Abstract
The electrical stimulation of specific brain targets has been shown to induce striking antidepressant effects. Despite that recent data have indicated that cerebellum is involved in emotional regulation, the mechanisms by which stimulation improved mood-related behaviors in the cerebellum remained largely obscure. Here, we investigated the stimulation effects of the ventromedial prefrontal cortex (vmPFC), nucleus accumbens (NAc), and lateral habenular nucleus on the c-Fos neuronal activity in various deep cerebellar and vestibular nuclei using the unpredictable chronic mild stress (CMS) animal model of depression. Our results showed that stressed animals had increased number of c-Fos cells in the cerebellar dentate and fastigial nuclei, as well as in the spinal vestibular nucleus. To examine the stimulation effects, we found that vmPFC stimulation significantly decreased the c-Fos activity within the cerebellar fastigial nucleus as compared to the CMS sham. Similarly, there was also a reduction of c-Fos expression in the magnocellular part of the medial vestibular nucleus in vmPFC- and NAc core-stimulated animals when compared to the CMS sham. Correlational analyses showed that the anxiety measure of home-cage emergence escape latency was positively correlated with the c-Fos neuronal activity of the cerebellar fastigial and magnocellular and parvicellular parts of the interposed nuclei in CMS vmPFC-stimulated animals. Interestingly, there was a strong correlation among activation in these cerebellar nuclei, indicating that the antidepressant-like behaviors were possibly mediated by the vmPFC stimulation-induced remodeling within the forebrain-cerebellar neurocircuitry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, electrical deep brain stimulation (DBS) is an emerging clinical treatment option for debilitating neurological and psychiatric diseases. In mood-related disorder, it has become an alternative therapeutic approach especially for treatment-resistant depression [1–3]. Converging evidence from experimental and clinical findings have shown that depression is a multisystem disorder affecting several integrated pathways including the cortical, subcortical, and limbic regions [4, 5]. For example, DBS has been shown to influence this network system by modulating other brain regions which causes various biochemical changes in the normalization of its pathological mood state [2, 5, 6].
In clinical and experimental animal models, the application of DBS in different brain regions has been reported to alleviate mood and anxiety-related symptoms [5, 7–9]. At present, studies in patients with treatment-resistant depression have shown promising results when stimulating electrode were implanted within the subcallosal cingulate gyrus or the ventromedial prefrontal cortex (vmPFC) [4, 10, 11], the inferior thalamic peduncle [12], the nucleus accumbens (NAc) [13–15], the anterior limb of the internal capsule [16, 17], the lateral habenula (LHb) [18], and the medial forebrain bundle [19]. Recently, we investigated the effects of electrical stimulation in different brain regions using the unpredictable chronic mild stress (CMS) paradigm as an animal model of depression [5]. The latter study has shown that high-frequency stimulation (HFS) of the NAc core, LHb, and of particular interest, the vmPFC produced significant antidepressant effects [5]. In addition, a wide range of brain regions has been reported to be specifically modulated by the HFS of the vmPFC, including the cortical, limbic structures, and the deep cerebellar nuclei [20].
The cerebellum is a brain region that functions mainly outside of the sphere of consciousness. Besides motor coordination, there is an accumulating body of evidence that supports the involvement of cerebellum in learning and emotional regulation [21, 22]. In addition, experimental animal and patient studies with cerebellar damage or lesioning have confirmed the roles of cerebellum in a number of cognitive tasks, such as language, working memory, and executive tasks and affective-related processes, for instance, the regulation of sadness, panic, stress, pain, empathy, and impulsive behavior [23–25]. Besides, Schmahman and Sherman also found that personality changes of either flattening of emotion or disinhibited and inappropriate behavior were commonly diagnosed in patients with lesions involving the cerebellum especially the anterior and posterior lobes of the cerebellum, as well as the vermal cerebellar pathology [26]. Indeed, converging lines of evidence from many studies have also demonstrated that the cerebellum is involved in the pathology of depression. Of particular interest, the reduction of cerebellar gray matter was seemingly observed in major depressive disorder [27] and functional MRI also detected abnormalities on the cerebellar activity of bipolar disorder, major depression, or geriatric depression patients [28–30]. In line with these studies, the depression scores were significantly increased in patients with cerebellar stroke [31] and cerebellar mass lesions [32]. Interestingly, some antidepressant medications have been shown to regulate the cerebellar function during improvement of affective symptoms and cognitive function [33] and normalization of the white matter volume reduction in patients with depression [34]. Furthermore, it is important to note that the patterns of cerebellum regulation can be considered also for the treatment comparison of antidepressant outcome effects [34].
In this study, we investigated the effects of electrical stimulation in different brain targets on the neuronal activity of the deep cerebellar and vestibular nuclei using immunohistochemical detection of c-Fos expression, a transcription factor expressed in response to both the intracellular and extracellular stimuli [35–37]. This neuroanatomical mapping method is widely used to identify the functional connectivity after an induction of electrical stimulation or behavioral responses [38–40]. Since pathological alterations in the cerebellum have been described in patients with mood-related problems, we hypothesized that effects of electrical stimulation on antidepressant response would induce a network remodeling of the dysfunctional neural circuitry within the deep cerebellar and vestibular nuclei.
Materials and Methods
Design of the Study
We aimed to investigate the effects of different stimulation brain targets on the neurocircuitry activity within the deep cerebellar and vestibular nuclei. The structure samples of the cerebelli and vestibular nuclei were obtained from previous experimental works [5]. In this study, using c-Fos immunohistochemistry, we compared the neuronal activation pattern of the deep cerebellar and vestibular nuclei in animals with exposure to either stressed or non-stressed condition. In each condition, animals received electrode implantation within the vmPFC, NAc core, NAc shell, and LHb. The sham animals were also implanted with electrodes but without the deliverance of electrical stimulus (Supplementary Table 1a).
Subjects
Sprague Dawley male rats (n = 62; Charles River, Sulzfeld, Germany) were kept in standard cages with control of room temperature (20–22 °C), humidity (60–70 %), and 12-h reversed dark or light cycle. The animals were having continuous ad libitum access to food and water. All experimental procedures were conducted in accordance to the guidelines of the institutional animal care provided by the Animal Experiments and Ethics Committee of Maastricht University.
