Abstract
Elevated temperature and water deficit are the major abiotic factors restricting plant growth. While in nature these two stresses often occur at the same time; little is known about their combined effect on plants. Therefore, the main objective of the current study was to observe the effect of these two stresses on phenology, dry matter and seed yield in soybean. Two soybean genotypes JS 97-52 and EC 538828 were grown under green-house conditions which were maintained at different day/night temperatures of 30/22, 34/24, 38/26 and 42/28 °C with an average temperature of 26, 29, 32 and 35 °C, respectively. At each temperature, pots were divided into three sets, one set was unstressed while second and third set were subjected to water stress at vegetative and reproductive stage, respectively. As compared to 30/22 °C increase in temperature to 34/24 °C caused a marginal decline in leaf area, seed weight, total biomass, pods/pl, seeds/pl, harvest index, seeds/pod and 100 seed weight. The decline was of higher magnitude at 38/26 and 42/28 °C. Water stress imposed at two growth stages also significantly affected dry matter and yield. The highest average seed yield (10.9 g/pl) was observed at 30/22 °C, which was significantly reduced by 19, 42 and 64% at 34/24, 38/24 and 42/28 °C, respectively. Similarly, compared to unstressed plants (11.3 g/pl) there was 28 and 74% reduction in yield in plants stressed at vegetative and reproductive stage. Thus, both temperature and water stress affected the growth and yield but the effect was more severe when water stress was imposed at higher temperatures. JS 97-52 was more affected by temperature and water stress as compared to EC 538828. Though drought is the only abiotic factor that is known to affect the water status of plants, but the severity of the effect is highly dependent on prevailing temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elevated temperature and water deficit are the major abiotic factors restricting plant growth, while in nature these two stresses often occur at the same time; little is known about their combined effect on plants (Shah and Paulsen 2003; Rizhsky et al. 2004). Water deficit and above optimal temperatures individually have deleterious effects and both interact strongly with each other. There is now a scientific consensus for an observed increase in average temperatures, change in distribution of rainfall and intensification of drought conditions which will lead to a decline of crop productivity (Chaves et al. 2003; Bai et al. 2004). Though drought is the only abiotic factor that is known to affect the water status of plants, the severity of the effect is highly dependent on prevailing temperature.
Soybean [Glycine max (L.) Merril] is the world’s most important seed legume and it contributes significantly to edible oil, protein concentrate for animal feed, food uses and various industrial products. Soybean is the major rainy season crop of India; currently it is grown in 12.2 million ha with an annual production of 11.9 million tons (FAO 2014). This makes soybean as the most important oilseed crop in the country and fourth largest in terms of area in the world. It is expected that soybean area will further expand in coming years to meet the demand for not only oil but also protein source. In this scenario, soybean is already playing a pivotal role and would continue to do so in the future. However, the productivity has remained low (about 1 t/ha) which is the major cause of concern. Occurrence of drought and high temperature conditions at one or the other stage of crop growth are considered to be the major factors limiting productivity of soybean in India (Bhatia et al. 2014a, b; Bhatia and Jumrani 2016; Jumrani et al. 2017).
The stress at vegetative phase has been reported to decline photosynthesis, leaf area and biomass and once the stress is over, plant can recover to a certain extent. However, stress at reproductive stage is known to influence reproductive processes such as abortion of flowers, reproductive efficiency, development of seeds and young pods. Also, stress at reproductive phase has often no chance of recovery and hence, results in severe loss of soybean productivity (Bhatia and Jumrani 2016; Jumrani et al. 2017). Also, the detrimental effects of high temperature and water stress on physiological processes, growth and yield may not be easy to evaluate due to its indeterminate growth habit. In whatever limited studies that have been carried out, the soybean crop was exposed for short duration of time to high temperature condition. For better understanding their response and adaptation potential to future high temperature, there is an urgent need to understand the magnitude of yield loss and impact on various physiological processes when soybean crop is grown for full crop season under elevated temperature conditions and also there is a need to understand the interaction of water stress with temperature in crops, particularly for a rainfed crop like soybean. Therefore, the main objectives of present study were to analyze the impact of increased temperatures and water stress imposed at different growth stages and their interaction on soybean phenology, growth, yield and its attributes.
