Abstract
Neuroimaging studies suggest that the cerebellum contributes to human cognitive processing, particularly procedural learning. This type of learning is often described as implicit learning and involves automatic, associative, and unintentional learning processes. Our aim was to investigate whether cerebellar transcranial direct current stimulation (tDCS) influences procedural learning as measured by the serial reaction time task (SRTT), in which subjects make speeded key press responses to visual cues. A preliminary modeling study demonstrated that our electrode montage (active electrode over the cerebellum with an extra-cephalic reference) generated the maximum electric field amplitude in the cerebellum. We enrolled 21 healthy subjects (aged 20–49 years). Participants did the SRTT, a visual analogue scale and a visual attention task, before and 35 min after receiving 20-min anodal and sham cerebellar tDCS in a randomized order. To avoid carry-over effects, experimental sessions were held at least 1 week apart. For our primary outcome measure (difference in RTs for random and repeated blocks) anodal versus sham tDCS, RTs were significantly slower for sham tDCS than for anodal cerebellar tDCS (p = 0.04), demonstrating that anodal tDCS influenced implicit learning processes. When we assessed RTs for procedural learning across the one to eight blocks, we found that RTs changed significantly after anodal stimulation (interaction “time” × “blocks 1/8”: anodal, p = 0.006), but after sham tDCS, they remained unchanged (p = 0.094). No significant changes were found in the other variables assessed. Our finding that anodal cerebellar tDCS improves an implicit learning type essential to the development of several motor skills or cognitive activity suggests that the cerebellum has a critical role in procedural learning. tDCS could be a new tool for improving procedural learning in daily life in healthy subjects and for correcting abnormal learning in neuropsychiatric disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since antiquity, humans have sought in various ways to improve their cognitive abilities [1, 2], the mental skills we use for gaining, processing, storing, and retrieving information. Among traditional ways used for improving cognitive performance are education, cognitive training, mastering psychological techniques, drinking coffee or energy drinks, meditation, exercise, sleep, and taking herbal or vitamin supplements. Research over recent years has proposed numerous novel ways for improving cognitive performance. Convincing evidence shows that transcranial direct current stimulation (tDCS) over the brain cortex enhances cognition and facilitates motor learning [3].
Although cognitive functioning (information processing) takes place mainly in the prefrontal cortex, other participating areas include the cerebellum [4–7]. The cerebellum participates also in procedural learning, a major learning type that takes place daily without our intent or conscious awareness, and plays a major role in structuring our skills, perceptions, and behavior [8, 9].
Despite extensive research, the cerebellar role in procedural learning remains controversial [10]. The original idea that the cerebellum is a learning machine [11, 12] receives support from data showing that this brain area is essential for adaptive changes in reflex behavior and is activated during motor learning [13–15].
In our previous studies, we showed that cerebellar tDCS modulates performance in a working memory task in healthy subjects [16] and is specifically involved in emotional processing [17]. A subsequent study using a different experimental approach confirmed that cerebellar tDCS induces beneficial effects and reported cerebellar–TMS-induced changes in motor cortical excitability [18]. No study has to our knowledge investigated whether and how cerebellar tDCS modulates procedural learning. Knowing more about cerebellar tDCS-induced changes in learning would help in developing new applications for tDCS in cognition, behavior, and psychiatric illness.
Our primary aim in this study addressing the cerebellar role in human learning was to investigate whether cerebellar tDCS influences procedural learning and to find out whether this brain area intervenes directly in procedural learning. To do so, in healthy adult volunteers, before cerebellar tDCS session and 35 min after it ended, we tested implicit procedural learning with a serial reaction time task (SRTT) [19, 20]. To investigate whether the primary outcome measure, cerebellar tDCS-induced changes in SRTT responses, were specific for learning or reflected changes in arousal or attention, before and after cerebellar tDCS, we tested all subjects with a visual attention task. To check whether tDCS influences mental fatigue and attention, we also tested subjects with a 100-mm visual analog scale (VAS).
Material and Methods
Subjects
Twenty-one healthy right-handed (assessed by the Edinburgh Handedness Inventory) volunteers (aged 20–49 years; 12 women and 9 men) participated in the study. All subjects provided informed consent. The procedures were approved by the institutional review board and were conducted in accordance with the Declaration of Helsinki. Before and after tDCS, all participants underwent a neurological examination using paper-and-pencil tests of motor-graphics (signature, Archimedes spiral, and horizontal lines test). None of the participants had a history of medical, neurological, or psychiatric disorders or were taking acute or chronic medications affecting the central nervous system.
