Abstract
Introduction
Venous thromboembolism (VTE) is a leading cause of preventable morbidity and mortality among hospitalized patients in the US. The objectives of this study were to examine VTE prophylaxis patterns and risk for VTE events during hospitalization and post-discharge among patients hospitalized for acute illnesses in the US.
Methods
Acutely ill hospitalized patients were identified from the MarketScan databases (January 1, 2012–June 30, 2015). Proportions of patients that received inpatient and/or outpatient VTE prophylaxis were determined. VTE rates were calculated for the overall study population and for each subpopulation with each acute illness type. Risk for VTE events after the index admission was determined by Kaplan–Meier analysis.
Results
Of the acutely ill patients (n = 17,895, mean age: 58.4 years), most were hospitalized for infectious diseases (40.6%), followed by respiratory diseases (31.0%), cancer (10.7%), heart failure (10.4%), ischemic stroke (6.4%), and rheumatic diseases (0.9%). Among the entire study population, 59.1% did not receive any VTE prophylaxis, and only 7.1% received both inpatient and outpatient prophylaxis. Among the overall study population, cumulative VTE rate, including during index admission and within 6 months post-discharge, was 4.6%. VTE risk in the inpatient and outpatient continuum of care remained elevated up to 30-40 days after hospital admission, with 60.1% of VTEs occurring within 40 days of hospital admission.
Conclusion
In this retrospective analysis of nearly 18,000 patients hospitalized for acute illnesses, 59.1% did not receive any VTE prophylaxis and only 7.1% received VTE prophylaxis in both the inpatient and outpatient continuum of care, despite significant VTE risk extending from hospitalization into the post-discharge period.
Funding
Portola Pharmaceuticals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE), encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE), is a leading cause of preventable morbidity and mortality among hospitalized patients in the United States [1]. According to a recent estimate, approximately 500,000 VTE events occur annually in the US, with 52% related to current or recent hospitalizations [2]. A cost analysis based on several studies conducted in the US estimated a person with a VTE event to have a direct medical cost of $12,000 to $14,000 (2014 USD) in the first year, which increases to between $18,000 and $23,000 per case when including subsequent complications [3]. In 2011 USD, total healthcare costs of VTE patients were estimated to range between $13.5 and $27.2 billion, with $4.5 to $14.2 billion predicted as preventable hospital-acquired costs [4].
When applying 2012 criteria of the American College of Chest Physicians (ACCP) to 2014 US hospital charges in the National Inpatient Sample, it was projected that 7.3 million acutely ill hospitalized patients were at risk for VTE in the US [5, 6]. Patients hospitalized for acute illnesses (such as stroke, heart failure, infectious disease, respiratory disease, or rheumatic disease) are at increased risk for VTE during hospitalization and for an extended duration following hospital discharge, primarily within 40 days following hospital admission [2, 7,8,9]. A recent observational study of medical and surgical patients showed that implementation of a hospital protocol to improve compliance with VTE prophylaxis guidelines achieved a decline in VTE events occurring during hospitalization [1]. However, a study of patients hospitalized at the Rochester Mayo Clinic hospital from 2005 to 2010 did not show a decrease in the VTE event rate when inpatient VTE prophylaxis was increased over time [2]. Among this study population, 75% of VTE events occurred after hospital discharge, with a median time to VTE of 19.5 days [2]. These findings led investigators to conclude that the short-duration in-hospital VTE prophylaxis (averaging 3 days) failed to provide acceptable VTE prevention [2].
Because of the significance of the clinical, healthcare, and economic burden of VTE and the uncertainty regarding appropriate prevention tactics, further study of VTE prophylaxis patterns and risk for VTE events in the continuum of care from hospitalization to the outpatient setting is warranted. Thus, the objectives of this study were to examine VTE prophylaxis patterns and VTE risk during hospitalization and post-discharge, as well as the frequency of hospital readmission among patients hospitalized for cancer, heart failure, infectious diseases, ischemic stroke, respiratory diseases, and rheumatic diseases using a large US claims database.