Materials and methods
Green houses/temperature treatments
The experiment was conducted under four green house conditions at ICAR-Indian Institute of Soybean Research, Indore (22.72°N, 75.83°E). Two contrasting soybean genotypes JS 97-52 and EC 538828 were used for this experiment. JS 97-52 is a soybean cultivar of India and it has white flower, yellow seed coat, semi-determinate growth habit, high yielding (2500–3000 kg/ha), late maturing type (110 days) and small seeded (100 seed weight 9–10 g). While EC 538828 is a germplasm of USA and it has purple flower, yellow seed coat, determinate growth habit, moderate in yield (2000–2300 kg/ha), early maturing (85 days) and bold seeded (100 seed weight 22–24 g). These genotypes were grown under green houses in cemented pots (45 cm height and 18 cm width) maintained at different day/night temperatures of 30/22, 34/24, 38/26 and 42/28 °C with an average temperature of 26, 29, 32 and 35 °C, respectively. Before sowing, the seeds were treated with fungicides viz. bavistin and dithane M and inoculated with slurry of Rhizobium japonicum. Ten seeds of uniform size were sown at 4 cm depth in each pot. Thinning was done after 1 week of sowing to 3 plants per pot which were maintained until maturity. Pots in the greenhouse were rotated frequently to minimize positional effects.
Imposition of soil moisture stress
In each greenhouse, pots were divided into three sets. One set was kept as control (unstressed), second set was subjected to water stress at vegetative stage and third set was subjected to water stress at reproductive stage. The water stress at vegetative stage was imposed as and when plants reached to fourth trifoliate stage (V4 stage). Water stress at reproductive stage was given as and when plants reached at initiation of seed fill stage (R5 stage). In both the stress treatments, the supply of water in the pots was stopped till the leaf water potential was reduced to − 2.5 MPa after which the stress was released by regular watering of plants till maturity.
Phenology
For observations on phenological development, five plants in each treatment were tagged and daily observations were recorded. The phenological data such as days to emergence (VO), days to flower initiation (R1 stage), initiation of seed fill (R5 stage), complete seed fill in the pod (R6 stage) and physiological maturity (R7 stage) were recorded (Fehr and Caviness 1977).
Water potential
In order to monitor the water status of the plants, leaf water potential (ΨL) was measured daily in stressed and unstressed (control) plants. The ΨL was recorded using psychrometers (Wescor Inc, USA) having eight C 52 sample chambers and a Psypro water potential data logger. Measurements were taken between 9.00 and 10.00 h from youngest, fully expanded leaf from the top from each treatment. Leaves were punched and the leaf discs were kept in sample chamber for 30 min and then readings were recorded.
Growth analysis
For growth analysis, three pots from each treatment were sampled. First sampling was carried out immediately after the release of water stress which was given when plants reached to fourth trifoliate stage (V4 stage). Second sampling was carried out after the release of water stress which was given when plants reached at initiation of seed fill stage (R5 stage). Third sampling was carried out as and when plants reached to full seed fill stage (R6 stage) in each treatment. The plant parts were separated into stem, leaf, pods and seeds and were oven dried at 70 °C for 72 h and dry weight was recorded. Leaf area was recorded at each sampling using automatic leaf area meter (Model LI-3100, LICOR, Inc., Nebaraska, USA).
Seed yield and yield attributes
At maturity, plants from remaining 5 pots (3 plants/pot) in each treatment were sampled and data on number of pods (0, 1, 2 and 3 seeded pods), number of seeds, seed weight, pod weight, straw weight and 100 seed weight were recorded.
Statistical analysis
Analysis of variance was carried out for all the data sets using SAS statistical software (ver. 9.2; SAS Institute, Cary, NC). Main effects of temperature (mean of water stress treatments and genotypes at each temperature), the main effects of water stress (mean of genotypes over all the temperature in each water stress treatment) and main effects of genotype (mean of each genotype over all the temperatures and water stress treatments) were analyzed. The treatment means (main effects of temperature, water stress and genotypes) and their interactions were compared based on least significant differences (LSD) at P ≤ .05 using Duncan multiple range test (DMRT).