Serial Reaction Time Task (SRTT)
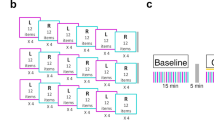
As the main outcome measure we used a SRTT (Wadsworth CogLab software Publishing, Belmont, CA, USA). Each subject was seated in front of a computer screen (visual distance, 50–70 cm), with two hands/four fingers positioned upon four keys (right hand: forefinger on K, middle finger on L; left hand: forefinger on S, middle finger on A) (Fig. 1a).
The SRTT is performed bimanually; subjects were instructed to press one of the four keys as soon as a circle appeared on the screen: “A” when it appeared on the left, “S” on the middle–left, “K” on the middle–right, and “L” on the right. The subjects had to keep the two fingers of the left and right hands on the keys and were asked to press the key corresponding to the position in which the circle appeared on the screen as quickly and accurately as possible.
The target circle disappeared only when the correct key was pressed and the new stimulus was displayed. In this version of the SRTT, we tested 12 blocks, comprising sequences containing 12 circle positions, each one repeated twice. In the tenth block, the circles appeared in random order, whereas in the other blocks they always appeared in the same sequence comprising 12 stimuli (K, L, S, K, A, S, A, L, K, S, L, A), i.e., each block began with a circle in location K, then a circle in location L, and so on. Because we wanted to test procedural learning, subjects were not told about this repeating sequence. For each session, we analyzed data from 288 trials. Each trial took only a few seconds, and after 24 trials, subjects were allowed to rest. The reaction times (RTs) were used for further analysis.
Visual Analog Scale (VAS)
Before and after each tDCS session, subjects described their attentional levels and perceived mental fatigue using a VAS that ranged from 0 (best attention; no fatigue) to 100 (worst attention ever; worst fatigue ever). The VAS consisted of a horizontal line, 100 mm in length, anchored at each end by word descriptors. The subject marked on the line the point they felt best represented at that moment. The VAS score was calculated by measuring in millimeters the distance from the left-hand end of the line to the point that the patient marked.
Visual Attention Task
We also used a visual attention task to investigate whether tDCS-induced changes in the primary outcome variable were specific for learning or reflected changes in arousal or attention. We used an endogenous cue version of the Posner paradigm [21] using a computer-controlled procedure (Wadsworth CogLab software Publishing, Belmont, CA, USA). In this task, the participants responded to targets displayed on the screen at one of two locations (left or right) on either side of the fixation mark. Before the target appeared, one location was cued with an arrow to attract subjects’ attention. For valid cues, the target appeared on the same side as indicated by the arrow, for invalid cues it appeared on the opposite side, and for neutral cues it appeared without a preceding arrow. The task required a right index finger response after each stimulus, regardless of its spatial frequency or location. Subjects were instructed to respond quickly and accurately and to maintain central eye fixation during the trials. The RTs for valid, invalid, and neutral cues were collected before and after tDCS and used for further analysis.
Cerebellar tDCS
Cerebellar tDCS [16, 17] was delivered with an electrical constant direct current stimulator via a pair of a rectangular saline-soaked synthetic sponge electrodes (5 × 7 cm): the active electrode was centered on the median line 2 cm below the inion with its lateral borders about 1 cm medially to the mastoid apophysis (over the cerebellum) and the reference electrode over the right arm. This extra-cephalic montage has already been used in previous cerebellar tDCS study [17] and avoids the confounding effects induced by two electrodes with opposite polarities over the scalp.
The stimulating current was an anodal direct current at 2 mA intensity delivered for 20 min over the cerebellum. We use only anodal tDCS because our previous experiments [16, 17] disclosed no difference between anodal and cathodal tDCS applied over the cerebellum.
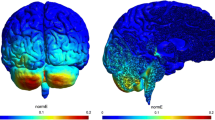
Electric fields (E) and current density (J) distributions were obtained by a computational method applied to a realistic human model. These variables were simulated using the platform SEMCAD X (Schmid & Partner Engineering AG, Zurich, Switzerland), solving the Laplace equation (\( \nabla \cdot \left( {\sigma \nabla \phi } \right)=0 \)) where σ is the electrical conductivity in human tissues. The E and J field distributions were obtained from the following equations: \( E=-\nabla \varphi \) and \( J=\sigma E \).
