Abstract
Mineral nutrient deficiencies induce a cascade of physiological, morphological, and biochemical changes in plants which reduce vegetative growth. In this work, the impact of P and K concentration levels on tomato plant development grown in hydroponic culture was investigated. Root morphology, chlorophyll a fluorescence, phosphorus (P) and potassium (K) content, and shoot and root biomass were analyzed. Root morphology showed significant differences among the plants grown in hydroponic culture with different concentrations of P and K. Plant root/shoot dry biomass ratio decreased by 22 and 35% for P15K0 and P30K0, respectively, compared to the control (P30K232). The deficiency of P and K (individually or both) reduced significantly the root mass density parameter. For example, root mass density decreased by 38% at P15K0 treatment compared to control. Correlation analysis showed that the P and K content ratio in shoot and root was significantly and positively correlated with root volume. Deficiencies in K and P decreased the relative size of the PSI final electron acceptor pool and the electron flow on the acceptor side of PSI. Tomato growth response depend on the availability of P and K, however, interactions between these two nutrients could influence their uptake and utilization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant development depend on many environmental variables while low nutrient status in the soil has been considered a major constraint in plant growth and productivity. Deficiency of one or more mineral nutrients could greatly impact many physiological, morphological and biochemical traits, which can reduce plant growth and induce biomass loss (Cakmak et al. 1994; Sanchez 2002; Hermans and Verbruggen 2005; Rietra et al. 2017). Nitrogen (N), phosphorus (P), and potassium (K) are important macronutrients for crops. However, to avoid low macronutrient availability, plants developed many strategies to maximize nutrient uptake and improving root features were reported to be the key of plant adaptations (Kim et al. 2015; Dai et al. 2019).
Potassium is involved in several plant physiological and biochemical processes (enzyme regulation, ion homeostasis, osmotic events, stomatal behavior, gas exchange, photosynthesis, and carbon metabolism) ensuring adequate plant growth and development (Cakmak 2005; Wang et al. 2018; Sohag et al. 2020). At the metabolic level, K+ plays an essential role in the activation of 50 to 60 enzymes, involved in critical metabolic processes (Amtmann et al. 2008; Wang et al. 2013). Potassium deficiency leads to suppression of various enzyme activities which limit plant growth and development (Hafsi et al. 2017; Chérel and Gaillard 2019). Plants confronted to K deficiency, increased their root length (Chen and Gabelman 2000; Hafsi et al. 2011) and it was observed that K+ accumulation was positively correlated to root length and root surface area (Tian et al. 2008).
Phosphorus is also involved in many important physiological and metabolic processes, including biomass production, photosynthesis, synthesis of membrane phospholipids (Plaxton and Tran 2011; Bernardi et al. 2015). P is essential for the maintenance of the photosynthetic machinery that includes PSI and PSII (Huchzermeyer and Koyro 2005). Under P deficiency, the rate of electron transport increases through PSII, which causes photoinhibition of PSII, and the unused electrons are diverted to photorespiration, which reduces CO2 uptake and fixation (Jacob and Lawlor 1993). In phosphorus deficiency situation, plant adaptation strategies consist of the modification of root morphology and architecture traits (Lynch 1995), the increase of root/shoot biomass ratio (Hermans and Verbruggen 2005), the improvement of root hair length (Zhu et al. 2001) or adapting metabolic costs and metabolic benefits in term of carbon metabolism (Lynch and Ho 2005; Li et al. 2019). Consequently, P deficiency cause reduction in plant growth and development by reducing the photosynthetic activity and the energy transfer (ATP formation) which in return reduce crops grain yield and quality (Karlsson et al. 2015; Chtouki et al. 2021).
Plant nutrition depends not only on the availability of nutrients but also on the interaction of macro and micronutrients (Sattari et al. 2014; Osanai et al. 2017). It has been reported that P deficiency in soil influenced K and Mg in rice uptake. An antagonistic interaction was observed when low P induced Mg deficiency in grapevines grown in lowpH soils (Li et al. 2004; Li and Rengel 2012). Niu et al. (2015) showed that primary root growth in Arabidopsis thaliana (L.) depends not only on the amount of P but also on Mg level in the medium. P and K are necessary for adequate plant growth and development. Usherwood and Segars (2001) have shown that an appropriate ratio between P and K is essential for obtaining a high yield of corn. Zhang et al. (2010) reported that N nutritional status influenced K uptake and high crop yield can be achieved by optimizing K:N nutrition ratios. Singh and Blanke (2000) mentioned that deficiencies of phosphorus and potassium increased the root/shoot ratio in the kohlrabi plant (Brassica oleracea var. gongylodes). Gremigni et al. (2003) proposed that the inhibitory effect of P on seed alkaloid concentrations in narrow-leafed lupin (Lupinus angustifolius L.) was significantly apparent when K was deficient. Interaction between K and N was found to have a significant effect on nutrition and production (Medina-Lara et al. 2008; Rasel et al. 2020). Medina-Lara et al. (2008) reported that the leaf K:N ratio less than 0.5 was found to be adequate for habanero pepper growth and production. In winter wheat grown in hydroponic culture, a negative influence of increasing N on root-to-shoot translocation of Zn was observed induced by a reducing shoot Zn level under severe Zn deficiency (Nie et al. 2019).
