Abstract
B-lymphoblastic leukemia/lymphoma (B-ALL/LBL), not otherwise specified, is a neoplasm of B-cell precursor lymphoid cells. Most of these tumors tend to manifest in children. B-LBL constitutes about 10% of the lymphoblastic lymphomas; however, in older adults, it is extremely rare. We present a case of a 46-year-old man with no pertinent medical or surgical history who presented with severe abdominal pain, nausea, and emesis. An abdominal CT scan was suggestive of strangulation of the distal small bowel and ischemia, showed extensive matted lymphadenopathy in the mesentery, and multiple left suprarenal periaortic nodes. A bowel resection was performed and there was an ileocolonic intussusception with the right colon filled with the intussuscepted terminal ileum. Opening the specimen revealed a submucosal ileal mass that measured 3 × 2.2 × 1.3 cm with a well-circumscribed, yellow-white cut surface. Histological examination showed a diffuse infiltrate composed of small- and medium-sized cells extending through the ileal wall from mucosa to subserosa, with irregular nuclear contours, open chromatin, inconspicuous nucleoli, occasional mitoses, and frequent apoptotic bodies. Flow cytometric analysis revealed 29% B-lymphoblasts corresponding to the diagnosis of B-LBL. BCR/ABL translocation by FISH was not detected. Cytogenetics analysis showed a normal male karyotype 46,XY. B-LBL is a rare neoplasm in older adults and can involve nodal and extranodal sites; however, the gastrointestinal tract is rarely compromised, with only a few cases reported in the literature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
B-lymphoblastic leukemia/lymphoma, not otherwise specified (B-ALL/LBL NOS), is a neoplasm of B-cell precursor lymphoid cells. The majority of these cases involve the bone marrow and blood (B-ALL), while the remainder includes nodal or extranodal sites (B-LBL). In addition, B-LBL constitutes only 10% of lymphoblastic lymphomas. The age of presentation is primarily in children [1], although in older adults, it is an extremely rare occurrence. The most common sites of involvement in B-LBL are the skin, soft tissue, bone, and lymph nodes [2]. Hence, we are presenting a rare case of B-LBL in an adult causing intussusception of the small intestine.
Clinical history
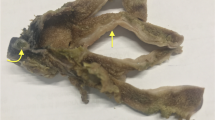
A 46-year-old man with no pertinent medical or surgical history presented with severe abdominal pain, nausea, and emesis. An abdominal CT scan was suggestive of strangulation of the distal small bowel and ischemia, which showed extensive matted lymphadenopathy in the mesentery, and multiple left suprarenal periaortic nodes measuring up to 1.1 cm. A bowel resection was performed and there was an ileocolonic intussusception with the right colon filled with the intussuscepted terminal 20 cm of ileum (Fig. 1a).
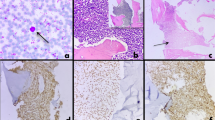
a Gross image of the ileocolonic intussusception. The image shows a demarcated transition of the unremarkable mucosa to a red congested mucosa. b Cut section of the mass. The mass measures 3 × 2.2 × 1.3 cm and the cut surface is yellow-white and fleshy. c High power microscopic histopathologic findings. Cells have irregular nuclear contours, open chromatin, inconspicuous nucleoli, occasional mitoses, and frequent apoptotic bodies. d TdT-positive nuclear stain in lymphoma cells
Materials, methods, and results
Opening the specimen revealed a submucosal ileal mass that measured 3 × 2.2 × 1.3 cm (Fig. 1b) with a well-circumscribed, yellow-white cut surface. Histological examination showed a diffuse infiltrate composed of small- and medium-sized cells extending through the ileal wall from mucosa to subserosa, with irregular nuclear contours, open chromatin, inconspicuous nucleoli, occasional mitoses, and frequent apoptotic bodies (Fig. 1c). Flow cytometric analysis revealed 29% blasts expressing CD19, CD10, dim partial CD20, CD38, cCD79a, dim cCD22, and nTdT consistent with B-lymphoblasts. Immunohistochemical profile showed positive focal dim CD19, subset dim PAX5, TdT (Fig. 1d), CD43, CD10, and CD79a and negative for CD3, CD4, CD5, CD20, CD34, CD56, cyclin-D1, CD138, HHV8, ALK, and EBER by in-situ hybridization. Kappa and lambda highlight a polytypic population of background plasma cells. BCR-ABL translocation by FISH was not detected. Cytogenetics analysis showed a normal male karyotype 46,XY. The findings were those of B-LBL, NOS.
Discussion
B-lymphoblastic lymphoma is a neoplasm of precursor B-cell lymphocytes with a tendency to form a mass. In a study by Lin et al. [3], they found the gastrointestinal tract an uncommon site for these tumors. Other authors like Iwamuro et al. [4] reported B-LBL in the stomach and Unnikrishnan et al. [5] described this lymphoma in the rectum.
