Abstract
Purpose
This review aims to explore and summarize the current clinical evidence about the use of regenerative medicine such as mesenchymal stem cells or platelet-rich plasma in intervertebral disc regeneration, in order to clarify the state of art of these novel approaches.
Materials and methods
We performed a research of the available literature about regenerative medicine strategies aiming to prevent intervertebral disc degeneration. All preclinical trials and in vitro studies were excluded. Only clinical trials were critically analysed.
Results
The manuscript selection produced a total of 7 articles concerning the use of regenerative therapies in intervertebral disc degeneration, covering the period between 2010 and 2016. Articles selected were 4 about the injection of mesenchymal stem cells-related results and 3 using platelet-rich plasma. The total population of patients treated with regenerative medicine strategies were 104 patients.
Conclusions
Regenerative medicine, such as the use of mesenchymal stem cells or platelet-rich plasma, in intradiscal disc degeneration has shown preclinical and clinical positive results. Randomized clinical trials studying the potential of MSCs intradiscal injection have not been conducted, and PRP effect has been studied only preliminarily. Additional more powered high-quality studies are needed to really appreciate the long-term safety and efficacy of regenerative medicine approaches in IDD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intervertebral disc degeneration (IDD) is considered one of the most important causes of low back pain [1]. Intervertebral disc (IVD) has the role of shock absorber of the spine and amortise compressive loading forces [2]. The IVD consists of a inner gelatinous-based nucleus pulposus (NP) surrounded by a fibro-cartilaginous ring: the annulus fibrosus (AF). Cells in the NP lie in proteoglycan-rich extracellular matrix (ECM) which plays an essential role maintaining the IVD hydrated [3–5]. Disc degeneration starts from degradation of proteoglycans in the NP, thus loss in water content. NP dehydration is a process associated with decreased cell number and change in phenotype of disc cells, resulting in loss of disc height, disc deformation and segmental instability [6–8]. This sequential process overloads surrounding structures of the IVD such as end plates, facet joints and ligaments [9]. Surgical strategies to address pain and disability from degenerated disc diseases are spinal fusion and disc arthroplasty. These procedures are expensive; they need invasive surgical treatment with possible complications [10–12]. This surgical techniques target the clinical symptoms instead of the degenerative cascade itself; additionally in spinal fusion, spine motion is not preserved and natural kinematics is altered [13, 14]. Early in degeneration progress, conservative therapies, such as bed rest, anti-inflammatory medications, analgesia and physical therapy, to invasive or interventional strategies, such as epidural injections and ablation techniques, are of value to reduce symptoms but not effective in preserving natural state of the disc.
On the other hand, regenerative medicine would have a curative intention aiming IVD regrowth. Tissue engineering approaches such as growth factors, chondrocyte transplantation, gene therapy and intracellular regulatory proteins are among the factors which have been demonstrated to play an essential role in regeneration of NP cells, both in vitro and in vivo [15–17].
In fact, IVD is avascular and during degeneration there is no intrinsic capacity to repair and restore the number of NP cells [5, 18]. Several authors demonstrated that regenerative medicine can lead to positive effects on intervertebral disc cells proliferation [19, 20] as well as in other anatomical districts [21–24]. Despite this cheering preclinical data, only few clinical trials aiming to evaluate tissue engineering approaches in IVD have been performed in the literature.
This review aims to explore and summarize the current clinical evidence about the use of regenerative medicine in intervertebral disc regeneration, in order to clarify the state of art of these novel approaches and to evaluate whether these promising strategies could now move from bench to current clinical practice.
Materials and methods
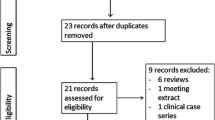
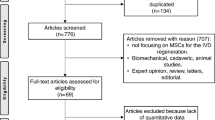
We performed a research of the available English literature on PubMed, MEDLINE, Google Scholar, Medscape and EMBASE databases using various combinations of the following keywords: intervertebral disc degeneration, mesenchymal stem cells, platelet-rich plasma, gene therapy, intervertebral disc regeneration, tissue engineering. We considered only clinical evidence including clinical trials, case series and case reports. Studies were selected according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). Two reviewers (M.B. and L.C.) independently screened the titles and abstracts from all identified articles to assess their appropriateness to the research focus. References from the identified articles were checked in order not to miss any relevant articles. A total of 1232 articles were identified; at the end of our review process only 7 articles were included. Reviews were excluded. The exclusion criteria were papers not evaluating the regenerative medicine potential in IDD. All preclinical trials and all in vitro studies were excluded. Studies without dose and source of stem cells specification as well as non-English papers were excluded. The PRISMA flow 2009 diagram illustrates the number of studies that have been identified, included and excluded as well as the reason for exclusion (Fig. 1). Clinical trials were divided in 2 tables according to the type of approach. Table 1 summarizes studies related to MSC and evaluate the following variables: study year, type of study and level of evidence, system used to obtain MSC, concentration method, volume or number of cells injected, duration of follow-up, number of patients treated, age range and clinical or radiological variables analysed. Table 2 includes studies that involve PRP evaluating study year, type of study and level of evidence, system used to obtain PRP, number of patients treated, mean age/range, follow-up, volume injected, analysed variables and results.