Understanding the influence of each mineral nutrient on other mineral element uptake is an important way to reveal the plant's responses and their strategies for adaptation to nutrient acquisition and utilization (Zemunik et al. 2015; Li et al. 2016; Moreira et al. 2018; Zhu et al. 2020). Improving the efficiency in the acquisition and utilization of major nutrients has been well studied, but the effect of their interaction in deficient conditions is still unclear (Aulakh and Malhi 2005; Li et al. 2009). This is a challenge in many regions around the world, where many deficient nutrients induced a loss in plant production, especially in highly alkaline and acidic soils.
We hypothesized that P and K interaction may influence their uptake and utilization by tomato plants grown in a hydroponic culture of each of these mineral nutrients. In this work, we emphasize how P and K levels affect growth physiology of tomato plant grown in hydroponic conditions and how the plants integrate the availability of P and K into growth adaptation. Root morphology, chlorophyll a fluorescence, macronutrient (P and K) content, and shoot and root biomass were analyzed. K/P ratio was also investigated to explore how this ratio affects plant growth.
Material and methods
Plant material and growth conditions
Tomato seeds (var. Campbell 33) were germinated in a commercial peat substrate in a controlled growth chamber with a day/night cycle of 16/8 h at 25 °C. The seeds were irrigated with distilled water twice a day. After 21 days, the plants were transplanted into 3-L basins with a half-concentrated Hoagland nutrient solution with pH 5.6 containing N: 242 mg l−1 as KNO3, Ca(NO3)2∙4H2O and NH4NO3; P: 30 mg l−1 as KH2PO4; K: 232 mg l−1 as KNO3 and KH2PO4; Ca: 224 mg l−1as Ca(NO3)2∙4H2O; Mg: 49 mg l−1as MgSO4∙7H2O; B: 0.45 mg l−1 as H3BO3; Cu: 0.02 mg l−1 as CuSO4.5H2O; Mn: 0.5 mg l−1 as MnCl2∙4H2O; Mo: 0.0106 mg l−1 as Na2MoO4. 2H2O; Zn: 0.48 mg l−1 as ZnSO4.7H2O; and Fe: 0.5% of (NH4)5[Fe(C6H4O7)2 used at rate 1 ml l−1 of nutrient solution) (Hoagland and Arnon 1938). A week later the seedlings received a fully concentrated nutrient solution. The combination of two concentrations of P (15 and 30 mg l−1) and K (0 and 116 and 232 mg l−1) was used 24 days after plant transplantation. The solution was renewed regularly every 5 days and a hydroponic experiment was organized according to a completely random plan with 4 repetitions per treatment.
The experiment was divided as follows: (1) the control treatment, in which plants were treated with sufficient nutrients along the experiment (P30K232); (2) the P-deficient treatment, in which plants were treated with all nutrients as in the control treatment but with a low P concentration (P15K232); (3) the K-deficient treatment, in which plants were treated with all nutrients as in the control treatment but with a low K concentration (P30K0 and P30K116); (4) the K and P-deficient treatment, in which plants were treated with all nutrients as in the control treatment but with a low K and P concentration (P15K0 and P15K116).
Root morphology parameters
Roots of plants grown in all treatments were cut carefully and were spread over a plastic box and scanned using an Epson Perfection LA2400 scanner. Root length, root average diameter, root surface area, and root volume characteristics were analyzed using the WinRHIZO image analyzing system (Regent Instructions, Quebec, Canada). Data were digitalized by processing the scanned root images and then analyzed. The root mass density (= root mass/root volume) was also calculated.
Root and shoot dry biomass
Roots and shoots of all treated plants were separated and dried in an oven at 70 °C for 2 days to determine dry weights.
Leaf chlorophyll a fluorescence transient OJIP
Tomato plants were kept in dark for 15 min to oxidize all electron transport chains before measuring chlorophyll a fluorescence (ChlF) transients. For each treatment, 10 independent measurements were made on the upper surface of the middle young fully developed leaves of plants by Handy PEA+ fluorometer (Handy PEA+, Hansatech instruments). One second saturating actinic light pulse of 3000 μmol s−1 m−2 (which is an excitation intensity to ensure closure of all PSII reaction centers) provided by an array of six light-emitting diodes (peak 650 nm) was applied on 4 mm2 surface of the middle of each young fully-developed leaves. Chlorophyll a fluorescence measurements were digitalized between 10 µs and 1 s by the instrument.