Electrode Implantation and Stimulation Procedures
The details of stereotactic surgery for electrode implantation procedure have been previously described [41, 42]. In brief, stimulating electrodes were implanted in the vmPFC (AP +2.70 mm; L ±0.60 mm; V −4.60 mm), NAc core (AP + 2.20 mm; L: ±1.50 mm; V −6.80 mm), NAc shell (AP +1.70 mm; L ±0.60 mm; V −7.20 mm), and LHb (AP −3.80 mm; L ±0.60 mm; V −5.00 mm) based on the rat brain atlas of Paxinos and Watson [43]. After surgery, animals were given a recovery period of 2 weeks.
For electrical stimulation, a digital stimulator (DS8000, World Precision Instruments or WPI, Berlin, Germany) and stimulus isolators (DLS100, WPI) were used to deliver the stimuli. The stimulation parameters of 100 μA (amplitude), 100 hz (frequency), and 100 μs (pulse width) were utilized based on previous experiments [5, 7].
The Unpredictable Chronic Mild Stress Model
The stressed animals were initially exposed to 3 weeks of chronic unpredictable stress paradigm, while the non-stressed controls were gently handled once daily. The CMS and the control non-CMS animals were separately placed in different rooms. The stress procedures were continued throughout the experimental period and details of the paradigm were described in a previous study [5]. In brief, the CMS protocol comprised sessions of intermittent illumination (on/off every 2 h), placement in mouse cage, regular flashes of light (stroboscopic lamp; frequency at 2.5 Hz), wet bedding with 300 ml of cold water, paired-housing in uncleaned cages, food and water deprivation, and without stress condition. The duration of each stressor ranged from 10 to 14 h. The order of the CMS stressors was randomized and it usually took place in the morning and followed by another in the evening.
Histological Processing
After the final experiment, animals were stimulated for 1 h and returned to their home-cage for 1 h before sacrifice. All rats were anesthetized with Nembutal (75 mg/kg), and then perfused intracardially with saline and fixative solution containing 4 % paraformaldehyde. Subsequently, all brains were removed, cryoprotected in sucrose solution, and stored in a −80 °C freezer prior to cryosectioning. Sections in coronal plane (10 μm) were obtained in a cryostat at −28 °C and mounted onto the SuperFrost/Plus slides (Menzek-Gläser, Braunschweig, Germany) and then stored again in the −80 °C freezer until further immunohistochemical staining. Histological study of the electrode tip localization was performed using the standardized hematoxylin–eosin (Merck, Darmstadt, Germany) method.
Immunohistochemistry
c-Fos immunohistochemical staining was performed on the microscopic slides using previously established methods with minor modifications [25, 44]. The coronal sections of cerebelli were obtained from Bregma −11.00 to −11.80 mm based on the rat brain atlas of Paxinos and Watson [43]. The cerebellar sections were incubated in the primary polyclonal rabbit anti-c-Fos antibody (sc-52; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at the concentration of 1:300 in room temperature for 48 h. Then, the sections were incubated in the secondary biotinylated donkey anti-rabbit antibody (Jackson Immunoresearch Laboratories Inc., Westgrove, PA, USA) at the concentration of 1:400 in room temperature for 24 h, followed by 3 h of incubation of avidin–biotin–peroxidase complex (Elite ABC-kit, Vectastatin; Vector, Burlingame, CA, USA). Finally, the immune complex reaction of horseradish peroxidase was visualized by 10 min of incubation of 3, 3′-diaminobenzidine tetrahydrochloride/nickel chloride solution. After staining, all sections were subsequently dehydrated and cover-slipped using Vectamount™ mounting medium (Vector Laboratories Inc., Burlingame, CA, USA). In the negative-control sections, no staining was detected when the primary antibody was omitted.
Image Acquisition and Analysis
The images were obtained using a BX-41 Olympus microscope that was connected to a DP-70 Olympus digital camera with objective of ×10 magnification power. The quantification of c-Fos immunopositive nuclei was performed using the ImageJ software (http://rsb.info.nih.gov/ij/) as previously described [23, 25]. The regions of interest from the deep cerebellar and vestibular nuclei, which included the dentate nucleus of the cerebellum (Dent); the interposed cerebellar nucleus, magnocellular part (IntMC); the interposed cerebellar nucleus, parvicellular part (IntPC); the fastigial cerebellar nucleus (Fast); the medial vestibular nucleus, parvicellular part (MVePC); the medial vestibular nucleus, magnocellular part (MVeMC); and the spinal vestibular nucleus or inferior vestibular nucleus (SpVe), were delineated (Fig. 1). The artifacts and control of background staining were taken into consideration in order to avoid false-positive findings. The optimal threshold for Gray value and particle size were determined for each area and remained the same for all sections. Quantification was conducted in six different sections per animal according to the rat brain atlas of Paxinos and Watson [43].
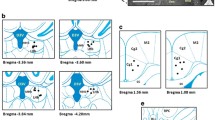
Representative microphotograph showing the digitally superimposed images of the deep cerebellar and vestibular nuclei at Bregma level −11.6 mm. The delineation of the regions of interest was conducted from Bregma −11.0 to −11.8 mm. Abbreviations: Dent dentate nucleus of the cerebellum, IntMC interposed cerebellar nucleus, magnocellular part, IntPC interposed cerebellar nucleus, parvicellular part, Fast fastigial cerebellar nucleus, MVeMC medial vestibular nucleus, magnocellular part, MVePC medial vestibular nucleus, parvicellular part, SpVe spinal vestibular nucleus. Scale bar: 500 μm
Statistical Analyses
The results were analyzed using the IBM SPSS Statistics 23 and the data were presented in boxplots with interquartile ranges. A multivariate analysis with general linear model and ANOVA with Bonferroni post hoc test were performed for detailed multiple comparisons. Independent sample t test was used to compare differences between the variables of the CMS and non-CMS groups. All p values <0.05 were considered significant. In addition, Pearson correlation coefficients were performed to study the association of antidepressant-related behaviors (forced swim test, home-cage emergence test, food intake test, sucrose intake test, and open-field test) and c-Fos activities within the cerebellar and vestibular nuclei. Bonferroni correction was calculated and the p value was adjusted for multiple variable comparisons.