Results
The manuscript selection produced a total of 7 articles concerning the use of regenerative therapies in IDD. Papers included in our review cover the period between 2010 and 2016.
We split these articles in two groups according to the regenerative approach used, obtaining two subgroups. The first one (Table 1) included 4 articles exploring MSC potential in IDD, while the second one (Table 2) consist of 3 studies focused on PRP.
Pettine et al. [25] (2016) evaluated a total of 26 patients (aged 18–61 years, 13 single level and 13 two level) with chronic low back pain (>6 months, non-responsive to other conservative treatment) and degenerative disc disease confirmed by magnetic resonance imaging (MRI) (Pfirrmann grade of IV–VII) and a visual analogue scale (VAS) more than 4 at first visit. They evaluated initial Oswestry Disability Index (ODI) and VAS score: 56.5% and 8.1 (0–10), respectively. ODI and VAS tests were repeated at 3,6,12 and 24 months following the procedure. MRI scans were repeated at 12 months, and a new evaluation of Pfirrmann grade was performed by a blinded independent reviewer. Autologous bone marrow aspiration (BMA) was collected from posterior iliac crest. BMA was then processed to concentrate cell preparation. One millilitre from a volume of 7 ml was used for cell analysis. The authors noted no complications associated with the injection and from 26 patients treated, only 5 needed surgical intervention of lumbar fusion or artificial disc replacement. Only one patient has reported clinical improvement after surgery. ODI and VAS reduction in remaining 21 patients was 67 and 72%, respectively (p < 0.001). Pfirrmann grade was improved only in 8 patients by one grade; all others patients maintained previous score. Authors noted that amelioration of clinical outcomes in terms of ODI and VAS score occurred within 3 months from disc injection. Moreover, these clinical improvements were higher in patients with greater than 2000 MSC/ml. Mochida et al. [26] in 2015 reported 3-year results of a prospective clinical study. In nine patients, aged 20–29 years with Pfirrman grade III disc degeneration (at the level adjacent to the scheduled posterior interbody fusion) viable NP cells were collected from the disc fused and co-cultured with autologous MSC. Seven days after fusion, one million activated NP cells were injected in the upper level fused. Clinical evaluations (using the Japanese Orthopaedic Association scoring system for low back pain) and follow-up imaging were performed at 1,2 and 4 weeks, 3 and 6 months, as well as 1,2 and 3 years after NP injection. No clinical adverse events were reported. Clinical outcome score (JOA) improved from 14.2 ± 4.8 points preoperatively to 27.2 ± 1.6 at 3 years. Degeneration of intervertebral disc treated was less than grade III according to Pfirrmann classification in all cases. Nevertheless, there was no significant variation in discs water content.
In 2011, Orozco et al. [27] conducted a clinical study of 10 patients (35 ± 7 years) treated using autologous bone marrow mesenchymal stem cell. All these patients suffered from chronic low back pain with disc degeneration with intact AF. Autologous MSC injection was guided with fluoroscopy into the NP area without any carrier. Before injection, stem cells were expanded in culture for 7–10 days. Follow-up period was 12 months, and clinical evaluation of low back pain, disability index and quality of life were performed. Radiological evaluation was focused on water content and improvement of disc height. Clinical improvement of pain, disability and quality of life were rapid (3 months). Water content of treated disc evaluated with T2-weighted MRI showed a significative elevation at 12 months. Nevertheless, disc height was not recovered.
Yoshikawa et al. [28] conducted the first report of two patients affected by degenerative disc diseases treated with autologous MSC transplantation. Patients selected satisfied selection criteria based on MRI, X-ray myelography and chronic low back pain. These patients were 70 and 67 years old, respectively. One of them suffered from adjacent segment disease after anterior interbody fusion of L4–L5. Five millilitres of bone marrow fluid was aspirated from ilium. After 2–4 weeks in culture, cells were placed in 10-ml injector with pieces of collagen sponge. After NP removal, under fluoroscopy, MSCs were grafted in the central regions of discs. Fenestration of AF was then sealed with acellular collagen sponge. Two years after surgery, although T1-weighted MRI failed to demonstrate improvement, T2-weighted MRI showed increased signal intensities, demonstrating increased water content in treated disc. Moreover, after surgery, low back pain, lower leg numbness and pain improved. VAS score decreased to 18% and Japanese Orthopaedic Association from 8 to 25 points.