Photosystem I biophysical parameters estimated by the JIP-test model
The JIP-test has been developed for the analysis and quantification of the polyphasic OJIP transient (Strasser et al. 2004; Tsimilli-Michael and Strasser 2008). Fo is the initial minimum value (when all reaction centers (RCs) were open and all QA oxidized) and FM is the maximum level (when all RCs were closed, and all QA reduced). The different phases O-J, J-I, and I-P of the polyphasic fluorescence rise indicate 3 different reduction processes of the electron transport chain (Strasser and Strasser 1995; Schansker and Strasser 2005). The first phase O-J phase has been related to the reduction of the acceptor side of PSII. I-P phase reflects the re-reduction of PC+ and P700+ in photosystem I (PSI) (Schreiber et al. 1989; Schansker et al. 2003). The J-I-phase was related to the reduction/oxidation of the PQ-pool (Joliot and Joliot 2002; Schansker and Strasser 2005; Tóth et al. 2007).
δRo has been estimated to indicate the efficiency with which an electron can move from the reduced intersystem electron acceptors to the PSI end electron acceptors can be presented as:
VI, relative variable Chl a fluorescence at the I-step; VJ, relative variable Chl a fluorescence at the J-step, and Vt is the relative variable fluorescence is defined as (Ft−Fo)/(FM−Fo) and this expression can be taken as a measure of the fraction of the primary quinone electron acceptor of PS II in its reduced state [QA−/QA (total)].
The quantum yield of reduction of end-acceptors (RE) of PSI is expressed as:
φPo (= FV/FM = FM–FO/FM) corresponds to the efficiency by which an absorbed photon will be trapped by PSII reaction centers. ψo (= 1−VJ) expressed the probability that the energy of a trapped exciton is used for electron transport beyond QA.
The electron transport from QA− to the PSI electron acceptors is expressed as REo/RC.
Chemical analysis
Elemental concentrations of P, and K were analyzed on a dry-weight basis. The dried plant samples (root and shoot separately) were powdered and digested by nitric acid and analyzed for P and K content using Inductively Coupled Plasma Optical Emission Spectrometry (Agilent 5110 ICP-OES, USA).
Statistical analysis
Analysis of variance (ANOVA) was done in SPSS 13.0 (SPSS Inc., USA) to examine the impacts of P and K interaction effects on ChlF parameters, dry matter, chlorophyll content, root morphological traits. Differences between treatment means (n = 4) were evaluated by the Student–Newman–Keuls test at a 0.05 probability level.
Results
Root and shoot biomass partitioning in P and K deficiencies
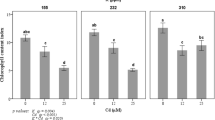
After 24 days of treatment, the dried shoots and roots were weighted (Fig. 1). Plants grown in hydroponic culture without K have shown significantly reduced root and shoot dry matter and highest reduction was observed in the root. At P15K0 and P30K0, the shoot dry weight decreased by 52 and 47%, respectively, compared to the treatment. The root dry weight decreased by 63 and 65%, respectively, at P15K0 and P30K232 compared to control treatment. However, plants grown in other treatments showed a significant increase in shoot and root dry weight compared to control (P30K232). The ratio of the aboveground biomass to the belowground biomass (root/shoot biomass ratio) is considered as the parameter that indicates direct biomass partitioning by plants. According to Fig. 1, the root/shoot biomass varied significantly among different P and K supplies. Our data showed that the root/shoot biomass ratio ranged from 0.13 to 0,17. As a result, the plant root/shoot dry biomass ratio was decreased significantly by 22 and 35% for P15K0 and P30K0, respectively, compared to the control. However, the root/shoot biomass increased with increasing concentrations of P and K together and plant grown in P30K232 treatment (control) showed a higher value.
Change in shoot dry biomass (A), root dry biomass (B), and shoot/root biomass ratio (C) in tomato plants grown in hydroponic culture with different levels of P and K concentration. Each value represents the mean ± SD of four independent repetitions. Asterisks indicate the level of statistical significance between control (P30K232) and treatments at p < 0.05
P and K deficiencies change root morphology
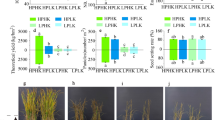
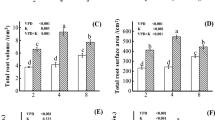
Root morphology analysis for tomato plants grown under different levels of P and K concentration can indicate dry matter allocation under different growth conditions and functional root trait adaptation to low nutrients. Root morphology showed significant differences among the plant grown in hydroponic culture with different concentrations of P and K. Root morphology included root length, volume, surface area, and diameter are presented in Fig. 2. Root length is increasing significantly for all treatments except for P30K0 compared to the control. The root length varied depending on the level of P and K concentration. In the case of P deficiency (P15), the root length increased as the concentration of K in the culture medium increased (Fig. 2A). However, in the case of K deficiency, the highest value is observed for the treatment P30K116. It seems that the P deficiency influences the root length compared to the K deficient treatment. The root diameter is also affected by the concentrations of P and K in the hydroponic culture (Fig. 2B). Nutrient deficiency decreased the root diameter significantly compared to the control except for the treatment P30K116. The surface and the root volume follow the same tendency observed for root length parameter with slight differences in terms of significance as compared to the control (Fig. 2C, D). Figure 3 clearly showed that the root mass density depends on the concentration of P and K in the hydroponic culture. The deficient level of P and K (individually or both) reduced the root mass density parameter significantly. For example, root mass density decreased by 38% at K and P deficiency (P15K0) compared to control treatment (P30K232).