These tumors are composed of small- to medium-sized blast cells with high nuclear:cytoplasmic ratio, irregular nuclear membranes, dispersed chromatin, inconspicuous nucleoli, and high mitotic rate [6]. Thus, these histological characteristics are indistinguishable from the T-lymphoblastic proliferations. The immunophenotype of B-LBL shows positivity for B-cell markers (CD19, CD20, cCD79a, cCD22, PAX5) and other markers such as CD10, CD22, CD24, PAX5, and TdT, such as in our case. Although CD79a can be positive in cases of T-lymphoblastic lymphomas, it is not a specific marker [7]. PAX5 is considered the most sensitive and specific marker for precursor and mature B-cell lineage [8]. Despite multiple studies, the exact etiology for B-LBL remains unknown. However, there is an increased risk in children with Down syndrome and other genetic disorders [9].
The prognosis of B-LBL, NOS appears to be better in children than in adults. Certain cases of B-ALL/LBL have specific recurrent genetic abnormalities and should be classified accordingly. One of these abnormalities is the t(9;22) (q34;q11) causing a reciprocal translocation between BCR and the ABL oncogene. This translocation produces a BCR-ABL fusion protein initiating the ABL tyrosine kinase [10, 11]. This fusion protein becomes a target for tyrosine kinase inhibitors, thus having a significant favorable effect on the outcome. Unfortunately, in our case, the translocation was not detected. The current WHO classification recognizes eight additional genetically defined B-ALL/LBL entities.
To our knowledge, we are presenting the first case report of B-LBL in an adult involving the small intestine and causing intussusception. It is essential to consider in these entities the adequate workup, such as FISH and chromosomal testing, for prognosis and to provide additional therapeutic options.
References
Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL (2005) A systematic literature review of the clinical and epidemiological burden of acute lymphoblastic leukaemia (ALL). Eur J Cancer Care (Engl) 14(1):53–62. https://doi.org/10.1111/j.1365-2354.2005.00513.x (PMID: 15698386)
Maitra A, McKenna RW, Weinberg AG, Schneider NR, Kroft SH (2001) Precursor B-cell lymphoblastic lymphoma. A study of nine cases lacking blood and bone marrow involvement and review of the literature. Am J Clin Pathol. 15(6):868–75. https://doi.org/10.1309/Q5GV-3K00-WAC6-BBUB (PMID: 11392884)
Lin P, Jones D, Dorfman DM, Medeiros LJ (2000) Precursor B-cell lymphoblastic lymphoma: a predominantly extranodal tumor with low propensity for leukemic involvement. Am J Surg Pathol 24(11):1480–1490. https://doi.org/10.1097/00000478-200011000-00003 (PMID: 11075849)
Iwamuro M, Kawai Y, Yamawaki Y, Takata K, Yamamoto K (2013) Precursor B lymphoblastic lymphoma involving the stomach. Case Rep Gastrointest Med. 2013:930918. https://doi.org/10.1155/2013/930918 (Epub 2013 Jun 11. PMID: 23840980; PMCID: PMC3693121)
Unnikrishnan P, Narayanan G, Kumar BS, Devi N (2017) B-lymphoblastic lymphoma of the rectum. Proc (Bayl Univ Med Cent) 30(3):336–337. https://doi.org/10.1080/08998280.2017.11929639.PMID:28670078;PMCID:PMC5468036
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiede J, Vardiman J (2008) WHO classification of tumours of hematopoietic and lymphoid tissues. Lyon. IARC Press, France, pp 294–295
Hashimoto M, Yamashita Y, Mori N (2002) Immunohistochemical detection of CD79a expression in precursor T cell lymphoblastic lymphoma/leukaemias. J Pathol 197(3):341–347. https://doi.org/10.1002/path.1126 (PMID: 12115880)
Torlakovic E, Torlakovic G, Nguyen PL, Brunning RD, Delabie J (2002) The value of anti-pax-5 immunostaining in routinely fixed and paraffin-embedded sections: a novel pan pre-B and B-cell marker. Am J Surg Pathol 26(10):1343–1350. https://doi.org/10.1097/00000478-200210000-00011 (PMID: 12360049)
Ziino O, Rondelli R, Micalizzi C, Luciani M, Conter V, Aricò M (2006) Acute lymphoblastic leukemia in children with associated genetic conditions other than Down’s syndrome. The AIEOP experience Haematologica 91(1):139–140 (PMID: 16434385)
Teachey DT, Pui CH (2019) Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol 20(3):e142–e154. https://doi.org/10.1016/S1470-2045(19)30031-2 (PMID: 30842058)
Ribeiro RC, Abromowitch M, Raimondi SC, Murphy SB, Behm F, Williams DL (1987) Clinical and biologic hallmarks of the Philadelphia chromosome in childhood acute lymphoblastic leukemia. Blood 70(4):948–953 (PMID: 3307953)
Acknowledgements
We would like to thank all members of the Arkadi M. Rywlin M.D. Department of Pathology and Laboratory Medicine, Mount Sinai Medical Center (Miami Beach, FL, USA) for their help with this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moreno, J.C.A., Pagacz, M., Oh, KS. et al. Rare presentation of B-lymphoblastic leukemia/lymphoma with intussusception in an adult. J Hematopathol 15, 101–103 (2022). https://doi.org/10.1007/s12308-022-00494-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-022-00494-8