Tuakli-Wosornu et al. [29] recently investigated the role of lumbar intradiscal PRP injection. They performed a prospective double-blind randomized controlled study to determine whether a single injection of PRP could improve pain and function of 36 patients with IDD. Twenty-nine of the 36 treated patients (80.6%) have been evaluated in terms of both pain and function using the Functional Rating Index (FRI), Numeric Rating Scale (NRS) for pain, the pain and physical function domains of the 36-item Short Form Health survey (SF-36) and the modified North American Spine Society (NASS) Outcome Questionnaire. They have been treated with injections of autologous PRP (1–2 ml) and compared with the control group treated with 1–2 ml of contrast agent. Data were collected at baseline, one, four and eight weeks, six months and one year. Statistically significant improvements were found 8 weeks after treatment with PRP compared with the control groups with regard to pain (NRS best pain), function (FRI) and patient satisfaction (NASS Outcome Questionnaire). Significant improvement was maintained at 6 months and 1 year for FRI function; NRS worst pain and SF36 pain and function.
In June of 2016, Levi et al. [30] performed a prospective trial, trying to assess changes in pain and function in patients after intradiscal injection of PRP. Twenty-two patients have been enrolled in this trial and f.u. period lasted 6 months. Authors considered as a positive results when patients achieved at least 50% improvement in VAS and 30% decrease in ODI. Positive results have been obtained in 14% of patients after 1 month, in 32% after 2 months and in 47% after 6 months.
Akeda et al. [31] performed the first clinical trial with the aim to determine the efficacy and feasibility of intradiscal PRP. Outcomes measures included the VAS scale, the Roland–Morris Disability Questionnaire at baseline and at 6 months after treatment. X-ray and MRI were obtained before and 4 months after treatment. At one month of f.u., VAS score and RDQ decreased from 7.1 ± 1.2 to 1.8 ± 2 (p < 0.01) and 11 ± 1.8 to 3.2 ± 2.4 (p < 0.01), respectively. These results sustained for 6 months after treatment. Despite these encouraging clinical results, MRI changes failed to change significantly.
Focusing our attention on the MSC-related results, the total population of patients was 47 patients, with only one study with more than 20 patients, which still represents a small population. All patients treated suffered from discogenic back pain for at least 3 months after conventional conservative therapy has failed. The highest follow-up period was 3 years with a mean f.u. of 21 months. In the majority of the included studies, benefits from MSC injection were noted starting from third month. All clinical trials have used bone marrow as source of MSCs, and all the studies used a minimal invasive method to obtain stem cells (BMA) from ilium (posterior iliac crest or iliac crest). In one study, MSC was used in co-culture with autologous NP cells, to upregulate the viability and the number of NP cells. This system is different from the other studies selected, and it could be considered as an ex vivo differentiation of MSC. None of the studies selected have a control group. In all the included studies, the mean concentration of cells ranges from 106 to 107/ml. Only Pettine et al. gave attention to different MSC concentration and their influence in clinical and radiological outcome. Nevertheless, Pettine et al. conducted a non-blinded study, with no control groups. In this trial, only MRI was used to diagnose and clearly identify the level of IDD. Other 3 studies selected the combination of X-ray and MRI. Only 2 studies evaluated Pfirrmann grade of patients. MRI evaluation was always used to monitor the overall recovery of disc height and water content. Results are controversial: Mochida et al. demonstrated no significative changes in water content, while Orozco demonstrated a significative elevation of water content at 12 months of f.u. System used to assess clinical outcome is heterogeneous (2 studies used a JOA scoring system for low back pain while other 2 studies used ODI). All studies evaluated pain with VAS scoring system. Only Orozco evaluated clinical outcome in terms of quality of life through the SF-36 questionnaire. No complication associated with the injection of MSC in diseased disc was quoted. The level of evidence of the included study was low with all of them settled on level 4. Yoshikawa et al. conducted a report of two cases treated with an alternative method of cellular therapy. MSC were seeded in collagen sponge. Despite the positive results obtained, an insufficient level of evidence affects this study.