Results
Electrode Localization and Behavior
The localization of electrode tips and the behavioral effects of antidepressant after electrical stimulation in different brain regions (vmPFC, NAc core, NAc shell, and LHb) have been previously reported [5]. The animals with misplacement of electrodes were discarded from the data analysis. A total of 62 rats were used and the data were derived from the average cell count of c-Fos positive nuclei per mm2 with respect to the deep cerebellar and vestibular nuclei (see Table 1; and Supplementary Table 1a).
Stimulation-Induced Changes of c-Fos Expression in Cerebellar–Vestibular Nuclei
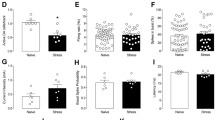
Electrical stimulation in specific brain structure has been previously shown to modulate the expression of c-Fos immediate-early gene within the deep cerebellar nuclei [23, 25]. Here, we further investigated the stimulation effects of the vmPFC, NAc core, NAc shell, and LHb on the c-Fos induction in various deep cerebellar and vestibular nuclei of non-CMS and CMS animal models of depression. Since no significant differences were observed in the c-Fos cell count between the ipsilateral and contralateral regions of the cerebellum (all p values >0.05), the results were pooled from the right and left hemispheres to create an overall estimation. The multivariate general linear model analysis revealed significant main effects for animals with either non-CMS or CMS condition (F = 4.609, p = 0.002), stimulation target group (F = 1.498, p = 0.070; marginal difference), and the interaction (F = 1.570, p = 0.050). In the CMS group, one-way ANOVA revealed significant effects in the Fast (F (4, 24) = 3.668, p = 0.018) and MVeMC (F(4, 22) = 4.780, p = 0.006) and marginally different in the IntMC (F (4, 23) = 2.746, p = 0.053). However, no remarkable differences were found in the Dent, IntPC, MVePC, and SpVe (all F (4, 22–24) < 1.167, p = n.s.) (Figs. 2 and 3; Supplementary Table 1b). Interestingly, the Bonferroni post hoc tests demonstrated that the HFS of the vmPFC decreased c-Fos expression in the Fast (p = 0.027) and MVeMc (p = 0.032) and a marginal reduction in the IntMC (p = 0.078) as compared to the sham (Fig. 4). Likewise, the HFS of the NAc core also decreased the c-Fos expression level within the MVeMC (p = 0.020), but not the other cerebellar and vestibular nuclei.
The boxplots show the high-frequency stimulation effects of the vmPFC, NAc core, NAc shell, and LHb on c-Fos activity within the deep cerebellar nuclei of the non-CMS and CMS animal models of depression. Note: In the CMS group, sham animals had remarkable increase of c-Fos-positive cells in the dentate (a) and fastigial (b) nucleus of the cerebellum. No significant result was found in the interposed cerebellar nuclei (c & d). Interestingly, vmPFC stimulation reduced significantly the c-Fos expression in the fastigial nucleus as compared to the sham. Indication: #significant difference from the non-CMS sham; *significant difference from the CMS sham
The boxplots show the high-frequency stimulation effects of the vmPFC, NAc core, NAc shell, and LHb on c-Fos activity within the vestibular nuclei of the non-CMS and CMS animal models of depression. Note: CMS sham animals significantly increased the c-Fos-positive cells in the spinal vestibular nucleus (c), and a marginal increase was also found in the magnocellular part of the medial vestibular nucleus (a). Interestingly, vmPFC and NAc stimulation showed a remarkable reduction of c-Fos activity within the magnocellular part of the medial vestibular nucleus (a). No significant result was found in the parvicellular part of the medial vestibular nucleus (b). Indication: #significant difference from the non-CMS sham; *significant difference from the CMS sham
Representative photomicrographs of superimposed images showing the effects of high-frequency stimulation of the vmPFC (c), NAc core (d), NAc shell (e), and LHb (f) on c-Fos neuronal activity within the deep cerebellar and vestibular nuclei (Bregma level from −11.30 to −11.60 mm) as compared to the sham CMS (a) and sham non-CMS (b) groups. Note: CMS sham animals significantly increased the number of c-Fos cells (a). Scale bar: 500 μm
In the non-CMS group, we detected no significant effects in the Dent, Fast, IntMC, IntPC, MVeMC, and MVePC (all F (4, 24–27) < 1.280, p = n.s.), except for the SpVe (F (4, 25) = 4.465, p = 0.007) (Supplementary Table 1b). The Bonferroni post hoc test analysis showed no statistical differences when compared to the sham.
For comparison between the sham non-CMS and CMS animals, Student’s t tests showed that CMS sham animals significantly increased the number of c-Fos-positive cells in the Dent (t14 = 3.092, p = 0.008), Fast (t13 = 4.340, p = 0.001), and SpVe (t13 = 2.979, p = 0.010); a tendency of increase was also found in the IntMC (t11 = 2.061, p = 0.064) and MVeMC (t12 = 2.139, p = 0.054). No significant differences were found in the IntPC (t13 = 0.127, p = n.s.) and MVePC (t13 = 0.609, p = n.s.) (Table 1). For additional comparison of results between individual stimulated-groups and the sham animals, see Supplementary Table 1c.
Correlation Analyses of Behavior and c-Fos Expression
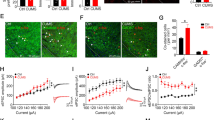
The correlations of the stimulation-induced antidepressant effects and the c-Fos expression within the cerebellar and vestibular nuclei are presented in Fig. 5 and Supplementary Table 2a–e. For correlational analyses, there was a significant positive correlation of the Fast with the IntMC (R 2 = 0.985, p = 0.0001) and IntPC (R 2 = 0.962, p = 0.001) after HFS of the vmPFC in the CMS group (Fig. 5; Supplementary Table 2b). Interestingly, CMS animals with vmPFC HFS showed a remarkable association between the IntMC and the IntPC (R 2 = 0.958, p = 0.0001), as well as association between the MVeMC and the MVeMC (R 2 = 0.988, p = 0.0001). To further investigate the stimulation-induced c-Fos expression on antidepressant-related behaviors, we found a significant positive correlation of the escape latency in the home-cage emergence test with c-Fos activity in the Fast (R 2 = 0.928, p = 0.002), IntMC (R 2 = 0.967, p = 0.0001), and IntPC (R 2 = 0.919, p = 0.003) (Fig. 5c–e; Supplementary Table 2b).