Change in root length (A), root diameter (B), root surface area (C) and root volume (D) in tomato plants grown in hydroponic culture with different levels of P and K concentration. Each value represents the mean ± SD of four independent repetitions. Asterisks indicate the level of statistical significance between control (P30K232) and treatments at p < 0.05
Change in volume mass density in tomato plants grown in hydroponic culture with different levels of P and K concentration. Each value represents the mean ± SD of four independent repetitions. Asterisks indicate the level of statistical significance between control (P30K232) and treatments at p < 0.05
P and K deficiencies alter electron transport around PSI
Youngest fully developed leaf of plant grown in the hydroponic culture at all treatments kept in dark for 15 min exhibit a typical polyphasic ChlF OJIP transient from Fo to FM (so-called kautsky curve) during the first second of red-light excitation (Fig. 4A). Polyphasic Chl a fluorescence transients of tomato plant grown in hydroponic culture with different treatments were recorded and plotted on a logarithmic time scale. The OJIP curves recorded in plant grown in deficient P (P15) did not show visual changes in their shape. However, in plant grown in sufficient P supply (P30), the OJIP transient shape changed little and the treatment of P30K0 decreased the fluorescence intensity at the different OJIP phases. Furthermore, the measured OJIP transient can be compared using ΔVt. The Chl a fluorescence transients of all treatment were double normalized between Fo (0.05 ms) and FM, which can be expressed as Vt = (Ft−Fo)/(FM−Fo). Subsequently, the control transient (P30K232) was subtracted from the transients of the treated leaves (ΔVt). The difference in transients are shown in Fig. 4B revealed a negative band at steps J and I. During the I-P phase recorded in plant grown in P15K0, the negative peak at the point I is followed by a positive band around 150 ms.
(A) Chlorophyll a polyphasic fluorescence rise OJIP, exhibited by the youngest fully developed leaf of plant grown in the hydroponic culture at all treatments kept in dark for 15 min. The transients are plotted on a logarithmic time scale. The signals are: the fluorescence intensity Fo (at 20 μs); the fluorescence intensities FJ (at 2 ms) and FI (at 30 ms); the maximal fluorescence intensity, FP = FM. Each transient represents the mean of 10 measurements. (B) differential plots of relative chlorophyll a fluorescence (ΔVt)
Figure 5 showed changes in biophysical parameters referring to the electron transport from intersystem acceptors to the PSI end-electron transport (I-P phase in OJIP transient). δRo (the efficiency with which an electron can move from the reduced intersystem electron acceptors to the PSI end-electron-acceptors), RE/RC (the electron transport from QA− to the PSI electron acceptors), and φRo (quantum yield with which electrons reduce the PSI end-electron-acceptor) parameters relayed to PSI decreased at the treatment of P15K0 compared to all other treatments. These results may indicate a loss of PSI reaction centers in response to K deficiency.
Change in photosystem I biophysical parameters estimated by the JIP-test model. (A) change in the efficiency with which an electron can move from the reduced intersystem electron acceptors to the PSI end electron acceptors (δRo), (B) change in the quantum yield of reduction of end-acceptors (RE) of PSI (ϕRo) and (C) the electron transport from QA− to the PSI electron acceptors (REo/RC). Each transient represents the mean of 10 measurements. Asterisks indicate the level of statistical significance between control (P30K232) and treatments at p < 0.05
P and K uptake influenced by the level of P and K concentrations
P content was significantly decreased for P15K116 and P15K232 by 49% and 40% in root and shoot, respectively, compared to the control (P30K232) (Fig. 6A). However, root P content showed a significant increase for the other treatments compared to the control, and no significant difference was observed for shoot P content.
Change in P content (A) and K content (B) in shoot and root in tomato plants grown in hydroponic culture with different levels of P and K concentration. Each value represents the mean ± SD of four independent repetitions. Asterisks indicate the level of statistical significance between control (P30K232) and treatments at p < 0.05
We here note, that in the control, P content was the same in shoot and root, while for P15K116, P30K0, and P30K116, P content in root was higher than in the shoot. Potassium content increased in shoot and root with an increasing level of K concentration in hydroponic culture. However, K content was higher in shoot than in root (Fig. 6B).