Methods
Study Population
This study was a retrospective database analysis to evaluate the patterns of pharmacologic VTE prophylaxis among patients hospitalized for acute medical illnesses in the real-world setting. Patients at risk of VTE due to hospitalization for acute medical illnesses of cancer, heart failure, infectious diseases, ischemic stroke, respiratory diseases, and rheumatic diseases, as the primary hospital discharge diagnosis, were identified from the Truven Health Analytics MarketScan Inpatient Drug Link databases between January 1, 2012 and June 30, 2015. Medical illnesses were identified by the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. The acute medical illnesses were based on ACCP guidelines [6] and other patient types studies in VTE clinical trials [10, 11].
The MarketScan Inpatient Drug Link databases match patients from the MarketScan Commercial and Medicare Supplemental healthcare claims databases with those in the Hospital Drug Database, which contains inpatient drug utilization data derived from hospital discharge records. The MarketScan Commercial and Medicare Supplemental claims databases encompass > 100 million employees, spouses, and dependents located in all ten US census regions. The databases consist of healthcare claims data from > 100 different insurance companies, Blue Cross Blue Shield plans, and third-party administrators. The claims data include inpatient and outpatient information, laboratory data, and detailed hospital drug data, reflecting real-world treatment patterns and costs. The MarketScan claims databases were further linked to the MarketScan Hospital Drug Database to allow researchers to evaluate the details of healthcare services, resource utilization, and costs, both inside and outside hospitalizations. While such a database-linking process has reduced the sample size in the final selected study population, the detailed inpatient and outpatient healthcare records from tens of thousands of selected patients were used for this study evaluation. In compliance with the Health Insurance Portability and Accountability Act of 1996, the databases utilized for this retrospective claims database analysis consist of fully de-identified datasets, with synthetic identifiers applied to patient-level and provider-level data to protect the identities of both the patients and data contributors. The study was exempt from requiring Institutional Review Board approval as it involved the assessment of retrospective and de-identified data.
Index hospitalization was defined as the earliest hospitalization for acute medical illnesses to occur during the index identification period. Patients were required to have 6 months of continuous medical and prescription insurance coverage prior to the index hospitalization (baseline period). Patients were additionally required to have 6 months of continuous insurance coverage after the index admission date (follow-up period). Patients were excluded if they had a pregnancy diagnosis during the baseline period or at the index hospitalization, death during the index hospitalization, or hip or knee replacement surgery during the index hospitalization.
Demographics, Patient Clinical Characteristics, and Hospital Characteristics
Patient demographics and clinical characteristics, including age, gender, health plan type, Charlson Comorbidity Index (CCI) score, and hospital length of stay (LOS), were evaluated during the 6-month baseline period and index hospitalization for the overall study population, as well as for each subpopulation with each acute illness type. Hospital characteristics (i.e., year of hospitalization, geographic region, urban/rural status, teaching status, and bed size) were additionally evaluated.
VTE Prophylaxis Patterns
The proportions of patients who received or did not receive inpatient and/or outpatient pharmacologic VTE prophylaxis were determined. VTE prophylaxis in the inpatient setting was determined based on pharmacy records for enoxaparin, warfarin, direct-acting oral anticoagulants (DOACs: apixaban, dabigatran, rivaroxaban, edoxaban), fondaparinux, or unfractionated heparin (UFH) during the index hospitalization. VTE prophylaxis in the outpatient setting was determined based on pharmacy claims for the above listed anticoagulants within 15 days after VTE diagnosis [9]. Among patients who received inpatient and/or outpatient prophylaxis, the proportions of patients who received enoxaparin only, warfarin only, enoxaparin and warfarin combined, a DOAC only, and “other” VTE prophylactic drug combinations or drugs (e.g., other anticoagulant combinations, fondaparinux, etc.) were evaluated.