Scatter plots show the correlational association between the deep cerebellar nuclei c-Fos expression and the antidepressant-like behavior. Note: In CMS animals, the fastigial nucleus is highly correlated with the magnocellular (a) and parvicellular (b) parts of the interposed cerebellar nucleus after high-frequency stimulation of the vmPFC. Interestingly, there is a positive correlation in the escape latency of the home-cage emergence test with the fastigial nucleus (c), as well as the magnocellular (d) and parvicellular (e) parts of the interposed cerebellar nucleus. Bonferroni correction was calculated and the p value (significant at p < 0.0036) was adjusted for multiple comparisons
In the non-CMS animals, after HFS of the LHb, there was a positive correlation between the IntMC and the open-field center zone (R 2 = 0.965, p = 0.003), as well as significant association between the IntPC and the escape latency in the home-cage emergence test (R 2 = 0.960, p = 0.003). In the non-CMS vmPFC HFS and CMS LHb HFS groups, no significant correlation was detected between the c-Fos activity of the cerebellar–vestibular nuclei and the antidepressant-related behaviors (Supplementary Table 2). Overall, no correlational association was found between the c-Fos expression in the cerebellar–vestibular nuclei and the antidepressant-related behaviors in the sham, NAc core HFS, and NAc shell HFS of both the CMS and non-CMS groups.
Discussion
The present findings show that the CMS paradigm induced a remarkable increase in the number of c-Fos-positive cells within the cerebellar Dent and Fast, as well as within the SpVe. Further, we have demonstrated that the HFS of the vmPFC reduced significantly the c-Fos neuronal activation within the Fast as compared to the CMS sham. Likewise, HFS of the vmPFC and NAc core also decreased the c-Fos expression levels within the MVeMC in comparison with the CMS sham. Interestingly, the activation of Fast, IntMC, and IntPC nuclei are highly correlated among each other and with the anxiety level of escape latency in the home-cage emergence test after HFS of the vmPFC in CMS animals.
In stress animal models, several studies have demonstrated the hemispheric lateralization that resulted from gender differences [45–47]. Despite the hemispherical asymmetries in the cerebellar or vestibular nuclei that have been described in patients with major and bipolar depression [28, 29, 48], the current CMS model of depression in male rats showed no major differences in c-Fos expression in the cerebellar hemispheres. This suggested a possible lack of cerebellar lateralization in mood-related behaviors of this animal model, and this finding was in line with our previous observations [23, 25, 44]. Currently, no studies of hemispheric lateralization have been reported to associate the mood-related behaviors in the cerebellum of rats. However, it would be an interesting subject to investigate the possibility of gender differences and other indicators in various brain regions in the animal models of depression for potential cerebellar lateralization.
In human imaging studies, there have been several reports demonstrating that the changes of cerebellar activities are associated with the resting frontal function in patients with social anxiety disorders [49, 50], posttraumatic stress disorder [51, 52], and generalized anxiety disorder [53]. The reciprocal connections between the deep cerebellar nuclei and the forebrain regions, namely, the limbic system and the frontal cortical areas, support the involvement of cerebellum in modulation of mood and anxiety responses. In particular, using functional connectivity magnetic resonance imaging (MRI), Allen et al. demonstrated the coherence of MR signal in the Dent to be correlated with signal in the cerebellar, thalamic, limbic, striatal, and cortical regions [54]. This study further provided a functional coherence and connectivity between the cerebellum and forebrain regions. It also supported the non-human primate findings of anatomical projections from the Dent to the prefrontal and posterior parietal cortical regions via the thalamus and the corticopontine–cerebellar connections. The corticopontine projections comprise the motor and associative cortical connections. From the cortical regions, the motor connection projects to the interpeduncular and peripeduncular nuclei and terminate in the anterior cerebellar lobe, while the associative cortical connection projects to the rostromedial pons (from prefrontal cortex); the rostrolateral pons (from parietal cortex); and the dorsolateral, lateral, and dorsal pons (from temporal and visual cortex), and their projections are terminated in the posterior cerebellar lobe which is involved in intellectual and emotional regulation [55, 56].
The Dent plays an important role in motor functions, and to a greater extent, it is also involved in processing cognitive and emotional responses [21]. In lower mammals, the Dent is connected to several associative areas in the forebrain, for instance, the vmPFC [57], and lesions within the Dent have been shown to produce cognitive deficits in the maze-learning tasks of rodents [58]. These findings were in accordance with the clinical data obtained from human studies, where Dent has expanded massively and interconnected with the cortical areas in the regulation of executive, language, mnemonic, and other cognitive functions [21, 22]. It has been demonstrated that the Dent projects to the prefrontal cortex in both the motor and non-motor cortical areas [59, 60]. Thus, stimulation of the Dent in patients produced subjective feelings of unpleasant sensation and fear, indicating the emotional component of this cerebellar nucleus [61]. Besides, the experience of fear sensation has also been reported when the Dent and superior cerebellar peduncle of patients were stimulated [62]. In rats, lesions of the Dent have caused incredible reduction of hedonic and purposive motivational interest [63]. Interestingly, our current data show activation of the Dent in the CMS animals, indicating a possible role of this nucleus in the pathophysiology of emotional responses.
The Fast receives strong afferent projection from Purkinje cells in the vermis, forming the “limbic cerebellum” [64, 65]. This neural circuit has robust anatomical connections with the reticular formation, hypothalamus, and other forebrain limbic structures, which plays a substantial role in the arousal system, autonomic functions, and emotional behaviors [64, 65]. It has been shown that lesions occurring within the Fast and the vermis caused mood dysregulation, and it is generally known as the cerebellar cognitive affective syndrome or the “Schmahmann syndrome” [26]. Likewise, the increase of activity within these areas was correlated with worsening of mood in humans, especially during the premenstrual dysphoric disorder [66].
In the present study of stressed animals, there was a significant increase of c-Fos neuronal activation within the Fast, and HFS of the vmPFC induced a remarkable reduction of its level of expression. In line with this observation of neuronal activity reduction, there was a study also demonstrating a decrease of ΔFosB in the Dent and Fast during the interictal phase of fully kindled rats for epileptic behavior [67]. Although both studies found a similar result of Fos activity reduction with respect to different experimental conditions of either antidepressant effect or epileptic interictal phase, it is important to highlight that the decrease of ΔFosB during the interictal phase can be possibly due to the neuronal network reorganization of the motor function after an episode of seizure attack. In support of this notion, previous studies have indicated that decreased neuronal activity promotes the synaptic strength of neuronal circuits [68, 69].