Results
Impact of temperature on leaf water status
When plants were subjected to soil moisture deficit at vegetative stage, the rate at which leaf water potential declined was significantly faster at high temperatures as compared to lower temperatures. The development of stress was similar in JS 97-52 and EC 538828 and it took about 17, 15, 12 and 10 days for plants to reach leaf water potential (ΨL) of − 2.5 MPa at temperatures 30/22, 34/24, 38/26 and 42/28 °C, respectively (Fig. 1a, b). However, when water stress was imposed at reproductive stage, the rate at which the stress developed was much faster as compared to when the water stress was imposed at vegetative stage. The reduction in plant water status was relatively faster in JS 97-52 as compared to EC 538828 with plants reaching to ΨL of − 2.5 MPa in 6, 5, 5 and 4, and 10, 9, 6 and 5 days after the stress was imposed at reproductive stage at 30/22, 34/24, 38/26 and 42/28 °C, respectively.
Impact of temperature and water stress on phenological stages
Increase in temperature resulted in a significant reduction in duration of each phenological stage in soybean. The average time to emergence was reduced from 6 days at 30/22 °C to 4, 3 and 2 days as the temperature increased to 34/24, 38/26 and 42/28 °C, respectively. The average days to flowering (R1) of these genotypes at 30/22 °C was 41 days which was significantly declined to 38, 29 and 28 days as the plants were grown at 34/24, 38/26 and 42/28 °C, respectively (Table 1). The average days to flowering in plants subjected to water stress at vegetative phase was delayed by 2 days (35 days) as compared to the average days observed for unstressed plants (33 days). Genotypes also differed significantly for days to flowering as EC 538828 flowered earlier with average value of 29 days as compared to JS 97-52 (39 days) (Table 1). The average days to reach R5 stage at 30/22 °C was 64 days which was significantly decreased to 61, 51 and 49 days as the plants were grown at 34/24, 38/26 and 42/28 °C, respectively. Similarly, average days to R6 stage at 30/22 °C was 86 days which was significantly reduced to 84, 72 and 70 days as the plants were grown at 34/24, 38/26 and 42/28 °C, respectively. The average days to physiological maturity was 94 days in plants grown at 30/22 °C, which was declined to 91, 84 and 82 days in plants grown at 34/24, 38/26 and 42/28 °C, respectively (Table 1). Imposing the water stress also significantly affected the R5, R6 stage and maturity in two soybean genotypes. As compared to unstressed plants, these phenological stages were significantly delayed in plants subjected to water stress at vegetative phase. However, a reverse trend was observed when water stress was imposed at reproductive stage where the stages were significantly declined as compared to unstressed plants. Between the two genotypes, there was difference in these phenological stages. Average days to R5, R6 and R7 stage was 44, 64 and 73 days in EC 538828 while, it was 68, 91 and 103 in JS 97-52, respectively. All the interactions were significant indicating that temperature and imposition of water stress at two stages differentially influenced the phenological stages in two soybean genotypes (Table 1).
Impact of temperature and water stress on growth parameters
Total leaf area
The total leaf area/pl in soybean was significantly affected by temperature and water stress in two genotypes. At vegetative stage, the average leaf area/pl in plants grown at 30/22 °C was 661 cm2 which declined by 3, 20 and 31% at temperatures 34/24, 38/26 and 42/28 °C, respectively (Table 2). At R5 stage the leaf area was 1803 cm2 at 30/22 °C which was reduced by 7, 33 and 48% whereas at R6 stage leaf area was 1413 cm2 at 30/22 °C which was declined by 4, 32 and 44% in plants grown at 34/24, 38/26 and 42/28 °C, respectively. As far as water stress treatments are concerned at vegetative, R5 and R6 stage, the reduction was 45, 34 and 31% as compared to unstressed plants, respectively (Table 2). Between the two soybean genotypes, the average leaf area at vegetative stage was 562 and 584 cm2/pl and the difference was statistically non-significant. However, at R5 and R6 stages, the average leaf area in JS 97-52 (2088 and 1649 cm2) was almost two to threefolds higher as compared to EC 538828 (715 and 609 cm2), respectively.
