Abstract
Conversion therapy for gastric cancer is a new therapeutic concept. We report a case of a patient with advanced gastric cancer who underwent conversion surgery due to a remarkable regression of multiple liver metastases following chemotherapy. A 71-year-old man was referred to our hospital with gastric cancer. Esophagogastroduodenoscopy (EGD) revealed an irregular, nodular, ulcerated lesion in the lower third of the stomach. Analysis of biopsy specimens revealed a poorly differentiated adenocarcinoma. Abdominal contrast-enhanced computed tomography (CT) showed multiple liver mass lesions. The patient was clinically diagnosed with advanced gastric cancer with liver metastases and received S-1 plus oxaliplatin chemotherapy. After 6 cycles of chemotherapy, CT and magnetic resonance imaging showed complete resolution of the liver metastases, and EGD detected mucosal irregularities only. Since there was no evidence of further metastatic lesions in other organs, the patient underwent distal gastrectomy with D2 lymphadenectomy. The gross appearance of the surgically resected specimen showed a slightly elevated tumor measuring 4.5 × 3.5 cm. Pathological examination confirmed the diagnosis of a moderately differentiated gastric adenocarcinoma invading the muscularis propria with no lymph node metastases. The postoperative course was uneventful. The patient has continued to receive S-1 and oxaliplatin chemotherapy, and there has been no evidence of recurrence for 3 months following the operation. We propose that conversion therapy might be an effective treatment for patients with advanced gastric cancer; however, further studies and assessments are needed to confirm and establish this treatment strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is currently the fourth most frequent cancer, and is the third leading cause of cancer-associated death worldwide [1]. Surgical resection with regional lymphadenectomy remains the most effective treatment for gastric cancer; however, systemic chemotherapy is the standard treatment for prolonging survival and improving quality of life if the cancer recurs or is an unresectable metastatic gastric cancer [2]. Although surgical removal of liver metastases of gastric cancer is still controversial, a recent phase III trial could not prove the superiority of palliative gastrectomy followed by chemotherapy over chemotherapy alone for stage IV gastric cancer [3].
Conversion therapy is a new therapeutic concept. This is defined as a surgical treatment, in which a curative resection is performed after chemotherapy in an attempt to remove tumors that were originally regarded as technically or oncologically unresectable or only marginally resectable [4]. Although this approach has played a crucial role in the field of colorectal cancer [5], the application of conversion surgery to gastric cancer is novel. We herein report a case of advanced gastric cancer with liver metastases that was successfully downgraded by systemic chemotherapy using S-1 and oxaliplatin and conversion surgery.
Case report
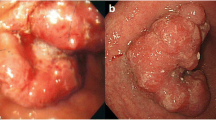
A 71-year-old man complaining of epigastric discomfort was referred to our hospital for further examination following diagnosis of gastric cancer by a local medical doctor. The laboratory findings on admission were as follows: normal red blood cell count (493 × 104/mm3; normal range, 435–555 × 104/mm3), normal white blood cell count (5.7 × 103/mm3; normal range, 3.3–8.6 × 103/mm3), and high C-reactive protein levels (0.61 mg/dl; normal range, < 0.3 mg/dl). High levels of the serum tumor markers alpha-fetoprotein (18.0 ng/ml; normal range, < 7.0 ng/ml) and carbohydrate antigen (CA) 72-4 (22.8 U/ml; normal range, < 6.9 U/ml) were detected, while levels of the serum tumor markers carcinoembryonic antigen, CA 19-9, and CA125 were within the normal ranges. Esophagogastroduodenoscopy (EGD) showed a nodular elevated lesion with an irregular central depression in the lower third of the stomach. Biopsy specimens of the lesion revealed a poorly differentiated adenocarcinoma (Fig. 1a), and immunohistochemical analysis of the tumor showed no reactivity for the human epidermal growth factor receptor 2 (HER2). Abdominal contrast-enhanced computed tomography (CT) revealed wall thickening with homogeneous enhancement in the lower part of the stomach, enlarged lymph nodes in the perigastric area, and multiple low-density liver mass lesions (Fig. 1b).
Initial esophagogastroduodenoscopy and computed tomography. Esophagogastroduodenoscopy shows an obvious elevated lesion with an irregular central depression in the lower third of the stomach (a). Abdominal contrast-enhanced computed tomography shows multiple liver metastases (arrows) and enlarged lymph nodes in the perigastric area (arrowhead) (b)
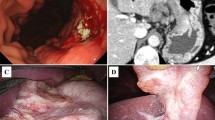
The clinical diagnosis was cT3N3M1, which is equivalent to stage IV in the Japanese classification system [6], and the patient received chemotherapy using S-1 plus oxaliplatin. We selected this combination regimen for chemotherapy from the viewpoint of renal function protection compared with the cisplatin based one, taking elderly patient into account. In addition, it allowed outpatient care, because it was not required a hydration therapy to preserve renal function. An 80 mg/m2/day dose of S-1 was administered orally twice daily for the first 2 weeks of a 3-week cycle. On day 1 of the 21-day cycle, the patient received 100 mg/m2 of intravenous oxaliplatin followed by 80 mg/m2/kg of S-1. After 4 courses of chemotherapy, the liver metastases had shrunk remarkably and EGD showed a slightly elevated lesion with mucosal irregularity in the area in which the initial tumor was located (Fig. 2a). After 6 courses of chemotherapy, the liver metastases were finally not detected by CT (Fig. 2b). The gastric wall thickening and lymphadenopathy in the perigastric area also showed remarkable reductions. In addition, magnetic resonance imaging (MRI) using gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid and 18F-fluorodeoxyglucose positron-emission tomography combined with computed tomography could not detect any liver mass lesions.