We used a realistic human model of the Virtual Family [22], based on high-resolution MRI for a healthy volunteer (“Ella”: a 26-year-old female) and segmented into a voxel-based format at a resolution of 1 mm. The models comprise up to 77 different tissues, whose dielectric properties were assigned according to a previously reported approach [23, 24]. The electrodes were modeled as rectangular pads of copper (σ = 5.9 × 107 S/m) with a rectangular sponge (σ = 0.3 S/m) which rested directly under the electrode. The potential difference between the electrodes was adjusted to inject a total current of 2 mA. For each simulation, the human model and the electrodes were inserted in a surrounding bounding box filled with air. The boundaries of the bounding box were treated as insulated, i.e., the normal component of current density was set equal to zero.
To illustrate the problem geometrically as viewed from the back and two sides (Fig. 2a), we plotted E amplitude distributions on the cortex and cerebellum (Fig. 2b). The maximum for the color scale was set to equal the peak field distribution on the cerebellum, the brain region inducing the highest E and J (Fig. 2b). Preliminary modeling studies demonstrated that electric field distribution generated by cerebellar tDCS symmetrically involved the cerebellum (under review).
When stimulation began, subjects felt the current at both electrodes as a mild itching sensation that disappeared after a few seconds thereafter leaving tDCS unperceived. For sham tDCS, electrodes were placed as for real stimulation, but the stimulator was turned off after 30 s. The subjects therefore felt the initial itching sensation when stimulation began but thereafter received no current.
Experimental Protocol
We used a within-subjects, randomized design in which two stimulation modalities (anodal and sham, i.e., placebo) were tested in separate sessions held at least 1 week apart (to avoid carry-over effects) and presented in a counterbalanced order, as described elsewhere [17]. The subjects were blind to the type of tDCS delivered in each session.
For each experimental session, we administered the visual attention task, the SRTT, and the VAS at baseline and 35 min after tDCS ended (post-stimulation) (Fig. 1b).
Statistical Analysis
Statistical calculations were done with Stata 12 SE (Statacorp, USA). All dependent variables were normally distributed and were therefore analyzed with parametric tests (repeated-measures ANOVA). Significance p level was set at 0.05. Because this was an exploratory study, values were not corrected for multiple comparisons. To compare whether RTs in the SRTT differed at baseline (pre-stimulation), we ran a two-way repeated-measures analysis of variance (ANOVA), with the factors “stimulation” (two levels: anodal and sham) and “blocks 1/12” (12 levels: B1, B2, B3, B4, B5, B6, B7, B8, B9, B10, B11, and B12) as independent variables.
In accordance with Robertson et al. [25], to estimate implicit motor learning, we used two SRTT indexes. Because implicit learning can be measured by assessing the delay in the sequence for the repeated block after the participant played out the sequential, repeated blocks [25], the first index assessed differences in RTs between random and repeated blocks. Similarly to Abrahamse et al. [26], we compared the RTs for the random block (i.e., block 10) versus the repeated blocks immediately before and after (i.e., blocks 9 and 11) the random block. We therefore generated the difference between the repeated and the random blocks for each stimulation condition (anodal, sham) and time (baseline, post-stimulation) and used t tests to compare them. Because implicit learning can also be assessed by assessing whether RTs decrease after each repeated block [25], the second SRTT index assessed the RT decrease during repeated blocks. For each stimulation condition (anodal and sham), we therefore used RTs as the dependent variable and the factors “time” (two levels: baseline, post-stimulation) and “blocks 1/8” (eight levels: B1, B2, B3, B4, B5, B6, B7, B8, and B8) as independent variables.
We also used a mixed-design ANOVA to analyze the difference in RT, with time (two levels: pre and post) and stimulation (two levels: anodal and sham) as the independent variables.
Finally, to find out whether cerebellar tDCS-induced changes were non-specific, i.e., secondary to changes in visual attention, we evaluated performance in the visual attention task by calculating the percentage (baseline = 100 %) change during the task as (post-stimulation-baseline)/(baseline). This value was used as a dependent variable to compare percentage changes across stimulation types through a one-way repeated-measures ANOVA (factor “stimulation”). We also evaluated effects in mental fatigue and attention (VAS scores) with the factors “stimulation” (two levels: anodal, sham) and “time” (two levels: baseline, post-stimulation) as independent variables.