When stress was imposed at reproductive stage, the average leaf area/pl was 1843 cm2 at 30/22 °C, which declined by 10, 37 and 51% at temperatures 34/24, 38/26 and 42/28 °C, respectively. The leaf area at R6 was reduced in a similar way as was the case at R5 stage indicating that there was no recovery in the leaf area. Among the water stress treatments, the average leaf area was higher in unstressed plants at both the growth stages and was significantly reduced due to water stress (Table 2). Between the two soybean genotypes, the average leaf area/pl at R5 and R6 stages was significantly very high in JS 97-52 (2087 and 1397 cm2) as compared to EC 538828 (693 and 551 cm2), respectively. However, the decline in leaf area was also very high under the influence of temperature and water stress for JS 97-52 as compared to EC 538828.
Total above ground biomass
At first sampling, the average above ground TBM was 3.12 g/pl at 30/22 °C and was not significantly different with plants grown at 34/24 °C. However, it declined significantly by 25 and 35% in plants grown at 38/26 and 42/28 °C (Table 3). At R5 stage, the average TBM was declined by 9, 30 and 41% whereas, at R6 stage, it was declined by 8, 32 and 51% at temperatures 34/24, 38/26 and 42/28 °C, respectively as compared to plants grown at 30/22 °C. The observed average TBM was significantly affected by water stress. At vegetative stage, the average TBM was 3.19 g/pl in unstressed plants and was reduced by 37% in plants subjected to water stress. At R5 and R6 stage, the total average TBM was reduced by 30 and 23% in water stressed plants as compared to unstressed plants. Between the two genotypes, average dry matter produced was relatively higher (25%) in EC 538828 (2.89 g/pl) as compared to JS 97-52 (2.32 g/pl) at vegetative stage. However, subsequently the rate of dry matter accumulation was very high in JS 97-52, which was about 55% higher at R5 and R6 stage as compared to EC 538828, respectively (Table 3).
The average TBM after the water stress given at R5 stage in plants grown at 30/22 °C was 23.39 g/pl which was significantly reduced by 10, 33 and 47% as the growing temperatures increased to 34/24, 38/26 and 42/28 °C, respectively. Similarly, at R6 stage, increase in growing temperatures to 34/24, 38/26 and 42/28 °C resulted in an average reduction of 9, 37 and 54% in TBM as compared to plants grown at 30/22 °C. Among the water stress treatments, the average biomass at R5 and R6 stage was significantly reduced by 38 and 53% in water stressed plants as compared to unstressed plants, respectively. Between the two genotypes, average dry matter at R5 and R6 stages was about two times higher in JS 97-52 as compared to EC 538828 (Table 3). Similar to the leaf area, the reduction in above ground biomass due temperature and water stress was of greater magnitude in JS 97-52 as compared to EC 538828.
