DNA: not applicable.
Inform consent
Informed consent was obtained from the deceased patient’s family for this report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12328_2023_1841_MOESM1_ESM.docx
Supplementary file1 Supplementary Table 1. Characteristics of five cases with gallbladder cancer with elevated G-CSF and PTHrP Abbreviations: F, female; M, male; Ca, calcium; PTHrP, parathyroid hormone-related protein; WBC, white blood cell; G-CSF, granulocyte colony-stimulating factor; NR, not reported (DOCX 19 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yanagi, M., Suda, T., Oishi, N. et al. Adenosquamous carcinoma of the gallbladder simultaneously producing granulocyte-colony-stimulating factor and parathyroid hormone-related protein. Clin J Gastroenterol 16, 901–907 (2023). https://doi.org/10.1007/s12328-023-01841-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-023-01841-7