Pooling data obtained from PRP clinical trial, and the total population treated was formed by 57 patients. The highest follow-up period was 12 months with a mean f.u. of 8 months. In all the evaluated studies, autologous PRP has been used and no adverse effect or complications such as spondylodiscitis, neurologic injury or progressive herniation after intradiscal injection have been reported. All these studies showed similar positive clinical results. All the included studies showed benefits from PRP injection starting from first month after therapy. Several differences in study design can be observed. These three studies used 3 different methods to evaluate clinical outcomes, which make it difficult to critically summarize all obtained results. Moreover, only Tuakli-Wosornu et al. compared patients treated with PRP with a control group reaching a level of evidence of IIa. In this study, no radiological examination has been obtained to strengthen its evidence. Although MRI is now considered of a paramount importance in the diagnosis and follow-up of these patients [32], MRI evaluation was used only during participant recruitment. Process for obtaining PRP has not been mentioned by authors.
Levi et al. used an evaluation method with a poor level of evidence, thereby rendering it very difficult to compare with other selected studies. Moreover, the 22 patients enrolled in the study and completed the 2 months of f.u.; only 19 reached 6 months of f.u.
Akeda et al. stated that intradiscal injection of PRP is safe and effective. They should consider the small number of patients treated and the short-term results (only 6 months of f.u.). Despite these limitations, this is the first clinical study that has been analysed MRI changes after PRP injection.
Discussion
IVD is a dynamic structure and similar to most cartilaginous structure, with low vascular support leading to poor regenerative potential, especially when metabolic homoeostasis is disrupted [33, 34]. At present, the gold standard in IDD treatment is fusion surgery with several techniques [35, 36], but these do not preserve the IVD. On the other hand, conservative treatment is based on physical therapy and cannot reverse the degenerative cascade [37, 38]. Therefore, current research is directed towards an interventional therapy that could inhibit degenerative changes of IVD [7, 8, 39]. The ideal interventional therapy should aim to achieve three objectives: resolve nociceptive disc pain, slowing or reversal of catabolic metabolism within IVD environment and partial or complete restoration of disc tissue [40]. In this context, several regenerative approaches have been attempted to address these issues, including growth factor delivery, gene therapy, tissue engineering and cell-based therapy.
Gene therapy could play a role modifying the gene expression of disc cell, resulting in sustained production of anabolic factors and gene regulators. Anabolic factors such as TGF-β, BMP-2, BMP-7 or IGF-1, and gene regulators such as SOX-9 and LMP-1 have demonstrated to modulate the metabolic activity of disc cells, increasing proteoglycans disc content [41, 42]. Several side effects have been described in preclinical evaluation of these gene therapy approaches; thus, safer systems of transfection and transduction should be tried before their clinical application [43, 44].
Actually, stem cell therapy or autologous growth factor injection is more attractive due to low harvest site morbidity, favourable modulation of cells and concentration and easier clinical application.
Tissue engineering approaches such as suitable scaffold for stem cells or growth factors have been tested in vitro and in vivo [45]. Architecture and mechanical properties of scaffolds should allow their implantation in high-pressure structure. Mercuri et al. [46] explored the use of a hydrogel as a scaffold with the aim to treat IVD. The authors demonstrated that the chemical stabilized elastin–glycosaminoglycan–collagen hydrogel was able to increase aggrecan and type II collagen synthesis in vitro inducing differentiation of human-derived adipose tissue stromal cells. In vivo evaluation performed by transplantation of the hydrogel into rats showed that the material was fully biocompatible. Nevertheless, preclinical studies testing these techniques are cost-effective and still far from possible clinical application.
The 7 clinical studies included in our brief review focused only on cell-based therapy with MSC or PRP. Regenerative medicine on spine has been tested clinically only with these approaches.
According to the International Society of Cellular Therapy, MSCs are known for their self-renewal ability as well as their capacity to sustain nearby cellular activity [47]. They also demonstrated the capacity to differentiate into osteoblasts, adipocytes, chondroblasts and cells with phenotypic features of IVD under proper in vitro conditions [48, 49]. MSCs have been sourced from different tissues. A great interest about mesenchymal cells derived from bone marrow, adipose or umbilical cord tissue could be detected in the literature even if none of these sources has clearly shown superiority [50]. In the selected clinical trials; all MSCs derived from bone marrow aspiration. Some authors consider adipose tissue as a superior source because of its relatively higher concentrations of MSCs, obtainable with moderately less invasive method and with better ability to acquire IVD phenotype [51, 52]. Strassburg et al. [53] demonstrated the capability of bone marrow-derived MSC to differentiate in NP-like cells as well as, if co-cultured with NP, they could stimulate NP cells to produce new cell matrix. Mochida et al. have clinically tested this therapeutic effect of MSCs [26].
In all MSC clinical studies, the concentration of injected cells in IVD has been mentioned and that was ranging from 106 to 107 cells per disc. Serigano et al., using dog models, further investigated the optimal number of MSC injection dose per disc. They stated that a dose of cells per disc ranging from 105 to 107 could better maintain survival and localization of MSC within the centre of NP region [54].