VTE Events
The proportions of patients with VTE events during the index hospitalization and within 6 months of hospital discharge were evaluated for the overall study population and for each subpopulation with each acute illness type. A VTE event during the index hospitalization was based on the presence of an ICD-9-CM code for DVT and/or PE at either primary or secondary position of discharge diagnosis codes. A VTE event during the post-discharge follow-up period was defined by the presence of a primary or secondary ICD-9-CM code for DVT and/or PE during an emergency room or inpatient admission, or on an outpatient claim with 1 or more of the following confirmatory events: a pharmacy claim for enoxaparin, fondaparinux, or UFH within 15 days after VTE diagnosis; or a pharmacy claim for warfarin or DOACs (apixaban, dabigatran, rivaroxaban, edoxaban) within 15 days after VTE diagnosis, and no evidence of atrial fibrillation or atrial flutter in the 6 months preceding the outpatient diagnosis for DVT and/or PE [9]. Cumulative VTE rates by time were also evaluated for the overall study population and for each subpopulation with each acute illness type with Kaplan–Meier analysis.
All-Cause and VTE-Related Hospital Readmissions
The proportions of patients with all-cause and VTE-related hospital readmissions in the 6-month post-discharge follow-up period were determined for the overall study population and for each subpopulation with each acute illness type.
Statistical Analyses
Descriptive statistics were utilized to evaluate differences in demographics, clinical characteristics, and hospital characteristics among the study groups with hospitalizations for each evaluated acute illness. ANOVA tests and Chi square tests were used to detect statistically significant differences in continuous and categorical variables, respectively. Cumulative rates for VTE events occurring after the index hospital admission date were evaluated using Kaplan–Meier analysis for all patients hospitalized for acute illnesses and for the patient populations with each particular acute illness. A critical value of 0.05 was used to determine statistical significance. All statistical analyses were carried out using SAS 9.4.
Results
Study Population
Among the overall study population (n = 17,895), most were hospitalized for infectious diseases (40.6%, n = 7268), followed by respiratory diseases (31.0%, n = 5539), cancer (10.7%, n = 1919), heart failure (10.4%, n = 1865), ischemic stroke (6.4%, n = 1148), and rheumatic diseases (0.9%, n = 156). The mean age of patients within the overall study population was 58.4 years, 55.4% were female, and mean CCI score was 2.2 prior to the index hospitalization (Table 1). The mean CCI score was 2.2 among all patients, and patients hospitalized for cancer or heart failure had the highest CCI scores of approximately 3 (Table 1). For the overall population, the mean index hospitalization LOS was 4.8 days, which ranged from 4.3 days for patients hospitalized for ischemic stroke to 5.2 days for patients hospitalized for infectious diseases (Table 1). Among the study population, most patients were cared for in urban (87.2%), non-teaching (95.4%), large (300 to ≥ 500 beds, 67.6%) hospitals, which were located in the South Census region (76.9%), reflecting the distribution of hospital records contained in the database (Table 1).
VTE Prophylaxis
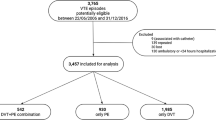
Among the overall hospitalized study population, the majority of patients did not receive any pharmacologic VTE prophylaxis (59.1%, n = 10,581), and only 7.1% (n = 1267) of patients received prophylaxis in both the inpatient and outpatient continuum of care (Fig. 1). Of those who received inpatient prophylaxis (38.2%, n = 6,843), the most common anticoagulant type was enoxaparin only (76.7%; Table 2). In the outpatient setting, 9.7% (n = 1738) of patients received VTE prophylaxis, among whom most received warfarin only (43.8%). Approximately one-quarter (n = 431) received other types of anticoagulant prophylaxis in the outpatient setting.
VTE Events
Among the overall hospitalized study population, 1.8% (n = 321) of patients had a VTE event rate during the index hospitalization, and 2.8% (n = 509) of patients had a VTE event within 6 months of hospital discharge, for a total of 4.6% of patients experiencing a VTE in the continuum of care (Fig. 2). VTE event rate during the index hospitalization was highest among patients with infectious diseases (2.4%) and lowest among patients with rheumatic diseases (0.6%). Within 6 months of hospital discharge, VTE event rate was highest among patients with cancer (5.4%), followed by patients with rheumatic diseases (5.1%).