Nevertheless, the present finding is well-supported by the established anatomical connection of the cerebellum, and importantly, it provides strong evidence on the role of Fast, as part of the “limbic cerebellum” for emotional processing. Interestingly, after vmPFC HFS, we found a positive correlation between the anxiety measure of escape latency in the home-cage emergence test and the deep cerebellar nuclei, in particular of the Fast, IntMC, and IntPC in CMS animals. To further examine the intrinsic relationship of these nuclei, our data revealed that a strong correlation was found among the Fast, IntMC, and IntPC in CMS animals after vmPFC HFS. In anatomical and functional connection, the forebrain–cerebellum relationship was previously established using the anatomical retrograde transneuronal transport of rabies virus [70] and classical eye blink conditioning experiments [71, 72]. Taken together, these observations further support the interactions between the prefrontal cortex and the roles of cerebellum in emotion and cognition.
Since vestibular nuclei have extensive afferent and efferent projections with the deep cerebellar nuclei, we investigated the stimulation effects of vmPFC, NAc, and LHb on these nuclei in both the CMS and non-CMS conditions. The vestibular nuclei received robust cerebellar inputs from the fastigial nuclei, and they were also involved in the control of emotional behaviors and vestibulocerebellum functions [73]. In clinical investigation, there were reports demonstrating that patients with vestibular impairment have high incidence of mood and anxiety-related disorders [74–76], as well as cognitive dysfunction [77]. In the present CMS model, there was a statistical increase of c-Fos expression found in the SpVe, while the MVeMC showed a marginal increase, when compared to the control non-CMS group. Interestingly, after HFS of the vmPFC and NAc core, we showed a reversal of the increased c-Fos neuronal expression in the MVeMC, but not the SpVe. Although we found no direct correlation between the c-Fos expression of the vestibular nuclei and the antidepressant-like behaviors, there was a possibility that the behaviors were modulated by the other brain regions. One of the possible explanations could be due to the non-cerebellar inputs of serotonergic and non-serotonergic pathways from the dorsal raphe nuclei [78, 79], as well as the polysynaptic pathways of hippocampal formation and amygdaloidal connection [80–82] to the vestibular nuclei. In support of this explanation, we have previously demonstrated that HFS of the vmPFC, which enhanced mood-related behaviors, modulated the serotonergic system within the dorsal raphe nucleus, as well as activation of certain brain areas associated with the vestibular nuclei in the CMS animal model of depression [5].
In addition, it is also postulated that the decrease of Fast and MVeMC c-Fos activity after vmPFC stimulation was allegedly caused by the influence of Purkinje cells. Purkinje cells play an inhibitory function on both the Fast and vestibular nuclei that originate from the anterior and posterior cerebellar vermis. It has been demonstrated that the neuronal activity of individual ensemble of Purkinje cells promotes the induction of plasticity and consolidation in the cerebellar and vestibular nuclei by neural interaction with either the mossy fiber or climbing fiber collaterals or among each other [83]. Besides the inhibitory role of Purkinje cells on motor function [84], our present results have suggested a possible antidepressant function of Fast and MVeMC within the deep cerebellar nuclei that is regulated by the inhibitory projections of Purkinje cells. Importantly, recent evidence from theta oscillation studies have revealed the synchronization of neuronal activities between the vmPFC and the cerebellum, particularly in experimental models of associative learning using trace eye blink conditioning [71, 72, 85]. Although the present study has implicated a potential antidepressant role of vmPFC stimulation on the cerebellum, its neural mechanisms by which the interaction of Purkinje cells with the vmPFC and deep cerebellar nuclei remain largely obscure, and comprehensive studies are required to unravel their neural–behavioral interactions.
In conclusion, our present CMS animal model induced specific phenotypic changes of c-Fos neuronal activation within the cerebellar Dent and Fast, as well as the SpVe. However, HFS targeted in the vmPFC significantly reduced the neuronal activity within the Fast as compared to the CMS sham. Our correlation analyses show that the antidepressant-like effects were highly associated with the activation of Fast, IntMC, and IntPC nuclei, and these nuclei were strongly correlated among each other. Taken together, the vmPFC stimulation-induced antidepressant-like behaviors were possibly mediated by a dynamic neurocircuitry remodeling within the forebrain–cerebellum interactions for emotional regulation.
Abbreviations
- CMS:
-
Chronic mild stress
- Dent:
-
Dentate nucleus of the cerebellum
- Fast:
-
Fastigial cerebellar nucleus
- HCET:
-
Home-cage emergence test
- HFS:
-
High-frequency stimulation
- IntMC:
-
Interposed cerebellar nucleus, magnocellular part
- IntPC:
-
Interposed cerebellar nucleus, parvicellular part
- LHb:
-
Lateral habenular nucleus
- MVePC:
-
Medial vestibular nucleus, parvicellular part
- MVeMC:
-
Medial vestibular nucleus, magnocellular part
- NAc core:
-
Nucleus accumbens core
- NAc shell:
-
Nucleus accumbens shell
- SpVe:
-
Spinal vestibular nucleus
- vmPFC:
-
Ventromedial prefrontal cortex
References
Temel Y, Hescham SA, Jahanshahi A, Janssen ML, Tan SK, van Overbeeke JJ, et al. Neuromodulation in psychiatric disorders. Int Rev Neurobiol. 2012;107:283–314. doi:10.1016/B978-0-12-404706-8.00015-2.
Temel Y, Lim LW. Neurosurgical treatments of depression. Curr Top Behav Neurosci. 2013;14:327–39. doi:10.1007/7854_2012_222.
Lim LW, Tan SK, Groenewegen HJ, Temel Y. Electrical brain stimulation in depression: which target(s)? Biol Psychiatry. 2011, 69:e5-6; author reply e7-8. doi 10.1016/j.biopsych.2010.09.056.
Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi:10.1016/j.neuron.2005.02.014.
Lim LW, Prickaerts J, Huguet G, Kadar E, Hartung H, Sharp T, et al. Electrical stimulation alleviates depressive-like behaviors of rats: investigation of brain targets and potential mechanisms. Transl Psychiatry. 2015;5:e535. doi:10.1038/tp.2015.24.