Impact of temperature and water stress on seed yield and yield attributes
Seed yield
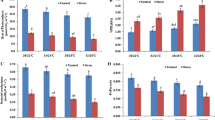
The seed yield in soybean was significantly affected by temperature, water stress imposed at vegetative and reproductive stage and the difference between the two genotypes was also significant (Table 4). The maximum average seed yield in soybean (10.9 g/pl) was observed in plants grown at 30/22 °C and there was a significant decline in seed yield as the plants were grown at higher temperatures. The reduction in seed yield was moderate (19%) in plants grown at 34/24 °C (8.8 g/pl) while the decline was severe (42 and 64%) when plants were grown at 38/26 °C (6.3 g/pl) and 42/28 °C (3.9 g/pl) as compared to the plants grown at 30/22 °C, respectively. Hence, the magnitude of yield loss was very severe when plants were grown at temperature beyond 34/24 °C. Among the water stress treatments, the imposition of water stress at vegetative and reproductive stage resulted in significant yield losses as compared to unstressed plants (Table 4). The average yield in unstressed plants was 11.3 g/pl and was reduced by 28 and 74% when plants were subjected to water stress at vegetative (8.1 g/pl) and reproductive stage (3.0 g/pl), respectively. Hence, the results clearly indicated that occurrence of water stress at reproductive phase was more detrimental to seed yield as compared to its occurrence at vegetative phase. Between two genotypes, JS 97-52 gave 36% higher yield (9.1 g/pl) as compared to EC 538828 (5.8 g/pl). The interaction of temperature with water stress was significant indicating that plants responded differentially to water stress given at two stages at four growing temperatures. The average yield of unstressed plants of two soybean genotypes grown at 30/22, 34/24, 38/24 and 42/28 °C was 16.4, 14.2, 9.3 and 5.6 g/pl which was significantly reduced to 11.2, 8.7, 7.5 and 5.0 g/pl in plants stressed at vegetative stage and 5.2, 3.6, 2.2 and 1.2 g/pl when plants were stressed at reproductive stage, respectively. This indicated that in plants water stressed at vegetative and reproductive stages at temperatures 30/22, 34/24, 38/24 and 42/28 °C, the seed yield was reduced by 31, 38, 19 and 10%, and 68, 75, 76 and 79% as compared to seed yield of unstressed plants at each temperature, respectively. Hence, the reduction in seed yield was very high at all the temperatures when plants were subjected to water stress at reproductive stage as compared to vegetative stage. The absolute yield was higher in JS 97-52 as compared to EC 538828 while reduction in yield as the temperatures increased and water stress imposed at vegetative and reproductive stages was relatively low in EC 538828. The temperature × genotype and water stress × genotype interactions were significant indicating that response of soybean genotypes to growing temperatures and water stress was different. Average seed yield of JS 97-52 in unstressed plants was 14.4 g/pl and reduced by 28 and 81% in the plants water stressed at vegetative and reproductive stage, respectively. Whereas in EC 538828 average seed yield was 8.3 g/pl in unstressed plants which was reduced by 29 and 59% in vegetative and reproductive stage water stressed plants, respectively. A significant interaction of temperature, water stress and genotype was observed for seed yield as shown in Fig. 2.
Total biomass
Similar to seed yield but to a lesser extent, the total above ground biomass (TBM) at harvest was significantly reduced by temperature and water stress treatments (Table 4). The average TBM in soybean was 27.0 g/pl in plants grown at 30/22 °C, which declined by 12, 35 and 54% in plants grown at 34/24, 38/26 and 42/28 °C, respectively. Among water stress treatments, the average TBM in unstressed plants was 26.9 g/pl, which was reduced by 23 and 52% when plants were subjected to water stress at vegetative and reproductive phase, respectively. Between the two genotypes, the average TBM was significantly higher (57%) in JS 97-52 (28.2 g/pl) as compared to EC 538828 (12.2 g/pl) (Table 4). The interaction of temperature with water stress was significant indicating that water stress imposed at two stages differentially influenced the TBM in plants grown at four temperatures. The average TBM in unstressed plants of two soybean genotypes grown at 30/22, 34/24, 38/26 and 42/28 °C was 35.9, 33.7, 22.8 and 15.4 g/pl and was significantly higher as compared to TBM observed in plants subjected to water stress at both the growth stages. As compared to unstressed plants, the reduction in TBM in plants subjected to water stress at vegetative and reproductive stage was 26, 33, 16 and 7%, and 48, 55, 55 and 48%, respectively. Hence, the influence of water stress in TBM at all the temperatures was significantly high in plants subjected to water stress at reproductive stage as compared to when stressed at vegetative stage. The interactions of temperature × genotype, water stress × genotype were significant indicating that TBM was differentially influenced by both temperature and water stress treatments in two soybean genotypes. In general, the TBM was significantly high in JS 97-52 at all the temperatures and water stress treatments as compared to EC 538828. Average TBM in JS 97-52 was 39.4 g/pl at 30/22 °C declined by 13, 42 and 60% as the temperature increased to 34/24, 38/24 and 42/28 °C, respectively. Average TBM was 14.7 g/pl in EC 538828 at 30/22 °C and the reduction was 10, 19 and 37% at these temperatures. Average TBM of JS 97-52 in unstressed plants was 38.0 g/pl and reduced by 22 and 56% in the plants stressed at vegetative and reproductive stage, respectively. Whereas in EC 538828 average TBM was 15.8 g/pl in unstressed plants which was reduced by 26 and 42% in vegetative and reproductive stage stressed plants, respectively. Though the total biomass was higher in JS 97-52 the degree of reduction was also high in this genotype. A significant interaction of temperature, water stress and genotype was observed for TBM as shown in Fig. 3.