Root volume and root/shoot biomass depend on K to P ratio
Figure 7 showed the correlation between root volume and P and K content in the shoot, root, and their ratios. As shown in Fig. 7, the correlation analysis showed that root volume and P and K content were significantly and positively correlated with each other. We observed that the highest correlation coefficient has been observed between root volume and shoot K content/root P content and with shoot K content/shoot P content ratios. The correlation coefficients R2 were 0.92 and 0.85, respectively, and have been found in the relationship between root volume and shoot K content/shoot P content ratio and between root volume and shoot K content/root P content ratio. It is well known that the root/shoot biomass ratio is an indicator of plant response to nutrient supply. Similarly, to the relationship between root volume and P and K content, we observed that the root/shoot biomass ratio was highly correlated with root K content/root P content and also with root K content/shoot P content ratios (Fig. 8). According to the correlation curve, the K/P ratio that varied between 6 and 8 showed the higher root/shoot biomass ratio.
Discussion
It has been well documented that deficiency of macronutrients modifies the allocation of carbohydrates in different plant organs and alters the root/shoot biomass ratio. The root/shoot biomass ratio under nutrients deficiencies depend on the species and conditions of culture (Gastal et al. 2015). Some studies reported that this ratio decreased, however, other studies reported that the root/shoot biomass ratio increased (Kudoyarova et al. 2015; Sainju et al. 2017). Under our conditions of K and P mineral imbalance, recognized by the root, the allocation of biomass is altered between shoot and root. Results presented in our study affirms that shoot and root biomass and their ratio were more affected by the deficiency of P and K together compared to individual deficiency. Then, the decrease in root/shoot biomass ratio significantly depend on the level of K/P concentration ratio in the hydroponic culture which confirms the synergistic interaction between K and P. Recently, Rietra et al. (2017) provided an overview on the interaction among plant nutrients by analyzing 94 peer-reviewed articles. These authors observed that a positive synergy was revealed between P and K for a wide range of crops. Our data suggest that the parameter of K content/P content ratio may be considered as a useful trait for probing the behavior of plant under nutrient deficiency of P and K.
In addition to modifying root/shoot ratio, K and P deficiencies significantly alter root system morphology. As shown in Fig. 2, P and K deficiency enhanced root development which is consistent with the observations that nutrient deficiency stimulates the import of both photosynthates and mineral nutrients that enhanced root development (Marschner et al. 1996). For example, K deficiency increases root length that contributed to increased surface exploration and then to improve the K absorption (Hafsi et al. 2011). Notably, root morphological alterations in tomato plant grown in hydroponic culture are orchestrated by K/P concentration ratio. Noted here that analysis of length, surface area, and diameter of root trait morphology may indicate the functionality of root under P and K deficiencies compared to control treatment (P30K232). However, it has appeared that the root mass density could be used as one of the functional root traits to explore the effect of nutrients deficiencies on crop plants. For P and K deficiency (P15, K0, and K116) treatments, tomato plants may accumulate dry matter through an enhanced efficiency of K and P content. Indeed, at P15K0 plants roots accumulate more P compared to P15K116 and P15K232 treatments. This could be considered as a mechanism for adaptationof low P and K together and to ensure adequate plant development. This mechanism for adaptation has been reported in previous studies (Chérel et al. 2014; Rao et al. 2016). Interestingly, K uptake and accumulation was more efficient at P deficiency (P15K232). We assume that the utilization efficiency of nutrients by the plant in deficient conditions is conditioned by the efficient acquisition by root. Wang et al. (2010) posed the question whether in such a situation phosphorus acquisition efficiency or phosphorus utilization efficiency is more critical. Answering this important question would still emphasize that plants faced nutrients deficiencies and their interactions.
It is well documented that chlorophyll a fluorescence, a non-invasive method, is sensitive to abiotic stress (Faseela et al. 2020). To test if K and P deficiency could be detected in plants using chlorophyll a fluorescence, the OJIP transient was measured in young fully developed leaves on plants grown in different hydroponic conditions with different levels of K and P concentration. We here note that the efficiency by which an absorbed photon will be trapped by PSII reaction centers (FV/FM) was unchanged in all transients and the values were close to the of 0.82 (data not shown). It has been reported that the I-P phase depends both on the electron flow through PSI as well as on the electron flow on the acceptor side of PSI (Schansker and Strasser 2005). Furthermore, I–P phase has been found to be linked to PSI relative content (Oukarroum et al. 2009). In our study, the numerical parameters related to I-P phase and referring to the electron transport from intersystem acceptors to the PSI end-electron transport is estimated by the parameter δRo, RE/RC, and φRo. In our experiment, deficiencies in K and P decreased the relative size of the PSI final electron acceptor pool and alter the electron flow on the acceptor side of PSI. This effect on the I-P phase and then on PSI end-electron-acceptor has been shown in Fig. 5 and the ΔVt curves (Fig. 4B). The same behavior of the impact of P deficiency treatment compared to the control plant when focusing on the I-step was also observed by Carstensen et al. (2018), Frydenvang et al. (2015) and Desotgiu et al. (2012). We assume that in K and P deficiency, PSI plays a pivotal role in managing the absorbed energy and the energetic electron flux between PSII and PSI intersystem. Probably this behavior was due to reduction in the absorbed light pressure on PSII to avoid any damage that can be caused by an over-light excitation.