Liu A, Jain N, Vyas A, Lim LW. Ventromedial prefrontal cortex stimulation enhances memory and hippocampal neurogenesis in the middle-aged rats. Elife. 2015, 4. doi 10.7554/eLife.04803.
Lim LW, Janssen ML, Kocabicak E, Temel Y. The antidepressant effects of ventromedial prefrontal cortex stimulation is associated with neural activation in the medial part of the subthalamic nucleus. Behav Brain Res. 2015;279:17–21. doi:10.1016/j.bbr.2014.11.008.
Schlaepfer TE, Bewernick BH, Kayser S, Hurlemann R, Coenen VA. Deep brain stimulation of the human reward system for major depression--rationale, outcomes and outlook. Neuropsychopharmacology. 2014;39:1303–14. doi:10.1038/npp.2014.28.
Holtzheimer 3rd PE, Mayberg HS. Deep brain stimulation for treatment-resistant depression. Am J Psychiatry. 2010;167:1437–44. doi:10.1176/appi.ajp.2010.10010141.
Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2008;64:461–7. doi:10.1016/j.biopsych.2008.05.034.
Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–8. doi:10.1001/archgenpsychiatry.2011.1456.
Jimenez F, Velasco F, Salin-Pascual R, Hernandez JA, Velasco M, Criales JL, Nicolini H. A patient with a resistant major depression disorder treated with deep brain stimulation in the inferior thalamic peduncle. Neurosurgery. 2005, 57:585-93; discussion -93.
Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–77. doi:10.1038/sj.npp.1301408.
Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–6. doi:10.1016/j.biopsych.2009.09.013.
Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–85. doi:10.1038/npp.2012.44.
Malone Jr DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–75. doi:10.1016/j.biopsych.2008.08.029.
Dougherty DD, Rezai AR, Carpenter LL, Howland RH, Bhati MT, O’Reardon JP, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78:240–8. doi:10.1016/j.biopsych.2014.11.023.
Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, et al. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi:10.1016/j.biopsych.2009.08.027.
Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–12. doi:10.1016/j.biopsych.2013.01.034.
Temel Y, Tan S, Vlamings R, Sesia T, Lim LW, Lardeux S, Visser-Vandewalle V, Baunez C. Cognitive and limbic effects of deep brain stimulation in preclinical studies. Front Biosci 2009;14(5):1891–1901. doi: 10.2741/3349
Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–2. doi:10.1093/brain/awh729.
Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996, 4:174-98. doi 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0.
Moers-Hornikx VM, Sesia T, Basar K, Lim LW, Hoogland G, Steinbusch HW, et al. Cerebellar nuclei are involved in impulsive behaviour. Behav Brain Res. 2009;203:256–63. doi:10.1016/j.bbr.2009.05.011.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–44. doi:10.1016/j.cortex.2009.11.008.
Moers-Hornikx VM, Vles JS, Lim LW, Ayyildiz M, Kaplan S, Gavilanes AW, et al. Periaqueductal grey stimulation induced panic-like behaviour is accompanied by deactivation of the deep cerebellar nuclei. Cerebellum. 2011;10:61–9. doi:10.1007/s12311-010-0228-z.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–79.
Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 2013;3:332–9. doi:10.1016/j.nicl.2013.08.016.
Liang MJ, Zhou Q, Yang KR, Yang XL, Fang J, Chen WL, et al. Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting-state FMRI. PLoS One. 2013;8:e79999. doi:10.1371/journal.pone.0079999.
Guo W, Liu F, Liu J, Yu L, Zhang Z, Zhang J, et al. Is there a cerebellar compensatory effort in first-episode, treatment-naive major depressive disorder at rest? Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:13–8. doi:10.1016/j.pnpbp.2013.06.009.
Jiang WH, Yuan YG, Zhou H, Bai F, You JY, Zhang ZJ. Abnormally altered patterns of whole brain functional connectivity network of posterior cingulate cortex in remitted geriatric depression: a longitudinal study. CNS Neurosci Ther. 2014;20:772–7. doi:10.1111/cns.12250.
Frank B, Andrzejewski K, Goricke S, Wondzinski E, Siebler M, Wild B, et al. Humor, laughter, and the cerebellum: insights from patients with acute cerebellar stroke. Cerebellum. 2013;12:802–11. doi:10.1007/s12311-013-0488-5.
Akil H, Statham PF, Gotz M, Bramley P, Whittle IR. Adult cerebellar mutism and cognitive-affective syndrome caused by cystic hemangioblastoma. Acta Neurochir (Wien). 2006;148:597–8. doi:10.1007/s00701-005-0646-8.
Diaconescu AO, Kramer E, Hermann C, Ma Y, Dhawan V, Chaly T, et al. Distinct functional networks associated with improvement of affective symptoms and cognitive function during citalopram treatment in geriatric depression. Hum Brain Mapp. 2011;32:1677–91. doi:10.1002/hbm.21135.
Zeng LL, Liu L, Liu Y, Shen H, Li Y, Hu D. Antidepressant treatment normalizes white matter volume in patients with major depression. PLoS One. 2012;7:e44248. doi:10.1371/journal.pone.0044248.
Chiu R, Boyle WJ, Meek J, Smeal T, Hunter T, Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988;54:541–52.
Hestermann D, Temel Y, Blokland A, Lim LW. Acute serotonergic treatment changes the relation between anxiety and HPA-axis functioning and periaqueductal gray activation. Behav Brain Res 2014, 273:155-65. doi 10.1016/j.bbr.2014.07.003.
Lim LW, Blokland A, van Duinen M, Visser-Vandewalle V, Tan S, Vlamings R, Janssen M, Jahanshahi A, Aziz-Mohammadi M, Steinbusch HW, Schruers K, Temel Y. Increased plasma corticosterone levels after periaqueductal gray stimulation-induced escape reaction or panic attacks in rats. Behav Brain Res 2011, 218:301-7. doi 10.1016/j.bbr.2010.12.026.
Lim LW, Temel Y, Visser-Vandewalle V, Blokland A, Steinbusch H. Fos immunoreactivity in the rat forebrain induced by electrical stimulation of the dorsolateral periaqueductal gray matter. J Chem Neuroanat. 2009;38:83–96. doi:10.1016/j.jchemneu.2009.06.011.