Results
No significant interaction was found between “stimulation” × “blocks 1/12” at baseline for SRRT RTs (p = 0.47). Hence, these variables had similar baseline values in all groups. For our primary outcome (difference in RTs between random and repeated blocks), no difference was found at baseline between anodal and sham stimulation (t(20) = 0.22, p = 0.82) (Fig. 3).
Conversely, after tDCS, the stimulation conditions were significantly different (t(20) = 2.1, p < 0.05). Post hoc tests comparing repeated versus random blocks disclosed that participants were significantly slower after anodal tDCS than after sham tDCS (M = 37 ms, SE = 14). Implicit learning therefore increased as subjects played out the random block and did so more “automatically” after anodal tDCS than after sham tDCS.
We have also assessed RTs for procedural learning across the one to eight blocks. The one-way repeated-measures ANOVA showed that RTs changed significantly after anodal stimulation (interaction “time” × “blocks 1/8”: anodal stimulation F(7,140) = 3.12; p < 0.01) (Fig. 4b); but after sham, tDCS remained unchanged (F(7,140) = 0.46; p = 0.86) (Fig. 4a). Post hoc analysis disclosed that anodal tDCS reduced RTs in all blocks except one (B1, t(20) = 4, p < 0.01; B2, t(20) = 4.8 p < 0.001; B3, t(20) = 5.4, p < 0.01; B4, t(20) = 3.6, p < 0.01; B5, t(20) = 6.2, p < 0.01; B6, t(20) = 0.81, p = 0.42; B7, t(20) = 2.3, p < 0.05; B8, t(20) = 2.6, p < 0.01).
We found no significant effects of the main effects of time (F(40,1) = 0.3, p = 0.54) and type of stimulation (F(40,1) = 1.3, p = 0.26), although we observed a significant time × stimulation interaction (F(40,1) = 4.6, p = 0.04), with post-stimulation RTs being significantly slower for sham tDCS than for anodal cerebellar tDCS.
Finally, no significant changes were found in visual attention performance for any stimulation condition: Anodal cerebellar tDCS induced no specific changes that differed significantly from those after sham cerebellar tDCS (p > 0.05). Likewise, VAS scores (mental fatigue and attention) remained unchanged over time.
Discussion
The main result in this study is that anodal cerebellar tDCS influenced procedural learning as indexed by the SRTT in healthy subjects. This new finding accords with current knowledge that anodal tDCS increases cortical excitability and focally improves cognitive function [3]. Hence, anodal tDCS presumably improved our healthy subjects’ performance in the SRTT test by focally improving cerebellar function. Because mood and fatigue VAS and visual attention task measures remained unchanged per time and stimulation the tDCS-induced changes in SRTT performance did not reflect changes in arousal or alertness. Hence, tDCS over the cerebellum modulated and improved our healthy subjects’ performance during procedural learning. Because our preliminary modeling study demonstrated that the stimulating electrode montage we used generated the maximum electric field density within the cerebellum, we can reliably attribute these learning changes to cerebellar tDCS.
This result also fit with our previous study [16] showing that cerebellar tDCS left visual evoked potentials unaffected.
Why cerebellar tDCS improved procedural learning as measured by the SRTT in our healthy subjects whereas in the study by Torriero et al. [27] cerebellar repetitive transcranial magnetic stimulation (rTMS), another non-invasive brain stimulation technique, impaired SRTT performance remains unclear. A possible explanation is that rTMS non-specifically triggers neuronal firing, leading to a pro-stochastic brain network that is thereby transiently impaired—a condition usually known as a “virtual lesion” [28]. Conversely, instead of triggering neuronal activity per se, tDCS fine-tunes brain network activity. Procedural learning performance might therefore have increased after anodal tDCS in our study but diminished after rTMS in the study by Torriero et al. because anodal tDCS induces stronger cerebellar changes than rTMS. Whatever the reason, both studies again underline that the cerebellum is a key structure in procedural learning that can be purposefully modulated by noninvasive brain stimulation.