Harvest index
As the yield and TBM were significantly affected by various temperature and water stress treatments in two soybean genotypes, these effects were manifested in significant change in harvest index (HI) (Table 4). The average harvest index was maximum at 30/22 °C (42.4%), which significantly declined to 38.6, 36.0 and 30.9% in plants grown at 34/24, 38/26 and 42/28 °C, respectively. This further confirms that the degree of reduction in seed yield due to increasing temperatures was more as compared to its impact on TBM resulting in considerable decline in HI. Among the water stress treatments, the imposition of water stress at vegetative and reproductive stage resulted in significant decline in HI as compared to unstressed plants (Table 4). The average HI in unstressed plants was 43.9%, which declined marginally to 42.1% and severely to 24.8% when plants were subjected to water stress at vegetative and reproductive stage, respectively. Between the two genotypes, the average HI was significantly higher in EC 538828 (45.6%) as compared to JS 97-52 (28.3%). The interaction of temperature and water stress was significant indicating that harvest index was differentially affected due to water stress imposed at two stages and at four different growing temperatures. Against the observed HI in unstressed plants at 30/22 (49.4%), 34/24 (46.0%), 38/26 (42.6%) and 42/28 °C (37.9%), the observed values in plants subjected to water stress at vegetative and reproductive stage was 46.8, 42.9, 41.4 and 37.5%, and 31.0, 26.9, 24.0 and 17.3%, respectively. The interaction of temperature × genotype was not significant while water stress × genotype interaction was significant indicating that HI was significantly higher in EC 538828 as compared to JS 97-52. Average HI of JS 97-52 in unstressed plants was 36.6% and reduced to 34.2 and 14% in the plants stressed at vegetative and reproductive stage, respectively. Whereas in EC 538828 average HI was 51.3% in unstressed plants which was reduced to 50.1 and 35.6% in vegetative and reproductive stage stressed plants, respectively. A non significant interaction of temperature, water stress and genotype was observed for HI (Fig. 4).
Number of pods
The number of pods/pl was significantly influenced by temperature and water stress imposed at different stages in two soybean genotypes (Table 4). The average number of pods/pl in soybean was maximum (47 pods/pl) in plants grown at 30/22 °C, which significantly declined to 44, 39 and 32 pods/pl when plants were grown at 34/24, 38/26 and 42/28 °C, respectively. Among the water stress treatments, there was a significant difference in average number of pods between unstressed plants (47 pods/pl) and plants stressed at reproductive stage (30 pods/pl) while no significant difference in number of pods between unstressed plants and plants stressed at vegetative stage (45 pods/pl) was observed. Between the two genotypes, the average number of pods was significantly higher (77%) in JS 97-52 (66 pods/pl) as compared to EC 538828 (15 pods/pl). The interaction of temperature with water stress was significant indicating that plants responded differentially to water stress at four growing temperatures. The average number of pods/pl of unstressed plants of two soybean genotypes grown at 30/22, 34/24, 38/26 and 42/28 °C were 52, 50, 47 and 40, which was at par with the number of pods observed in plants water stressed at vegetative stage (49, 49, 44 and 38 pods/pl, respectively). However, compared to unstressed plants at these temperatures, pods/pl was significantly reduced to 42, 34, 26 and 19, respectively in plants water stressed at reproductive stage (Table 4). Temperature × genotype and water stress × genotype interactions were significant indicating that temperatures and water stress treatments differentially influenced the number of pods formed. The number of pods in genotype JS 97-52 were significantly more at all the temperatures and water stress treatments as compared to EC 538828. In response to growing temperatures, the number of pods declined significantly in genotype JS 97-52 from 79 to 73, 62 and 50 as the temperatures increased from 30/22 to 34/24, 38/26 and 42/28 °C, respectively. As against this, no significant influence of temperature on number of pods was observed (~ 15 pods/pl) in EC 538828. Similarly, compared to unstressed plants, no significant difference in number of pods in plants stressed at vegetative stage was observed in both the genotypes while it differed significantly only in JS 97-52 in plants water stressed at reproductive stage (39%). A significant interaction of temperature, water stress and genotype was observed for pods/pl as shown in Fig. 5.