Temel Y, Blokland A, Lim LW. Deactivation of the parvalbumin-positive interneurons in the hippocampus after fear-like behaviour following electrical stimulation of the dorsolateral periaqueductal gray of rats. Behav Brain Res. 2012;233:322–5. doi:10.1016/j.bbr.2012.05.029.
Lim LW, Temel Y, Sesia T, Vlamings R, Visser-Vandewalle V, Steinbusch HW and Blokland A. Buspirone induced acute and chronic changes of neural activation in the periaqueductal gray of rats. Neuroscience. 2008, 155:164-73. doi 10.1016/j.neuroscience.2008.05.038.
Lim LW, Blokland A, Tan S, Vlamings R, Sesia T, Aziz-Mohammadi M, Visser-Vandewalle V, Steinbusch HW, Schruers K, Temel Y. Attenuation of fear-like response by escitalopram treatment after electrical stimulation of the midbrain dorsolateral periaqueductal gray. Exp Neurol. 2010, 226:293-300. doi 10.1016/j.expneurol.2010.08.035.
Tan S, Vlamings R, Lim L, Sesia T, Janssen ML, Steinbusch HW, Visser-Vandewalle V, Temel Y. Experimental deep brain stimulation in animal models. Neurosurgery. 2010, 67:1073-9; discussion80. doi 10.1227/NEU.0b013e3181ee3580.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. China: Academic Press of Elsevier; 2007.
Moers-Hornikx VMP, Vles JSH, Hemmes RJM, Lim LW, Hoogland G, Steinbusch HWM, et al. c-Fos expression in the deep cerebellar nuclei in a rat model of conditioned fear. J Exp Clin Med. 2012;29:201–7. doi:10.5835/jecm.omu.29.03.007.
Sullivan RM, Dufresne MM, Waldron J. Lateralized sex differences in stress-induced dopamine release in the rat. Neuroreport. 2009;20:229–32. doi:10.1097/WNR.0b013e3283196b3e.
Weathington JM, Puhy C, Hamki A, Strahan JA, Cooke BM. Sexually dimorphic patterns of neural activity in response to juvenile social subjugation. Behav Brain Res. 2013;256:464–71. doi:10.1016/j.bbr.2013.08.042.
Schulz KM, Andrud KM, Burke MB, Pearson JN, Kreisler AD, Stevens KE, et al. The effects of prenatal stress on alpha4 beta2 and alpha7 hippocampal nicotinic acetylcholine receptor levels in adult offspring. Dev Neurobiol. 2013;73:806–14. doi:10.1002/dneu.22097.
Soza Ried AM, Aviles M. Asymmetries of vestibular dysfunction in major depression. Neuroscience. 2007;144:128–34. doi:10.1016/j.neuroscience.2006.09.023.
Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE, et al. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31:2243–53. doi:10.1038/sj.npp.1301053.
Warwick JM, Carey P, Jordaan GP, Dupont P, Stein DJ. Resting brain perfusion in social anxiety disorder: a voxel-wise whole brain comparison with healthy control subjects. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1251–6. doi:10.1016/j.pnpbp.2008.03.017.
Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–95. doi:10.1176/ajp.156.11.1787.
Bonne O, Gilboa A, Louzoun Y, Brandes D, Yona I, Lester H, et al. Resting regional cerebral perfusion in recent posttraumatic stress disorder. Biol Psychiatry. 2003;54:1077–86.
Blair K, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, et al. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am J Psychiatry. 2008;165:1193–202. doi:10.1176/appi.ajp.2008.07071060.
Allen G, McColl R, Barnard H, Ringe WK, Fleckenstein J, Cullum CM. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. Neuroimage. 2005;28:39–48. doi:10.1016/j.neuroimage.2005.06.013.
Kamali A, Kramer LA, Frye RE, Butler IJ, Hasan KM. Diffusion tensor tractography of the human brain cortico-ponto-cerebellar pathways: a quantitative preliminary study. J Magn Reson Imaging. 2010;32:809–17. doi:10.1002/jmri.22330.
Leergaard TB, Bjaalie JG. Topography of the complete corticopontine projection: from experiments to principal Maps. Front Neurosci. 2007;1:211–23. doi:10.3389/neuro.01.1.1.016.2007.
Rogers TD, Dickson PE, Heck DH, Goldowitz D, Mittleman G, Blaha CD. Connecting the dots of the cerebro-cerebellar role in cognitive function: neuronal pathways for cerebellar modulation of dopamine release in the prefrontal cortex. Synapse. 2011;65:1204–12. doi:10.1002/syn.20960.
Lalonde R, Strazielle C. The effects of cerebellar damage on maze learning in animals. Cerebellum. 2003;2:300–9. doi:10.1080/14734220310017456.
Dum RP, Li C, Strick PL. Motor and nonmotor domains in the monkey dentate. Ann N Y Acad Sci. 2002;978:289–301.
Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–9. doi:10.1152/jn.00626.2002.
Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 1982;2010(20):236–60. doi:10.1007/s11065-010-9142-x.
Nashold Jr BS, Slaughter DG. Effects of stimulating or destroying the deep cerebellar regions in man. J Neurosurg. 1969;31:172–86. doi:10.3171/jns.1969.31.2.0172.
Bauer DJ, Kerr AL, Swain RA. Cerebellar dentate nuclei lesions reduce motivation in appetitive operant conditioning and open field exploration. Neurobiol Learn Mem. 2011;95:166–75. doi:10.1016/j.nlm.2010.12.009.
Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum. 2007;6:254–67. doi:10.1080/14734220701490995.
Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–53. doi:10.1093/brain/awl359.
Rapkin AJ, Berman SM, Mandelkern MA, Silverman DH, Morgan M, London ED. Neuroimaging evidence of cerebellar involvement in premenstrual dysphoric disorder. Biol Psychiatry. 2011;69:374–80. doi:10.1016/j.biopsych.2010.09.029.
Rijkers K, Moers-Hornikx VM, Hemmes RJ, Aalbers MW, Temel Y, Vles JS, et al. Sustained reduction of cerebellar activity in experimental epilepsy. Biomed Res Int. 2015;2015:718591. doi:10.1155/2015/718591.