As described in our previous study [16], we speculate that tDCS acts to influence cerebellar function in at least two ways. First, tDCS could alter the membrane potential fine tuning needed for long-term depression (LTD) [29]. Current knowledge suggests that Purkinje cell LTD can play a role not only in motor function but also in cognitive tasks [30]. Alternatively, tDCS could directly alter cerebellar cortex neuronal cell membrane properties perturbing signal processing in the cerebellar cortex. In rats, changes in membrane properties and ionic conductances lead to changes in Purkinje cell intrinsic pacemaking and ultimately interfere with their synaptic information [31]. Cerebellar deficits are characterized by abnormalities in pacemaking mechanisms in the Purkinje cells, and when pacemaking is normalized, normal cerebellar function can be restored [32]. By analogy, we therefore argue that tDCS subtly impairs Purkinje-cell pacemaking, thus interfering with the way in which they process cognitive information.
Our findings also provide insights on the role of specific brain areas in the various memory learning systems. The cerebellar tDCS-induced changes in implicit sequence learning we identified in healthy volunteers also fit in well with neuroimaging study showing frontoparietal activation during explicit learning (for facts and events) [33] and cerebellar activation during procedural learning [34]. The brain encodes explicit memories as facts or events; these memories may form even after a single exposure, are available to conscious recollection, and receive distinct support from regions within the medial temporal lobe. In contrast, implicit memories, the memory form tested by the SRRT, underpin skill acquisition, develop slowly with practice, and are inaccessible to conscious recall [35, 36].
Our finding that tDCS over the cerebellum specifically enhanced our subjects’ learning ability during the first phase corroborates and extends current knowledge on the cerebellum’s role in learning. Clinical data and several studies using functional neuroimaging show that the neural substrate mediating procedural learning involves a brain network including the cerebellum, striatum, and frontal lobe motor areas [34, 37–40]. Each of these has distinct roles in acquiring or retaining motor skills or both. The cerebellum is active during the first phase in the learning process [34, 41], and the brain recruits the neural system, including the striatum and motor cortical areas when motor skills are learned well or automatized for long-term storage [37, 42]. These observations indicate that the cerebellum has a specific role in acquiring novel motor/sequence tasks. The cerebellar role in the first learning phase receives support also from lesion studies. Clear deficits in procedural spatial learning have been documented in a study analyzing how hemi-cerebellectomized rats perform in the Morris Water Maze [43]. The investigators suggest that the cerebellum may act as the site not for storing but for acquiring spatial procedural strategies, whereas other studies reported that what impairs procedural learning is brainstem and cerebellar dysfunction combined [44].
Collectively, our results and current knowledge underline the cerebellum as a key component in a brain network responsible for implicit learning and particularly for procedural learning. The cerebellum improves cognitive abilities related to procedural learning by activating interconnected areas or inhibiting competing networks (temporal medial lobe structures), or doing both. As well as providing useful theoretical insights, this study provides practical information implying that brain stimulation might also be an efficient means of promoting plastic changes at both cortical [3, 45–47] and cerebellar level [16–18, 48, 49]. Such interventions may be part of the treatment repertoire offered by future neurorehabilitation units for the benefit of disabled people or, perhaps in gymnastics for athletes’ training (golfers or tennis players), or alternatively, on the list of illegitimate doping remedies [50].
Cerebellar dysfunction is associated with many motor syndromes and neuropsychiatric disorders. Hence, one interesting proposal would be to evaluate cerebellar tDCS’s role in dyslexia, a neurodevelopmental disorder in which the reading, writing, and spelling deficits could be related to cerebellar dysfunction [51]. Another disorder is schizophrenia, a condition with a variety of symptoms that includes impairment in procedural learning. Accordingly, a meta-analysis showed that patients with schizophrenia perform worse than healthy controls in the SRTT [52]. Future studies could test whether cerebellar tDCS – alone or associated with customary interventions – has a therapeutic role for patients with these conditions. Although preliminary, our findings encourage further studies in healthy and clinical samples.
Limitations
A limitation of our study is that, because we did not measure the studied outcome variables 35 min after cerebellar tDCS, we could not assess how long the cerebellar tDCS-induced changes in learning lasted. The relatively small sample size also warrants further studies. Finally, because we did not correct for multiple comparisons, our results should be primarily considered as hypothesis-driven findings for further studies evaluating the cognitive effects of cerebellar stimulation. Another important limitation is the lack of an awareness evaluation, to account for the single moment at which an individual is transformed from being unaware to being aware of learning given that this moment can be measured, whereas awareness may be a continuum.