Some authors have investigated how autologous bone marrow-derived MSC could migrate to the injured IVD and play a role in healing and regeneration. Daisuke Sakai et al. have studied this “homing” process of MSCs. Their study provides evidence to suggest that although MSCs are recruited during disc degeneration, only a limited number of MSCs migrate to the IVD, presumably because of its avascular nature [55].
Yim et al. have published a systematic review of comparative controlled studies regarding the potential benefits of using MSCs for disc regeneration. Twenty-four animal studies (including smaller and larger size animal model) were included, and 862 discs injected with MSCs were evaluated and compared with 1603 control discs. All types of MSCs (bone marrow, adipose or synovial tissue derived) demonstrated a significative inhibition of disc degeneration. Moreover, bone marrow-derived MSC showed better quality of repair compared to non-MSC treatments [56].
Authors should keep in mind that in vivo animal models are helpful to better accomplish safety, efficacy and feasibility of MSC injection therapy, but an iatrogenic model will never precisely reproduce a degenerated disc and its complex microenvironment. Additionally, human and animal have several differences such as tissue size, spine biomechanics and cell populations.
Evidence supporting disc tissue regeneration by intradiscal MSCs injection exists, and there are also several examples of positive preclinical results confirmed by clinical trials.
Differentiation behaviour of MSC is regulated by several grow factors. Growth factors are responsible for the morphological and functional modification of NP cells. The literature suggests how the process of differentiation and regeneration could be moderate by many grow factors, rarely by a single growth factor. Imbalance between anabolic grow factors such as insulin-like growth factor-1(IGF-1), transforming growth factors β (TGF β) or bone morphogenetic proteins (BMPs) and catabolic enzymes as matrix metalloproteinases (MMPs) and metalloproteinase with thrombospondin motifs (ADAMTS) are implied in degenerative processes of IVD [57, 58].
Several in vitro and in vivo studies demonstrated the effects of growth factors in regulating IVD cell proliferation and chondrogenic matrix metabolism [59].
Thus, the efficacy of intradiscal injection of MSC could be enhanced by combination with growth factors cocktails such as PRP. Chen et al. created an ex vivo porcine model of a degenerated IVD to test the regenerative ability of three different therapeutic regimens including MSC, PRP and MSC/PRP combined treatment. They concluded that MSC or PRP, if used alone, leads to chondrogenic-related mRNA expression and matrix synthesis. Curiously, the combination of both resulted in an osteogenic differentiation. These results were confirmed in ex vivo and in vivo model [60].
Platelet-rich plasma (PRP) was defined as growth factors cocktail with potential effect on NP cells in terms of promoting cell differentiation and reconstitution of human NP tissue [61, 62]. We previously conducted a preclinical review about the role of PRP injection in IVD degeneration. Twelve articles concerning the use of PRP in IVD were included in the review (6 in vitro and 6 in vivo studies). All the included studies underlined the positive histological results, and, when performed, MRI analysis of in vivo studies underlined therapeutic effect of PRP [63]. At present, only 3 clinical studies have been performed with positive midterm results demonstrating that PRP, if used alone, could induce ECM regeneration and cell proliferation [29–31]. PRP has been tested also in combination with MSC as a therapeutic agent able to regenerate NP [60].
Our systematic review has several limitations. Due to the methodological heterogeneity of the included studies, it is very difficult to compare obtained results and therefore draw definitive conclusions. Moreover, no firm consensus on evaluation of disc regeneration, patients’ selection and clinical outcome improvement is identifiable. Lastly, the low number of clinical studies, their poor level of evidence and the extremely few tested population do not allow reaching firm evidence. In order to confirm the promising efficacy of regenerative medicine in IDD treatment, we advocate for the standardization and implementation of clinical studies, to better move this encouraging regenerative therapy from bench to bedside.
In summary, this small review of the literature highlights that intradiscal injection of MSC or PRP should be considered as possible treatments of chronic low back pain caused by a degenerative disc disease. To the best of our knowledge, this is the first review that considers and summarizes current clinical evidence about regenerative medicine approaches in IDD. The procedure to obtain PRP, or to expand and transplant MSCs, is feasible and relatively safe with many advantages over more invasive surgical strategies. Intradiscal injection has demonstrated to be a simple technique, conservative of spine biomechanics, cost-effective, readily available and do not require long hospitalization of the patient. Since MSC or PRP is autologous derived, they could avoid transmission of diseases or immunological reaction [56].
However, randomized clinical trials studying the potential of MSCs intradiscal injection have not been conducted until now. PRP effect has been studied with a sufficient level of evidence without considering MRI modification only in one trial. Additional more powered high-quality studies are needed to really appreciate the long-term safety and efficacy of regenerative medicine approaches in IDD. If further larger clinical trial will confirm preliminary positive results, we believe that these strategies will find their right place in IDD treatment especially as interventional techniques prior to open standard surgery.