Cumulative VTE Rate
Among the overall study population, cumulative VTE rate within 6 months of index admission was 4.6% (Fig. 3). High cumulative VTE event rates were seen in the follow-up period among patients hospitalized for cancer (6.9%), followed by those hospitalized for rheumatic diseases (5.8%), infectious diseases (5.3%), heart failure (4.6%), respiratory diseases (3.3%), and ischemic stroke (3.1%). Of the cumulative VTE events that occurred among the overall study population, 60.1% occurred within 40 days of index admission, and 39.9% occurred after 40 days until the end of the 6-month follow-up period.
Hospital Readmissions
Within 6 months of hospital discharge, 26.8% (n = 4790) of the overall study population had a hospital readmission for any cause of which 7.0% (n = 336) were VTE-related (Fig. 4). VTE-related hospital readmissions were most frequent among patients with cancer (13.7%) and rheumatic diseases (11.5%).
VTE Event Risk
Among the overall study population, VTE risk remained elevated up to 30–40 days after hospital admission across all patient admission types (Fig. 5).
Risk of VTE events (hazard function) by days after the Index hospital admission date of the overall hospitalized study population and stratified by acute illness type. The hazard function by time for patients hospitalized for rheumatic dieseases was not determined on account of the small sample size
Discussion
In this retrospective analysis of nearly 18,000 patients with acute medical illnesses who received care in US hospitals, the VTE event rate in the inpatient and outpatient continuum of care was 4.6%:1.8% in the inpatient setting and 2.8% within 6 months of hospital discharge. Approximately 60% of VTE events occurred after hospital discharge, with most happening within 40 days. Our findings on VTE event rates during hospitalization and after hospital discharge are consistent with those observed in other previously conducted real-world studies in the US [2, 9, 12]. In a study of acutely ill hospitalized patients between 2005 and 2008, Amin et al. reported a 3.3% overall VTE rate, with 56.6% of VTE events having occurred after hospital discharge [9]. Additionally, in another database claims analysis of 141,628 patients hospitalized for acute illnesses between 2005 and 2009, the VTE rate was 1.9% 90 days post-discharge [12]. In a population-based cohort study within a well-defined geographic area of Olmsted County, MN, USA, between 2005 and 2010, the average annual VTE event rate related to hospitalization was 282 per 10,000 person-years, with 75% of events having occurred after hospital discharge [2].
Among the entire study population of patients hospitalized for acute illnesses, nearly 60% did not receive any pharmacologic VTE prophylaxis, while 38% received inpatient prophylaxis, 10% received outpatient prophylaxis, and only 7% received both inpatient and outpatient prophylaxis. Not all hospitalized acutely ill patients included in this study may have been considered at high risk for VTE based on the ACCP criteria [6]. Future real-world studies are warranted to identify such patient groups. In this study, we also did not evaluate VTE occurrences among patients who received or did not receive prophylaxis; however, the impact of VTE prophylaxis in terms of reduction of VTE risk with prophylaxis has been well demonstrated by randomized clinical trials [6]. The frequency of inpatient VTE prophylaxis observed in our study is lower than that previously observed in other studies of patients hospitalized for acute medical illnesses in the US [9, 12, 13, 15,16,17]. Amin et al. reported inpatient and outpatient pharmacological VTE prophylaxis rates of 46.7% and 8.8%, respectively [9]. A second study by Amin et al. reported an inpatient VTE prophylaxis rate of 65.9% among patients hospitalized for medical illnesses identified from the Premier hospital database in years 2005 to 2006 [15]. Mahan et al. reported an inpatient VTE prophylaxis rate of 41.5% among 141,628 hospitalized medical patients between 2005 and 2009 [12]. Other studies have reported inpatient VTE prophylaxis rates ranging from only 18% within the first 48 h of hospital admission [14] to between 40% and 60% for at-risk patients during hospitalizations [16, 17]. The variation in VTE prophylaxis rates across studies may in part be attributed to differences in study populations (e.g., proportions with particular acute illnesses, such as infectious diseases, age distributions of study populations, and hospital characteristics) and also whether or not mechanical prophylaxis was measured. However, our data relative to that of the earlier conducted studies reflect that use of pharmacologic VTE prophylaxis has not increased in either the inpatient or outpatient setting in the last several years, and may be declining. This possible decline could be related to the possible uncertainty surrounding the impact of VTE prophylaxis and its duration on patient outcomes [6].