Conclusion
Collectively, our new findings along with current knowledge suggest that the cerebellum plays a critical role in procedural learning. In this context, tDCS could be a useful new tool for studying cerebellar functions. Our study may also help to explain impaired implicit sequence learning in cerebellar pathologies [53], and the learning benefits provided by anodal tDCS may have promising implications for designing motor learning protocols in patients undergoing neurorehabilitation. Even though implicit learning processes differ in healthy subjects and patients, anodal tDCS might also help to provide more information on deficits in procedural learning in conditions such as dyslexia and schizophrenia.
Abbreviations
- rTMS:
-
Repetitive transcranial magnetic stimulation
- SRRT:
-
Serial reaction time task
- tDCS:
-
Transcranial direct current stimulation
- VAS:
-
Visual analogue scale
References
Patten BM. The ancient art of memory. Usefulness in treatment. Arch Neurol. 1972;26:25–31.
Patten BM. The history of memory arts. Neurology. 1990;40:346–52.
Utz KS, Dimova V, Oppenlander K, et al. Electrified minds: transcranial direct current stimulation (tDCS) and galvanic vestibular stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—a review of current data and future implications. Neuropsychologia. 2010;48:2789–810.
Baillieux H, De Smet HJ, Paquier PF, et al. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110:763–73.
Manto M. The cerebellum, cerebellar disorders, and cerebellar research—two centuries of discoveries. Cerebellum. 2008;7:505–16.
Manto M, Haines D. Cerebellar research: two centuries of discoveries. Cerebellum. 2012;11:446–8.
O’Halloran CJ, Kinsella GJ, Storey E. The cerebellum and neuropsychological functioning: a critical review. J Clin Exp Neuropsychol. 2012;34:35–56.
Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–54.
Meltzoff AN, Kuhl PK, Movellan J, et al. Foundations for a new science of learning. Science. 2009;325:284–8.
Welsh JP, Harvey JA. Cerebellar lesions and the nictitating membrane reflex: performance deficits of the conditioned and unconditioned response. J Neurosci. 1989;9:299–311.
Albus JS. Theory of cerebellar function. Math Biosci. 1971;10:25–61.
Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–70.
Gilbert PF, Thach WT. Purkinje cell activity during motor learning. Brain Res. 1977;128:309–28.
Kitazawa S, Kimura T, Yin PB. Cerebellar complex spikes encode both destinations and errors in arm movements. Nature. 1998;392:494–7.
Seidler RD, Purushotham A, Kim SG, et al. Cerebellum activation associated with performance change but not motor learning. Science. 2002;296:2043–6.
Ferrucci R, Marceglia S, Vergari M, et al. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci. 2008;20:1687–97.
Ferrucci R, Giannicola G, Rosa M, et al. Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cogn Emot 2012;26:786-799.
Galea JM, Jayaram G, Ajagbe L, et al. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–22.
Niessen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cogn Psychol. 1987;19:1–32.
Pascual-Leone A, Grafman J, Clark K, et al. Procedural learning in Parkinson’s disease and cerebellar degeneration. Ann Neurol. 1993;34:594–602.
Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25.
Christ A, Kainz W, Hahn EG, et al. The Virtual Family—development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. 2010;55:N23–38.
Parazzini M, Fiocchi S, Rossi E, et al. Transcranial direct current stimulation: estimation of the electric field and of the current density in an anatomical human head model. IEEE Trans Biomed Eng. 2011;58:1773–80.
Parazzini M, Rossi E, Rossi L, et al. Numerical estimation of the current density in the heart during transcranial direct current stimulation. Brain Stimul. 2012. doi:10.1016/j.brs.2012.05.007.
Robertson EM. The serial reaction time task: implicit motor skill learning? J Neurosci. 2007;27:10073–5.
Abrahamse EL, van der Lubbe RH, Verwey WB, et al. Redundant sensory information does not enhance sequence learning in the serial reaction time task. Adv Cogn Psychol. 2012;8:109–20.
Torriero S, Oliveri M, Koch G, et al. Interference of left and right cerebellar rTMS with procedural learning. J Cogn Neurosci. 2004;16:1605–11.
Silvanto J, Muggleton NG. New light through old windows: moving beyond the "virtual lesion" approach to transcranial magnetic stimulation. NeuroImage. 2008;39:549–52.
Paulus W. Outlasting excitability shifts induced by direct current stimulation of the human brain. Suppl Clin Neurophysiol. 2004;57:708–14.
Vigot R. Cerebellar long-term depression: a mechanism for learning and memory. Med Sci (Paris). 2003;19:437–41.