References
de Schepper EI, Damen J, van Meurs JB, Ginai AZ, Popham M, Hofman A, Koes BW, Bierma-Zeinstra SM (2010) The association between lumbar disc degeneration and low back pain: the influence of age, gender, and individual radiographic features. Spine 35:531–536 (Phila Pa 1976)
Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P (2000) Mechanical initiation of intervertebral disc degeneration. Spine 25:1625–1636 (Phila Pa 1976)
Roberts S, Evans H, Trivedi J, Menage J (2006) Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am 2(88 Suppl):10–14
Biyani A, Andersson GBJ (2004) Low back pain: pathophysiology and management. J Am Acad Orthop Surg 12:106–115
Shankar Hariharan, Scarlett Jeremy A, Abram Stephen E (2009) Anatomy and pathophysiology of intervertebral disc disease. Tech Reg Anesth Pain Manag 13:67–75
Urban JP, McMullin JF (1988) Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine 13:179–187 (Phila Pa 1976)
Di Martino A, Vaccaro AR, Yung Lee J, Denaro V, Lim MR (2005) Nucleus pulposus replacement: basic science and indications for clinical use. Spine 30(16 Suppl):S16–S22
Di Martino A, Merlini L, Faldini C (2013) Autoimmunity in intervertebral disc herniation: from bench to bedside. Expert Opin Ther Targets 17(12):1461–1470
Adams MA, Roughley PJ (2006) What is intervertebral disc degeneration, and what causes it? Spine 31:2151–2161 (Phila Pa 1976)
Siepe CJ, Heider F, Wiechert K, Hitzl W, Ishak B, Mayer MH (2014) Mid- to long-term results of total lumbar disc replacement: a prospective analysis with 5- to 10-year follow-up. Spine J. doi:10.1016/j.spinee.2013.08.028
Karppinen J, Shen FH, Luk KD, Andersson GB, Cheung KM, Samartzis D (2011) Management of degenerative disk disease and chronic low back pain. Orthop Clin North Am 42:513–528
Formica M, Cavagnaro L, Zanirato A, Felli L, Formica C (2016) Proximal junctional spondylodiscitis after pedicle subtraction osteotomy. Spine J 2:e49–e51. doi:10.1016/j.spinee.2015.09.050
Punt IM, Visser VM, van Rhijn LW, Kurtz SM, Antonis J, Schurink GW, van Ooij A (2008) Complications and reoperations of the SB Charite lumbar disc prosthesis: experience in 75 patients. Eur Spine J 17:36–43
Formica M, Cavagnaro L, Basso M, Zanirato A, Felli L, Formica C (2015) Is it possible to preserve lumbar lordosis after hybrid stabilization? Preliminary results of a novel rigid-dynamic stabilization system in degenerative lumbar pathologies. Eur Spine J 24(Suppl 7):849–854. doi:10.1007/s00586-015-4264-8
Thompson JP, Oegema TR, Bradford DS (1991) Stimulation of mature canine intervertebral disc by growth factors. Spine 16:253–260 (Phila Pa 1976); [PMID: 2028297]
Gou S, Oxentenko SC, Eldrige JS, Xiao L, Pingree MJ, Wang Z, Perez-Terzic C, Qu W (2014) Stem cell therapy for intervertebral disk regeneration. Am J Phys Med Rehabil 93(11 Suppl 3):S122–S131
Masuda K (2008) Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J 17(Suppl 4):441–451
Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K et al (2008) Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res 26(5):589–600
Zhang YG, Guo X, Xu P, Kang LL, Li J (2005) Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res 430:219–226
Yang H, Wu J, Liu J, Ebraheim M, Castillo S, Liu X, Tang T, Ebraheim NA (2010) Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-[beta]1 decrease rabbit intervertebral disc degeneration. Spine J 10:802–810
Emre TY, Atbasi Z, Demircioglu DT, Uzun M, Kose O (2016) Autologous osteochondral transplantation (mosaicplasty) in articular cartilage defects of the patellofemoral joint: retrospective analysis of 33 cases. Musculoskelet Surg. doi:10.1007/s12306-016-0448-6
D’Ambrosi R, Palumbo F, Paronzini A, Ragone V, Facchini RM (2016) Platelet-rich plasma supplementation in arthroscopic repair of full-thickness rotator cuff tears: a randomized clinical trial. Musculoskelet Surg 100(Suppl 1):25–32
Di Matteo B, Filardo G, Kon E, Marcacci M (2015) Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy–a systematic review. Musculoskelet Surg 99(1):1–9. doi:10.1007/s12306-014-0340-1
Felli L, Garlaschi G, Muda A, Tagliafico A, Formica M, Zanirato A, Alessio-Mazzola M (2016) Comparison of clinical, MRI and arthroscopic assessments of chronic ACL injuries, meniscal tears and cartilage defects. Musculoskelet Surg 100(3):231–238