Esophagogastroduodenoscopy and computed tomography after systemic chemotherapy. Esophagogastroduodenoscopy reveals a slightly elevated lesion with an indistinct margin in the area where the tumor was initially located (a). Abdominal contrast-enhanced computed tomography shows no liver mass lesions and a remarkable reduction in lymph node swelling (b)
As there was no evidence of further metastatic lesions in any other organs, the patient underwent distal gastrectomy with D2 lymphadenectomy followed by a Billroth I reconstruction. There was a slight sclerotic change of tissue in the perigastric area during dissection of the lymph nodes, while it did not require further technical artifice for the surgical procedure compared with conventional operation. The operating time was 275 min, and blood loss was 200 ml.
The gross appearance of the surgically resected specimen showed a slightly elevated tumor measuring 4.5 × 3.5 cm (Fig. 3). Microscopic examination of the specimen confirmed the diagnosis of a moderately differentiated adenocarcinoma in the stomach with invasion of the muscularis propria and no lymph node metastases (Fig. 4). Viable tumor cells were found in less than one-third of the section where the tumor was located prior to chemotherapy. Following preoperative therapy, the tumor was classified as ypT2N0M0, stage IB. In addition, the histological response after preoperative therapy meant that the primary tumor could be downgraded to grade 2, according to the Japanese classification system [6]. The postoperative course was uneventful and there has been no evidence of recurrence for 4 months following the operation. The patient has been well and continues to receive S-1 plus oxaliplatin chemotherapy.
Discussion
Herein we described a case of a patient with advanced metastatic gastric cancer who was treated with conversion surgery due to the complete response of the liver metastases after systemic chemotherapy using S-1 plus oxaliplatin. We searched the Medline and PubMed databases for English-language literature, published between 2000 and 2016, describing the use of conversion surgery for advanced gastric cancer. We used the keywords “advanced gastric cancer”, “chemotherapy” and “conversion surgery”. We obtained a range of data on age, gender, tumor location, depth of invasion, histological type, treatments, and outcomes for each patient. To the best of our knowledge, our case report is only the fifth case describing the use of conversion surgery to treat advanced gastric cancer to be reported in the English literature, and the first case using S-1 plus oxaliplatin chemotherapy.
The clinicopathological features of the 4 previously reported cases [7,8,9] and the present case are listed in Table 1. From these cases we found that the median patient age was 57 years (range 54–71 years). Patients were predominantly male, with a male-to-female ratio of 4:1. Tumors were located in the lower one-third of the stomach in all cases and the gross appearance type was elevated in 4 cases and ulcerated in 1 case. Treatment consisted of total gastrectomy in 1 patient and distal gastrectomy in 4 patients. Histological analyses of the gastric adenocarcinomas revealed 3 intestinal types and 2 diffuse-type carcinomas. The initial chemotherapy treatment regimen was S-1 plus cisplatin in 3 cases, S-1 plus cisplatin with trastuzumab in 1 case, and S-1 plus oxaliplatin in 1 case. The median course of chemotherapy was 6 courses (range 1–18 courses).
In recent years, several studies have demonstrated the surgical impact on patients initially diagnosed with unresectable gastric cancer, which then became resectable during the course of receiving systemic chemotherapy [10,11,12,13,14]. Yoshida et al. devised a system to help clarify the indications for conversion therapy by proposing 4 new classification categories for stage IV gastric cancer based on the biology and heterogeneous characteristics of the tumors. [4]. Although patient eligibility remains unclear, proposed indications included patients from category 2, some patients from category 3, and a very small number of patients from category 4. The present case was initially presented as a marginally resectable metastasis without macroscopic peritoneal dissemination, which could be classified as category 2 under these new guidelines.
S-1-based combination therapy is used mainly as a first-line treatment for unresectable advanced gastric cancer [15, 16]. S-1 is an orally active combination of tegafur (a prodrug that is converted by cells to fluorouracil), gimeracil (an inhibitor of dihydropyrimidine dehydrogenase), and oteracil (which inhibits the phosphorylation of fluorouracil in the gastrointestinal tract, thereby reducing the toxic gastrointestinal effects of fluorouracil). S-1 plus oxaliplatin chemotherapy has shown promising efficacy against unresectable advanced gastric cancer in several large trials [17, 18]. Therefore, even if conversion therapy can be performed, chemotherapy should be continued for as long as possible after tumors have been resected until the tumors acquire resistance to chemotherapy or until uncontrollable adverse events occur in the patients [4, 14, 18, 19]. In the present patient, the postoperative time may be not enough to evaluate whether the conversion surgery might be beneficial. Further careful medical follow-up is needed to determine the benefit of conversion surgery.