Womack M, Khodakhah K. Active contribution of dendrites to the tonic and trimodal patterns of activity in cerebellar Purkinje neurons. J Neurosci. 2002;22:10603–12.
Walter JT, Alvina K, Womack MD, et al. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–97.
Honda M, Deiber MP, Ibanez V, et al. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain. 1998;121(Pt 11):2159–73.
Jenkins IH, Brooks DJ, Nixon PD, et al. Motor sequence learning: a study with positron emission tomography. J Neurosci. 1994;14:3775–90.
Petri HL, Mishkin M. Behaviorism, cognitivism and the neuropsychology of memory. Am Sci. 1994;82:30–7.
Squire LR. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. J Cogn Neurosci. 1992;4:232–43.
Doyon J, Song AW, Karni A, et al. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci U S A. 2002;99:1017–22.
Exner C, Koschack J, Irle E. The differential role of premotor frontal cortex and basal ganglia in motor sequence learning: evidence from focal basal ganglia lesions. Learn Mem. 2002;9:376–86.
Poldrack RA, Sabb FW, Foerde K, et al. The neural correlates of motor skill automaticity. J Neurosci. 2005;25:5356–64.
Rauch SL, Whalen PJ, Savage CR, et al. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Hum Brain Mapp. 1997;5:124–32.
Matsumura M, Sadato N, Kochiyama T, et al. Role of the cerebellum in implicit motor skill learning: a PET study. Brain Res Bull. 2004;63:471–83.
Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–64.
Petrosini L, Molinari M, Dell’Anna ME. Cerebellar contribution to spatial event processing: Morris water maze and T-maze. Eur J Neurosci. 1996;8:1882–96.
Daum I, Rockstroh B, Birbaumer N, et al. Behavioural treatment of slow cortical potentials in intractable epilepsy: neuropsychological predictors of outcome. J Neurol Neurosurg Psychiatry. 1993;56:94–7.
Brunoni AR, Nitsche MA, Bolognini N, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–95.
Nitsche MA, Paulus W. Transcranial direct current stimulation—update 2011. Restor Neurol Neurosci. 2011;29:463–92.
Polania R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp 2012;33:2499-2508.
Pope PA, Miall RC. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul 2012;5:84-94.
Hamada M, Strigaro G, Murase N, et al. Cerebellar modulation of human associative plasticity. J Physiol. 2012;590:2365–74.
Nielsen JB, Cohen LG. The Olympic brain. Does corticospinal plasticity play a role in acquisition of skills required for high-performance sports? J Physiol. 2008;586:65–70.
Nicolson RI, Fawcett AJ, Brookes RL, et al. Procedural learning and dyslexia. Dyslexia. 2012;16:194–212.
Siegert RJ, Weatherall M, Bell EM. Is implicit sequence learning impaired in schizophrenia? A meta-analysis. Brain Cogn. 2008;67:351–9.
Gomez-Beldarrain M, Garcia-Monco JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 11):2202–5.
Conflict of Interest
Roberta Ferrucci is a stakeholder in Newronika s.r.l., a spin-off company of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and the Università degli Studi di Milano.
Andre R. Brunoni reported no financial interests or potential conflicts of interest.
Marta Parazzini reported no financial interests or potential conflicts of interest.
Maurizio Vergari is a stakeholder in Newronika s.r.l., a spin-off company of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and of the Università degli Studi di Milano.
Elena Rossi is supported by “Dote ricerca”: FSE, Regione Lombardia and Newronika srl., a spin-off company of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and the Università degli Studi di Milano.
Manuela Fumagalli reported no financial interests or potential conflicts of interest.
Francesca Mameli is a stakeholder in Newronika s.r.l., a spin-off company of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and the Università degli Studi di Milano.
Manuela Rosa reported no financial interests or potential conflicts of interest.
Gaia Giannicola reported no financial interests or potential conflicts of interest.
Stefano Zago reported no financial interests or potential conflicts of interest.
Alberto Priori is a stakeholder in Newronika s.r.l., a spin-off company of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and of the Università degli Studi di Milano.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrucci, R., Brunoni, A.R., Parazzini, M. et al. Modulating Human Procedural Learning by Cerebellar Transcranial Direct Current Stimulation. Cerebellum 12, 485–492 (2013). https://doi.org/10.1007/s12311-012-0436-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-012-0436-9