Pettine K, Suzuki R, Sand T, Murphy M (2016) Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up. Int Orthop 40(1):135–40. doi:10.1007/s00264-015-2886-4. [Epub 2015 Jul 10]
Mochida J, Sakai D, Nakamura Y, Watanabe T, Yamamoto Y, Kato S (2015) Intervertebral disc repair with activated nucleus pulposus cell transplantation: a three-year, prospective clinical study of its safety. Eur Cell Mater 29:202–212 discussion 212
Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J (2011) Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92(7):822–828. doi:10.1097/TP.0b013e3182298a15
Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y (2010) Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine 35(11):E475–E480. doi:10.1097/BRS.0b013e3181cd2cf4 (Phila Pa 1976)
Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, Harrison JR, Gribbin CK, LaSalle EE, Nguyen JT, Solomon JL, Lutz GE (2016) Lumbar intradiskal platelet-rich plasma (PRP) injections: a prospective, double-blind, randomized controlled study. PM R 8(1):1–10; quiz 10. doi:10.1016/j.pmrj.2015.08.010. [Epub 2015 Aug 24]
Levi D, Horn S, Tyszko S, Levin J, Hecht-Leavitt C, Walko E (2016) Intradiscal platelet-rich plasma injection for chronic discogenic low back pain: preliminary results from a prospective trial. Pain Med 17(6):1010–1022. doi: 10.1093/pm/pnv053 [Epub 2015 Dec 26]
Akeda K, Imanishi T, Ohishi K, Masuda K, Uchida A, Sakakibara T, Kasai Y, Sudo A (2012) Intradiscal injection of autologous platelet-rich-plasma for the treatment of lumbar disc degeneration preliminary prospective clinical trial for discogenic low back pain patients. Poster No. 2194. ORS 2012 Annual Meeting
Quattrocchi CC, Giona A, Di Martino A, Gaudino F, Mallio CA, Errante Y, Occhicone F, Vitali MA, Zobel BB, Denaro V (2015) Lumbar subcutaneous edema and degenerative spinal disease in patients with low back pain: a retrospective MRI study. Musculoskelet Surg. 99(2):159–163. doi:10.1007/s12306-015-0355-2
Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y (2005) The pathogenesis of discogenic low back pain. J Bone Jt Surg Br 87(1):62–67
Formica M, Basso M, Cavagnaro L, Formica C, Zanirato A, Felli L (2016) Kümmell disease: illustrative case for definition criteria. Spine J 16(10):e707–e708. doi:10.1016/j.spinee.2016.03.035
Formica M, Berjano P, Cavagnaro L, Zanirato A, Piazzolla A, Formica C (2014) Extreme lateral approach to the spine in degenerative and post traumatic lumbar diseases: selection process, results and complications. Eur Spine J 23(Suppl 6):684–692
Allain J, Delecrin J, Beaurain J, Poignard A, Vila T, Flouzat-Lachaniette CH (2014) Stand-alone ALIF with integrated intracorporeal anchoring plates in the treatment of degenerative lumbar disc disease: a prospective study on 65 cases. Eur Spine J 23(10):2136–2143
Cavagnaro L, Basso M, Alessio Mazzola M, Formica M (2014) Lumbar Traction in the management of low back pain: a survey of latest results. J Nov Physiother 4:5
Wegner I, Widyahening IS, van Tulder MW, Blomberg SE, de Vet HC (2013) Traction for low-back pain with or without sciatica. Cochrane Database Syst Rev 8(8). doi:10.1002/14651858.CD003010.pub5
Wang Z, Perez-Terzic CM, Smith J, Mauck WD, Shelerud RA, Maus TP, Yang TH, Murad MH, Gou S, Terry MJ, Dauffenbach JP, Pingree MJ, Eldrige JS, Mohammed K, Benkhadra K, van Wijnen AJ, Qu W (2015) Efficacy of intervertebral disc regeneration with stem cells—A systematic review and meta-analysis of animal controlled trials. Gene 564(1):1–8
Depalma M (2012) Biologic treatments for discogenic low back pain. SpineLine 13(2):19–23
Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH (1999) Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine 24:2419–2425 (Phila Pa 1976)
Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, Zhou L, Breyer B, Feng T, Gupta P, He TC, Phillips FM (2003) Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine 28:755–763 (Phila Pa 1976)
Wallach CJ, Kim JS, Sobajima S, Lattermann C, Oxner WM, McFadden K, Robbins PD, Gilbertson LG, Kang JD (2006) Safety assessment of intradiscal gene transfer: a pilot study. Spine J 6:107–112