Nakayama K, Kiyosue K, Taguchi T. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J Neurosci. 2005;25:4040–51. doi:10.1523/JNEUROSCI.4115-04.2005.
Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–8. doi:10.1038/nature01242.
Lu X, Miyachi S, Takada M. Anatomical evidence for the involvement of medial cerebellar output from the interpositus nuclei in cognitive functions. Proc Natl Acad Sci U S A. 2012;109:18980–4. doi:10.1073/pnas.1211168109.
Wu GY, Yao J, Fan ZL, Zhang LQ, Li X, Zhao CD, et al. Classical eyeblink conditioning using electrical stimulation of caudal mPFC as conditioned stimulus is dependent on cerebellar interpositus nucleus in guinea pigs. Acta Pharmacol Sin. 2012;33:717–27. doi:10.1038/aps.2012.32.
Wang YJ, Chen H, Hu C, Ke XF, Yang L, Xiong Y, et al. Baseline theta activities in medial prefrontal cortex and deep cerebellar nuclei are associated with the extinction of trace conditioned eyeblink responses in guinea pigs. Behav Brain Res. 2014;275:72–83. doi:10.1016/j.bbr.2014.08.059.
Carleton SC, Carpenter MB. Afferent and efferent connections of the medial, inferior and lateral vestibular nuclei in the cat and monkey. Brain Res. 1983;278:29–51.
Godemann F, Linden M, Neu P, Heipp E, Dorr P. A prospective study on the course of anxiety after vestibular neuronitis. J Psychosom Res. 2004;56:351–4. doi:10.1016/S0022-3999(03)00079-5.
Godemann F, Siefert K, Hantschke-Bruggemann M, Neu P, Seidl R, Strohle A. What accounts for vertigo one year after neuritis vestibularis—anxiety or a dysfunctional vestibular organ? J Psychiatr Res. 2005;39:529–34. doi:10.1016/j.jpsychires.2004.12.006.
Kalueff AV, Ishikawa K, Griffith AJ. Anxiety and otovestibular disorders: linking behavioral phenotypes in men and mice. Behav Brain Res. 2008;186:1–11. doi:10.1016/j.bbr.2007.07.032.
Smith PF, Zheng Y, Horii A, Darlington CL. Does vestibular damage cause cognitive dysfunction in humans? J Vestib Res. 2005;15:1–9.
Halberstadt AL, Balaban CD. Organization of projections from the raphe nuclei to the vestibular nuclei in rats. Neuroscience. 2003;120:573–94.
Balaban CD. Neural substrates linking balance control and anxiety. Physiol Behav. 2002;77:469–75.
Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septohippocampal System, 2nd Edition..Oxford University Press 2000.
Halberstadt AL, Balaban CD. Anterograde tracing of projections from the dorsal raphe nucleus to the vestibular nuclei. Neuroscience. 2006;143:641–54. doi:10.1016/j.neuroscience.2006.08.013.
Halberstadt AL, Balaban CD. Serotonergic and nonserotonergic neurons in the dorsal raphe nucleus send collateralized projections to both the vestibular nuclei and the central amygdaloid nucleus. Neuroscience. 2006;140:1067–77. doi:10.1016/j.neuroscience.2006.02.053.
Wulff P, Schonewille M, Renzi M, Viltono L, Sassoe-Pognetto M, Badura A, et al. Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat Neurosci. 2009;12:1042–9. doi:10.1038/nn.2348.
Verduzco-Flores S. Stochastic synchronization in purkinje cells with feedforward inhibition could be studied with equivalent phase-response curves. J Math Neurosci. 2015;5:25. doi:10.1186/s13408-015-0025-6.
Chen H, Wang YJ, Yang L, Sui JF, Hu ZA, Hu B. Theta synchronization between medial prefrontal cortex and cerebellum is associated with adaptive performance of associative learning behavior. Sci Rep. 2016;6:20960. doi:10.1038/srep20960.
Acknowledgments
The authors are thankful to Gara Lopez and Maria Ruiz for technical assistance in histochemical works. The scientific works were funded by the Netherlands Organization for Scientific Research (NWO-VENI no. 016.096.032), the University of Hong Kong Seed Funding Program for Basic Research (201604159006), and the Hong Kong UGC-ECS Grant (27104616).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
• CMS increased c-Fos expression in the Dent Fast and SpVe.
• vmPFC HFS decreased c-Fos expression in the Fast and MVeMC.
• In CMS, HCET anxiety is correlated with the Fast, IntMC, and IntPC after vmPFC HFS.
• In CMS, the Fast is correlated with the IntMC and IntPC after vmPFC HFS.
• vmPFC HFS induced antidepressant via forebrain-cerebellar neurocircuitry remodeling.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(a) shows the groups and the number of animals used for the non-CMS and CMS treatment conditions. Animals were implanted with stimulating electrodes in the vmPFC, NAc core, NAc shell, and LHb based on the rat brain atlas of Paxinos and Watson [43]. (b) shows the significant effects of data analyzed by a multivariate General Linear Model to determine the within- and between-subjects factor groups, as well as oneway ANOVA with Bonferroni post hoc tests for detailed multiple comparisons. All p values < 0.05 were considered statistically significance. (c) shows the significant effects of data comparison between the individual stimulated-group and sham animals. The data were analyzed by independent sample t-test, and all p values < 0.05 were considered statistically significance. (DOC 81 kb)
Supplementary Table 2
The tables show the Pearson correlation coefficients between variables related with the antidepressant-like behaviors and the c-Fos neuronal activities within the deep cerebellar and vestibular nuclei. The tables display correlations for sham groups (a), and animals subject to high-frequency stimulation of the vmPFC (b), NAc core (c), NAc shell (d), and the LHb (e). The lower-left side of the tables shows correlations of non-CMS animals, while the upper-right side shows correlations of animals with CMS. For statistical significance, Bonferroni correction was calculated and the p-value was adjusted for multiple comparisons. Indication: *, correlation is significant with p < 0.0036. (DOC 222 kb)
Rights and permissions
About this article
Cite this article
Huguet, G., Kadar, E., Temel, Y. et al. Electrical Stimulation Normalizes c-Fos Expression in the Deep Cerebellar Nuclei of Depressive-like Rats: Implication of Antidepressant Activity. Cerebellum 16, 398–410 (2017). https://doi.org/10.1007/s12311-016-0812-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-016-0812-y