In general, the term “conversion therapy” is used a therapeutic concept in which the treatment strategy is converted by chemotherapy to curative treatment through an onco-surgical approach. Considering the achievements of adjuvant chemotherapy, which is called for chemotherapy when a complete resection without residual tumor has been performed, the term ‘adjuvant’ can be used when the tumor does not exist macroscopically. On the basis of this sense, the term ‘adjuvant surgery’ could be defined as the curative surgery after complete response was detected by chemotherapy in metastatic advanced cancer [20, 21]. Further discussion seems to be required to determine the most appropriate terminology.
In conclusion, conversion surgery is a challenging but promising treatment strategy for patients with initially unresectable gastric cancer and can contribute to improved treatment outcomes for patients with metastatic advanced gastric cancer. However, further investigations including case–control studies with large sample sizes and prospective cohort studies and/or randomized control trials are needed to verify this.
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Namikawa T, Munekage E, Munekage M, et al. Evaluation of systemic inflammatory response biomarkers in patients receiving chemotherapy for unresectable and recurrent advanced gastric cancer. Oncology. 2016;90:321–6.
Fujitani K, Yang HK, Mizusawa J, for the REGATTA study investigators. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309–18.
Yoshida K, Yamaguchi K, Okumura N, et al. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016;19:329–38.
Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. 2009;27:1829–35.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–12.
Takahashi N, Nimura H, Aoki H, et al. Successful preoperative chemotherapy with S-1 plus low-dose cisplatin for advanced gastric cancer with synchronous liver metastases: report of 2 cases. Chemotherapy. 2007;53:378–82.
Li ZY, Shan F, Zhang LH, et al. Preoperative chemotherapy with a trastuzumab-containing regimen for a patient with gastric cancer and hepatic metastases. Genet Mol Res. 2014;13:10952–7.
Tsunematsu M, Takahashi N, Murakami K, et al. Successful conversion surgery for gastric cancer with multiple liver metastases treated after S-1 plus cisplatin combination chemotherapy: a case report. Surg Case Rep. 2017;3:95.
Mieno H, Yamashita K, Hosoda K, et al. Conversion surgery after combination chemotherapy of docetaxel, cisplatin and S-1 (DCS) for far-advanced gastric cancer. Surg Today. 2017. https://doi.org/10.1007/s00595-017-1512-z (published online ahead of print April 1, 2017).
Kinoshita J, Fushida S, Tsukada T, et al. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol. 2015;41:1354–60.
Terashima M. Conversion therapy for gastric cancer: who can make conversion as successful as Goromaru? Gastric Cancer. 2016;19:685–6.
Fukuchi M, Mochiki E, Ishiguro T, et al. Efficacy of conversion surgery following S-1 plus cisplatin or oxaliplatin chemotherapy for unresectable gastric cancer. Anticancer Res. 2017;37:1343–7.
Yamaguchi K, Yoshida K, Tanahashi T, et al. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2017. https://doi.org/10.1007/s10120-017-0738-1 (published online ahead of print April 14, 2017).
Namikawa T, Shiga M, Ichikawa K, et al. Metachronous liver and bone metastasis from small early gastric carcinoma without lymph node involvement: a case report. Mol Clin Oncol. 2013;1:249–52.
Namikawa T, Fukudome I, Ogawa M, et al. Clinical efficacy of protein-bound polysaccharide K in patients with gastric cancer undergoing chemotherapy with an oral fluoropyrimidine (S-1). Eur J Surg Oncol. 2015;41:795–800.
Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–8.
Koizumi W, Takiuchi H, Yamada Y, et al. Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study). Ann Oncol. 2010;21:1001–5.
Namikawa T, Munekage E, Munekage M, et al. Evaluation of a trastuzumab-containing treatment regimen for patients with unresectable advanced or recurrent gastric cancer. Mol Clin Oncol. 2016;5:74–8.
Yoshida K, Yamaguchi K, Okumura N, et al. The roles of surgical oncologists in the new era: minimally invasive surgery for early gastric cancer and adjuvant surgery for metastatic gastric cancer. Pathobiology. 2011;78:343–52.
Suzuki T, Tanabe K, Taomoto J, et al. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol Lett. 2010;1:743–7.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Namikawa, T., Tsuda, S., Fujisawa, K. et al. Conversion surgery after S-1 plus oxaliplatin combination chemotherapy for advanced gastric cancer with multiple liver metastases. Clin J Gastroenterol 11, 297–301 (2018). https://doi.org/10.1007/s12328-018-0842-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-018-0842-8