Vadalà G, Sowa GA, Smith L, Hubert MG, Levicoff EA, Denaro V, Gilbertson LG, Kang JD (2007) Regulation of transgene expression using an inducible system for improved safety of intervertebral disc gene therapy. Spine 32:1381–1387 (Phila Pa 1976)
Tsaryk R, Gloria A, Russo T, Anspach L, De Santis R, Ghanaati S, Unger RE, Ambrosio L, Kirkpatrick CJ (2015) Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater 20:10–21
Mercuri J, Addington C, Pascal R, Gill S, Simionescu D (2014) Development and initial characterization of a chemically stabilized elastin glycosaminoglycan-collagen composite shape-memory hydrogel for nucleus pulposus regeneration. J Biomed Mater Res A 102:4380–4393
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement”. Cytotherapy 8(4):315–317
Alini M, Roughley PJ, Antoniou J, Stoll T, Aebi M (2002) A biological approach to treating disc degeneration: not for today, but maybe for tomorrow. Eur Spine J 11(Suppl 2):S215–S220
Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS (2011) Sources of adult mesenchymal stem cells applicable for musculoskeletal applications—a systematic review of the literature. Open Orthop J 5:242–248
Sobajima S, Vadala G, Shimer A, Kim JS, Gilbertson LG, Kang JD (2008) Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J 8(6):888–896
Gimble JM, Katz AJ, Bunnell BA (2007) Adipose-derived stem cells for regenerative medicine. Circ Res 100(9):1249–1260
Longo UG, Papapietro N, Petrillo S, Franceschetti E, Maffulli N, Denaro V (2012) Mesenchymal stem cell for prevention and management of intervertebral disc degeneration. Stem Cells Int 2012. doi:10.1155/2012/921053
Strassburg S, Richardson SM, Freemont AJ, Hoyland JA (2010) Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med 5(5):701–711
Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J (2010) Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J Orthop Res 28:1267–1275
Sakai D, Nishimura K, Tanaka M, Nakajima D, Grad S, Alini M, Kawada H, Ando K, Mochida J. (2015) Migration of bone marrow-derived cells for endogenous repair in a new tail-looping disc degeneration model in the mouse: a pilot study. Spine J 15(6):1356–65. doi:10.1016/j.spinee.2013.07.491 [Epub 2014 Nov 24]
Yim RL, Lee JT, Bow CH, Meij B, Leung V, Cheung KM, Vavken P, Samartzis D (2014) A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: insights and future directions for regenerative therapeutics. Stem Cells 23(21):2553–67. doi:10.1089/scd.2014.0203 [Epub. Review]
Kim DJ, Moon SH, Kim H, Kwon UH, Park MS, Han KJ, Hahn SB, Lee HM (2003) Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine 28:2679–2684. doi:10.1097/01.BRS.0000101445.46487.16 (Phila Pa 1976); [PMID: 14673369]
Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M (1996) The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98:996–1003. doi:10.1172/JCI118884 [PMID: 8770872]
Masuda K, Oegema TR, An HS (2004) Growth factors and treatment of intervertebral disc degeneration. Spine 29:2757–2769. doi:10.1097/01.brs.0000146048.14946.af (Phila Pa 1976); [PMID: 15564925]
Chen WH, Liu HY, Lo WC, Wu SC, Chi CH, Chang HY, Hsiao SH, Wu CH, Chiu WT, Chen BJ, Deng WP (2009) Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials 30(29):5523–5533
Marx RE (2001) Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent 10:225–228
Akeda K, An HS, Pichika R, Attawia M, Thonar EJ, Lenz ME, Uchida A, Masuda K (2006) Platelet-rich plasma (PRP) stimulates the extracellular matrix metabolism of porcine nucleus pulposus and anulus fibrosus cells cultured in alginate beads. Spine 31(9):959–966 (Phila Pa 1976)
Formica M, Cavagnaro L, Formica C, Mastrogiacomo M, Basso M, Di Martino (2015) What is the preclinical evidence on platelet rich plasma and intervertebral disc degeneration? A. Eur Spine J 24(11):2377–86. doi:10.1007/s00586-015-4189-2 [Epub 2015 Aug 14]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Basso, M., Cavagnaro, L., Zanirato, A. et al. What is the clinical evidence on regenerative medicine in intervertebral disc degeneration?. Musculoskelet Surg 101, 93–104 (2017). https://doi.org/10.1007/s12306-017-0462-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-017-0462-3