In conclusion, focusing on how a specific essential nutrient affects the crop plant yield is primordial in plant nutrition. However, it is in our interest to focus more on the effects of interactions between nutrients on plant development. It seems to be fundamental to better understand nutrient interaction and how plants can grow in more than one mineral nutrient deficiency and how to remediate and reverse this negative constraint.
Abbreviations
- Fo (F50 μ s) and FM :
-
Initial and maximum Chl a fluorescence
- F2ms :
-
Chl a fluorescence measured at 2 ms;
- FV :
-
Maximum variable Chl fluorescence
- PS II:
-
Photosystem II
- φPo :
-
Maximum quantum yield of primary photochemistry
- ψo :
-
Efficiency with which a trapped exciton can move an electron into the electron transport chain
- δRo :
-
The efficiency with which an electron can move from the reduced intersystem electron acceptors to the PSI end electron acceptors
- QA and QB :
-
Primary and secondary quinone electron acceptors of photosystem II, respectively
- RC:
-
Reaction center
- OJIP-transient:
-
Fluorescence induction transient defined by the names of its intermediate steps
- VI :
-
Relative variable chl a fluorescence at the I-step
- VJ :
-
Relative variable chl a fluorescence at the J-step
- Vt :
-
Relative variable chl a fluorescence at time t
References
Amtmann A, Troufflard S, Armengaud P (2008) The effect of potassium nutrition on pest and disease resistance in plants. Physiol Plant 133:682–691
Aulakh MS, Malhi SS (2005) Interactions of nitrogen with other nutrients and water: effect on crop yield and quality, nutrient use efficiency, carbon sequestration, and environmental pollution. Adv Agron 86:341–409
Bernardi ACDC, Carmello QADC, Carvalho SA, De MEC, Medina CL, Gomes MDMDA, Lima DM (2015) Nitrogen, phosphorus and potassium fertilization interactions on the photosynthesis of containerized citrus nursery trees. J Plant Nutr 38:1902–1912
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530
Cakmak I, Hengeler C, Marschner H (1994) Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J Exp Bot 45:1245–1250
Carstensen A, Herdean A, Schmidt SB, Sharma A, Spetea C, Pribil M, Husted S (2018) The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol 177:271–284
Chen J, Gabelman WH (2000) Morphological and physiological characteristics of tomato roots associated with potassium-acquisition efficiency. Sci Hortic 83:213–225
Chérel I, Lefoulon C, Boeglin M, Sentenac H (2014) Molecular mechanisms involved in plant adaptation to low K+ availability. J Exp Bot 65:833–848
Chérel I, Gaillard I (2019) The complex fine-tuning of K+ fluxes in plants in relation to osmotic and ionic abiotic stresses. Int J Mol Sci 20:715
Chtouki M, Naciri R, Garré S, Nguyen F, Oukarroum A (2021) Chickpea plant responses to polyphosphate fertiliser forms and drip fertigation frequencies: effect on photosynthetic performance and phenotypic traits. Funct Plant Biol. https://doi.org/10.1071/fp21035
Dai W, Huang D, Dai W (2019) Plant response to mineral nutrient deficiency. In: Stress Physiology of Woody Plants. pp 133–153
Desotgiu R, Pollastrini M, Cascio C, Gerosa G, Marzuoli R, Bussotti F (2012) Chlorophyll a fluorescence analysis along a vertical gradient of the crown in a poplar (Oxford clone) subjected to ozone and water stress. Tree Physiol 32:976–986
Faseela P, Sinisha AK, Brestič M, Puthur JT (2020) Chlorophyll a fluorescence parameters as indicators of a particular abiotistress in rice. Photosynthetica 58:293–300
Frydenvang J, van Maarschalkerweerd M, Carstensen A, Mundus S, Schmidt SB, Pedas PR, Laursen KH, Schjoerring JK, Husted S (2015) Sensitive detection of phosphorus deficiency in plants using chlorophyll a fluorescence. Plant Physiol 169:353–361
Gastal F, Lemaire G, Durand JL, Louarn G (2015) Quantifying crop responses to nitrogen and avenues to improve nitrogen-use efficiency. In: Crop Physiology: applications for genetic improvement and agronomy: Second Edition. pp 161–206
Gremigni P, Hamblin J, Harris D, Cowling WA (2003) The interaction of phosphorus and potassium with seed alkaloid concentrations, yield and mineral content in narrow-leafed lupin (Lupinus angustifolius L.). Plant Soil 253:413–427
Hafsi C, Atia A, Lakhdar A, Debez A, Abdelly C (2011) Differential responses in potassium absorption and use efficiencies in the halophytes catapodium rigidum and hordeum maritimum to various potassium concentrations in the medium. Plant Prod Sci 14:135–140