To address the unmet need for appropriate VTE prophylaxis strategies among acutely ill hospitalized patients, new paradigms have been proposed [18]. These paradigms involve an individualized and more patient-centered approach for the assessment of VTE and bleeding risks, such as the incorporation of validated Risk Assessment Models (RAMs) during hospital admission [18]. RAMs are used to identify acutely ill hospitalized patients who are at increased risk for VTE and would benefit most from VTE prophylaxis [18]. As of January 1, 2017, the Center for Medicare and Medicaid Services mandated the use of standardized VTE RAMs for US hospitals [18]. Also, the use of elevated D-dimer, a biomarker of coagulation activity, is undergoing investigation and validation for the potential utility in the identification of high VTE risk acutely ill patients and may aid in the identification of patients who may benefit from extended (~ 45 days) VTE prophylaxis after hospital discharge [18,19,20,21].
Four randomized controlled studies have been conducted that examined the potential for extended VTE prophylaxis versus standard of care in acutely ill patients hospitalized for a medical condition [10, 11, 22, 23]. Studies that evaluated extended-duration (admission-to-home) enoxaparin, rivaroxaban, and apixaban failed to demonstrate a reduction in VTE without an increase in major bleeding in acutely medically ill hospitalized patients when compared with standard-duration enoxaparin [11, 22, 23]. The phase 3 multinational APEX trial (Acute Medically Ill VTE Prevention With Extended Duration Betrixaban Study) showed that admission-to-home, extended-duration prophylaxis with betrixaban (up to 35–42 days) reduced VTE events among acutely ill hospitalized patients, without an increase in the risk for major bleeding, compared with standard-duration (6–14 days) enoxaparin [10]. Among patients hospitalized for heart failure, respiratory failure, infectious disease, ischemic stroke, or rheumatic disease, admission-to-home, extended-duration betrixaban, relative to standard-duration enoxaparin, was associated with a reduction in the primary outcome of asymptomatic proximal DVT and symptomatic VTE [5.3% vs. 7.0%; relative risk: 0.76; confidence interval (CI): 0.63–0.92, p = 0.006], and no significant difference in major bleeding rate (0.7% vs. 0.6%; relative risk: 1.19; 95% CI, 0.67–2.12; p = 0.55) [10]. The net clinical benefit (a composite of any component of the primary efficacy end point or principal safety outcome) occurred in 5.8% of the betrixaban group and 7.3% of the enoxaparin group (relative risk: 0.78; 95% CI, 0.65–0.95; p = 0.01; number needed to treat to reduce one composite endpoint = 67) [10]. In a follow-up analysis of the APEX trial, it was additionally shown that extended-duration betrixaban versus standard-duration enoxaparin reduced all fatal or irreversible ischemic or bleeding events by approximately 30%, and showed that 65 patients would require treatment with betrixaban to prevent 1 fatal or irreversible event versis enoxaparin [24]. Furthermore, extended-duration betrixaban versus standard-duration enoxaparin reduced the risk of VTE-related rehospitalization at 42 days (0.25% vs. 0.75%) and at 77 days (0.45% vs. 1.04%) [25].