Hafsi C, Falleh H, Saada M, Ksouri R, Abdelly C (2017) Potassium deficiency alters growth, photosynthetic performance, secondary metabolites content, and related antioxidant capacity in Sulla carnosa grown under moderate salinity. Plant Physiol Biochem 118:609–617
Hermans C, Verbruggen N (2005) Physiological characterization of Mg deficiency in Arabidopsis thaliana. J Exp Bot 56:2153–2161
Hoagland DR, Arnon DI (1938) Agricultural experiment station the water-culture method for growing plants without soil. California Experiment Station, C347,1
Huchzermeyer B, Koyro HW (2005) Salt and drought stress effects on photosynthesis: enzyme cohesion and high turnover metabolite shuttling, essential for functioning of pathways, is impaired by changes in cytosolic water potential. In: Handbook of Photosynthesis, Second Edition. pp 751–777
Jacob J, Lawlor DW (1993) Extreme phosphate deficiency decreases the In Vivo CO2/O2 specificity factor of ribulose 1,5-bisphosphate carboxylase-oxygenase in intact leaves of sunflower. J Exp Bot 44:1635–1641
Joliot P, Joliot A (2002) Cyclic electron transfer in plant leaf. Proc Natl Acad Sci USA 99:10209–10214
Karlsson PM, Herdean A, Adolfsson L, Beebo A, Nziengui H, Irigoyen S, Ünnep R, Zsiros O, Nagy G, Garab G, Aronsson H, Versaw WK, Spetea C (2015) The Arabidopsis thylakoid transporter PHT4;1 influences phosphate availability for ATP synthesis and plant growth. Plant J 84:99–110
Kim SA, Han H, Shin SH (2015) Classroom activities for learners of Korean as a foreign language: from the perspective of multiple intelligences. Electron J Foreign Lang Teach 12:391–417
Kudoyarova GR, Dodd IC, Veselov DS, Rothwell SA, Veselov SY (2015) Common and specific responses to availability of mineral nutrients and water. J Exp Bot 66:2133–2144
Li L, Rengel Z (2012) Soil acidification as affected by phosphorus sources and interspecific root interactions between wheat and chickpea. Commun Soil Sci Plant Anal 43:1749–1756
Li L, Tang C, Rengel Z, Zhang FS (2004) Calcium, magnesium and microelement uptake as affected by phosphorus sources and interspecific root interactions between wheat and chickpea. Plant Soil 261:29–37
Li SX, Wang ZH, Malhi SS, Li SQ, Gao YJ, Tian XH (2009) Chapter 7 nutrient and water management effects on crop production, and nutrient and water use efficiency in dryland areas of China. Adv Agron 102:223–265
Li X, Zeng R, Liao H (2016) Improving crop nutrient efficiency through root architecture modifications. J Integr Plant Biol 58:193–202
Li D, Zhang T, Wang M, Liu Y, Brestic M, Chen THH, Yang X (2019) Genetic engineering of the biosynthesis of glycine betaine modulates phosphate homeostasis by regulating phosphate acquisition in tomato. Front Plant Sci 9:1995
Lynch J (1995) Root architecture and plant productivity. Plant Physiol 109:7–13
Lynch JP, Ho MD (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56
Marschner H, Kirkby EA, Cakmak I (1996) Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J Exp Bot 47:1255–1263
Medina-Lara F, Echevarría-Machado I, Pacheco-Arjona R, Ruiz-Lau N, Guzmán-Antonio A, Martinez-Estevez M (2008) Influence of nitrogen and potassium fertilization on fruiting and capsaicin content in habanero pepper (Capsicum chinense Jacq.). HortScience 43:1549–1554
Moreira A, Moraes LAC, Moretti LG, Aquino GS (2018) Phosphorus, potassium and sulfur interactions in soybean plants on a typic hapludox. Commun Soil Sci Plant Anal 49:405–415
Nie Z, Zhao P, Shi H, Wang Y, Qin S, Liu H (2019) Nitrogen supply enhances zinc uptake and root-to-shoot translocation via up-regulating the expression of TaZIP3 and TaZIP7 in winter wheat (Triticum aestivum). Plant Soil 444:501–517
Niu Y, Jin G, Li X, Tang C, Zhang Y, Liang Y, Yu J (2015) Phosphorus and magnesium interactively modulate the elongation and directional growth of primary roots in Arabidopsis thaliana (L.) Heynh. J Exp Bot 66:3841–3854
Osanai Y, Tissue DT, Bange MP, Anderson IC, Braunack MV, Singh BK (2017) Plant-soil interactions and nutrient availability determine the impact of elevated CO2 and temperature on cotton productivity. Plant Soil 410:87–102
Oukarroum A, Schansker G, Strasser RJ (2009) Drought stress effects on photosystem i content and photosystem II thermotolerance analyzed using Chl a fluorescence kinetics in barley varieties differing in their drought tolerance. Physiol Plant 137:188–199
Plaxton WC, Tran HT (2011) Metabolic adaptations of phosphate-starved plants. Plant Physiol 156:1006–1015
Rao IM, Miles JW, Beebe SE, Horst WJ (2016) Root adaptations to soils with low fertility and aluminium toxicity. Ann Bot 118:593–605
Rasel M, Tahjib-Ul-Arif M, Hossain MA, Hassan L, Farzana S, Brestic M (2020) Screening of salt-tolerant rice landraces by seedling stage phenotyping and dissecting biochemical determinants of tolerance mechanism. J Plant Growth Regulat 40:1853–1868
Rietra RPJJ, Heinen M, Dimkpa CO, Bindraban PS (2017) Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun Soil Sci Plant Anal 48:1895–1920
Sainju UM, Allen BL, Lenssen AW, Ghimire RP (2017) Root biomass, root/shoot ratio, and soil water content under perennial grasses with different nitrogen rates. Field Crop Res 210:183–191
Sanchez PA (2002) Soil fertility and hunger in Africa. Science 295:2019–2020
Sattari SZ, van Ittersum MK, Bouwman AF, Smit AL, Janssen BH (2014) Crop yield response to soil fertility and N, P, K inputs in different environments: testing and improving the QUEFTS model. Field Crop Res 157:35–46
Schansker G, Strasser RJ (2005) Quantification of non-QB-reducing centers in leaves using a far-red pre-illumination. Photosynth Res 84:145–151
Schansker G, Srivastava A, Govindjee SRJ (2003) Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct Plant Biol 30:785–796
Schreiber U, Neubauer C, Klughammer C (1989) Devices and methods for room-temperature fluorescence analysis. Philos Trans Royal Soc London B, Biol Sci 323:241–251
Singh P, Blanke MM (2000) Deficiency of potassium but not phosphorus enhances root respiration. Plant Growth Regul 32:77–81
Sohag AAM, Tahjib-Ul-Arif M, Afrin S, Khan MK, Hannan MA, Skalicky M, Mortuza MG, Brestic M, Hossain MA, Murata Y (2020) Insights into nitric oxide-mediated water balance, antioxidant defence and mineral homeostasis in rice (Oryza sativa L.) under chilling stress. Nitric Oxide Biol Chem 100–101:7–16
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP-test. In: Photosynthesis: from Light to Biosphere.pp 4869–4872
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. pp 321–362
Tian XL, Wang GW, Zhu R, Yang PZ, Duan LS, Li ZH (2008) Conditions and indicators for screening cotton (Gossypium hirsutum L.) varieties tolerant to low potassium. Acta Agron Sin 34:1435–1443
Tóth SZ, Schansker G, Strasser RJ (2007) A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynth Res 93:193–203
Tsimilli-Michael M, Strasser RJ (2008) Experimental resolution and theoretical complexity determine the amount of information extractable from the chlorophyll fluorescence transient OJIP. In: Photosynthesis. Energy from the Sun. pp 697–701
Usherwood NR, Segars WI (2001) Nitrogen interactions with phosphorus and potassium for optimum crop yield, nitrogen use effectiveness, and environmental stewardship. The Sci World J 1(Suppl 2):57–60
Wang X, Shen J, Liao H (2010) Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci 179:302–306
Wang M, Zheng Q, Shen Q, Guo S (2013) The critical role of potassium in plant stress response. Int J Mol Sci 14:7370–7390
Wang X, Hao L, Zhu B, Jiang Z (2018) Plant calcium signaling in response to potassium deficiency. Int J Mol Sci 19(11):3456
Zemunik G, Turner BL, Lambers H, Laliberté E (2015) Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat Plants 1:15050
Zhang F, Niu J, Zhang W, Chen X, Li C, Yuan L, Xie J (2010) Potassium nutrition of crops under varied regimes of nitrogen supply. Plant Soil 335:21–34
Zhu YG, Smith SE, Smith FA (2001) Plant growth and cation composition of two cultivars of spring wheat (Triticum aestivum L.) differing in P uptake efficiency. J Exp Bot 52:1277–1282
Zhu Z, Li D, Wang P, Li J, Lu X (2020) Transcriptome and ionome analysis of nitrogen, phosphorus and potassium interactions in sorghum seedlings. Theor Exp Plant Physiol 32:271–285
Acknowledgements
This work was funded by University Mohamed VI Polytechnic. We are especially grateful to Aziz Soulaimani and Sabah Fathallah for assistance with P and K chemical analysis.
Author information
Authors and Affiliations
Contributions
AO designed the study. WR, RN, and MC conducted the experiments, collected the samples, and performed the physiological measurements. AO, WR, RN, MC and YZ discussed the data. AO wrote the manuscript with contributions from the other authors. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naciri, R., Rajib, W., Chtouki, M. et al. Potassium and phosphorus content ratio in hydroponic culture affects tomato plant growth and nutrient uptake. Physiol Mol Biol Plants 28, 763–774 (2022). https://doi.org/10.1007/s12298-022-01178-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-022-01178-4