Claims and hospitalization records in the MarketScan databases are subject to coding errors, coding for the purpose of rule-out rather than actual disease, and under-coding, either by the healthcare provider or due to limitations imposed by the database. Additionally, the MarketScan databases may not be representative of the US population as a whole; for example, this study used claims data from MarketScan commercial and Medicare supplemental databases, which may not generalize to patients insured by Medicaid. Also, the majority of claims in the MarketScan databases are from patients located in the South Census region and thus may not account for regional differences in patient care. Despite the potential limitations, the MarketScan databases are robust in data, which likely represent real-world patterns associated with clinical practice.
Whether hospitalized patients were considered at low to high risk for VTE could not be ascertained due to current limitations of the databases and lack of use of standardized VTE RAMs during the study period. Also, the MarketScan database claims do not indicate patients’ immobility status, which is a risk factor for VTE, and therefore VTE risk among the study population may not be comprehensive. Availability of the CCI score, however, provided additional information on the severity of comorbidities of the study population, to support the notion of some patients being at higher risk for VTE. Some patients evaluated in this study might have also received anticoagulation therapy for other disease indications, such as atrial fibrillation. In such cases, since the anticoagulation might have served both the purpose of stroke prevention and VTE prophylaxis, these patients were counted as having received VTE prophylaxis. This might have led to an overestimation of VTE prophylaxis rates, but still reflects the anticoagulation patterns in real-world patient populations. Additionally, VTE prophylaxis rates may be higher when mechanical VTE prophylaxis is considered, which was not measured in this study as the data sources do not contain reliable information on mechanical VTE prophylaxis. The observational design of this study is susceptible to various biases, such as information or classification bias (e.g., identification of false-positive VTE events). Lastly, as this study was a retrospective, observational analysis, causality between VTE prophylaxis and VTE event occurrence cannot be established.
Conclusions
In this retrospective analysis of nearly 18,000 patients with acute medical illnesses who received care in US hospitals, the risk for VTE was present in both the inpatient and outpatient settings. Despite significant VTE risk extending from hospitalization into the post-discharge period, with most VTE events occurring within 40 days, only a small portion of at-risk patients (7.1%) received VTE prophylaxis in both the inpatient and outpatient continuum of care. In spite of an accumulation of the evidence of the elevated risk for VTE remaining for at least 40 days after hospital admission, the proportion of patients who received VTE prophylaxis in the last few years was low, and thus a significant number of at-risk patients still do not receive any prophylaxis [2, 9, 13]. The results of this real-world study imply that there is a significant unmet medical need for effective VTE prophylaxis in both the inpatient and outpatient continuum of care among patients hospitalized for acute medical illnesses and who are at risk of VTE.
References
Cardoso LF, Krokoscz DV, de Paiva EF, et al. Results of a venous thromboembolism prophylaxis program for hospitalized patients. Vasc Health Risk Manag. 2016;12:491–6.
Heit JA, Crusan DJ, Ashrani AA, et al. Effect of a near-universal hospitalization-based prophylaxis regimen on annual number of venous thromboembolism events in the US. Blood. 2017;130:109–14.
Grosse SD, Nelson RE, Nyarko KA, et al. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10.
Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108:291v302.
Huang W, Cohen A, Zayaruzny M, et al. Impact of evolving ACCP guidelines on estimates of venous thromboembolism risk in US hospitals. International Society on Thrombosis and Haemostatsis (ISTH). July 8-13, 2017. Berlin Germany. Res Pract Thromb Haemost. 2017;1(Suppl S1):1–1451.
Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: american College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–226S.
Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164:963–8.
Anderson FA, Zayaruzny M, Heit JA, et al. Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol. 2007;82:777–82.
Amin AN, Varker H, Princic N, et al. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med. 2012;7:231–8.
Cohen AT, Harrington RA, Goldhaber SZ, APEX Investigators, et al. Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med. 2016;375:534–44.
Cohen AT, Spiro TE, Buller HR, The MAGELLAN Investigators, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–23.
Mahan CE, Fisher MD, Mills RM, et al. Thromboprophylaxis patterns, risk factors, and outcomes of care in the medically ill patient population. Thromb Res. 2013;132:520–6.
Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174:1577–84.
Pendergraft T, Liu X, Edelsberg J, et al. Prophylaxis against venous thromboembolism in hospitalized medically ill patients. Circ Cardiovasc Qual Outcomes. 2013;6:75–82.
Amin AN, Stemkowski S, Lin J, et al. Inpatient thromboprophylaxis use in U.S. hospitals: adherence to the seventh American College of Chest Physician’s recommendations for at-risk medical and surgical patients. J Hosp Med. 2009;4:E15–21.
Tapson VF, Decousus H, Pini M, et al. IMPROVE Investigators. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132:936–45.
Cohen AT, Tapson VF, Bergmann JF, et al. ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371:387–94.
Spyropoulos AC, Raskob GE. New paradigms in venous thromboprophylaxis of medically ill patients. Thromb Haemost. 2017;117:1662–70.
Cohen AT, Spiro TE, Spyropoulos AC, et al. D-dimer as a predictor of venous thromboembolism in acutely ill, hospitalised patients: a subanalysis of the randomized controlled MAGELLAN trial. J Thromb Haemost. 2014;12:479–87.
Mebazaa A, Spiro TE, Büller HR, et al. Predicting the risk of venous thromboembolism in patients hospitalised with heart failure. Circulation. 2014;130:410–8.
Clark CL, Shams AH, Chang AM, et al. D-dimer in acute medically ill adults and current thromboprophylaxis: a multicenter observational study evaluating the prevalence of elevated D-dimer in acute medically ill, hospitalized adults and current thromboprophylaxis trends; the DAMIACT study, initial data analysis. International Society on Thrombosis and Haemostatsis (ISTH). July 8-13, 2017. Berlin Germany. Res Pract Thromb Haemost. 2017; 1(Suppl S1):1 − 1451.
Hull RD, Schellong SM, Tapson VF, et al. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18.
Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–77.
Gibson CM, Korjian S, Chi G, et al. Comparison of fatal or irreversible events with extended-duration betrixaban versus standard dose enoxaparin in acutely ill medical patients: an APEX trial substudy. J Am Heart Assoc. 2017;6:e006015.
Chi G, et al. Extended-duration betrixaban reduces the risk of rehospitalization associated with venous thromboembolism among acutely ill hospitalized medical patients: findings from the APEX trial. Circulation. 2018;137:91–4.
Acknowledgements
Funding
Sponsorship for this study, development of this manuscript and article processing charges were funded by Portola Pharmaceuticals. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Alpesh Amin is a research consultant and/or speaker for Novosys Health, Portola, BI, BMS, and Pfizer. Alpesh Amin did not receive funding for manuscript development. W Richey Neuman is an employee of Portola Pharmaceuticals. Melissa Lingohr-Smith is an employee of Novosys Health, who has received research funds from Portola Pharmaceuticals in connection with conducting this study and development of this manuscript. Brandy Menges is an employee of Novosys Health, who has received research funds from Portola Pharmaceuticals in connection with conducting this study and development of this manuscript. Jay Lin is an employee of Novosys Health, who has received research funds from Portola Pharmaceuticals in connection with conducting this study and development of this manuscript.
Compliance with Ethics Guidelines
In compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA), the databases utilized for this retrospective claims database analysis consist of fully de-identified data sets, with synthetic identifiers applied to patient-level and provider-level data to protect the identities of both the patients and data contributors. The study was exempt from requiring Institutional Review Board approval as it involved the assessment of retrospective and de-identified data.
Data Availability
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7284827.
Rights and permissions
About this article
Cite this article
Amin, A., Neuman, W.R., Lingohr-Smith, M. et al. Venous Thromboembolism Prophylaxis and Risk in the Inpatient and Outpatient Continuum of Care Among Hospitalized Acutely Ill Patients in the US: A Retrospective Analysis. Adv Ther 36, 59–71 (2019). https://doi.org/10.1007/s12325-018-0846-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0846-2