Number of seeds/pl
The average number of seeds/pl in soybean was maximum (105 seeds/pl) in plants grown at 30/22 °C, which significantly declined to 93, 74 and 51 seeds/pl when plants were grown at 34/24, 38/26 and 42/28 °C, respectively (Table 4). Among the water stress treatments, average number of seeds/pl in unstressed plants was 106 and declined to 97 seeds/pl in plants stressed at vegetative stage and 40 seeds/pl in plants stressed at reproductive stage. Hence, the imposition of stress at vegetative stage (8%) did not affect the number of seeds while at reproductive stage the reduction was severe (62%). Between the two genotypes, the average number of seeds was significantly higher (80%) in JS 97-52 (135 seeds/pl) as compared to EC 538828 (27 seeds/pl). The interaction of temperature with water stress was non-significant indicating a similar response of these two stresses on number of seeds. Temperature × genotype and water stress × genotype interactions were significant indicating that temperatures and water stress treatments differentially influenced the number of seeds/pl. The number of seeds in genotype JS 97-52 were significantly more at all the temperatures and water stress treatments as compared to EC 538828. In JS 97-52 at 30/22 °C average number of seeds/pl was 180 and declined to 158, 122 and 80 as the temperatures increased to 34/24, 38/26 and 42/28 °C, respectively. As against this number of seeds/pl in EC 538828 at 30/22 °C was 31 which, declined to 29, 26 and 23 as the temperatures increased to 34/24, 38/26 and 42/28 °C, respectively. Average number of seeds/pl in unstressed plants of JS 97-52 was 177 reduced to 168 and 60 seeds/pl in plants water stressed at vegetative and reproductive stage, respectively. While average number of seeds/pl in EC 538828 was 35 in unstressed plants which was declined to 26 and 21 seeds/pl in plants water stressed at vegetative and reproductive stage, respectively. A non-significant interaction of temperature, water stress and genotype was observed for seeds/pl (Fig. 6).
Hundred seed weight
Hundred seed weight was significantly affected by temperature and water stress imposed at different stages in two genotypes. The average 100 seed weight was maximum (16.0 g) in plants grown at 30/22 °C and it declined significantly by 12, 21 and 38% when the plants were grown at 34/24, 38/26 and 42/28 °C, respectively (Table 4). Among the water stress treatments, the average 100 seed weight in unstressed plants was 15.5 g and was reduced by 9 and 36% when plants were subjected to water stress at vegetative and reproductive stages, respectively. Between the two genotypes, the average 100 seed weight was 249% higher in EC 538828 (20.5 g) as compared to JS 97-52 (5.9 g) (Table 4). The interaction of temperature with water stress was significant indicating that 100 seed weight in soybean was differentially influenced by water stress treatments at four growing temperatures. The average 100 seed weight of unstressed plants of two soybean genotypes grown at 30/22, 34/24, 38/26 and 42/28 °C was 18.2, 17.0, 14.9 and 11.8 g which was reduced to 16.4 (10%), 14.3 (16%), 14.1 (6%) and 11.6 g (2%) in plants water stressed at vegetative stage, and to 13.4 (27%), 10.9 (36%), 9.0 (40%) and 6.5 g (45%) in plants water stressed at reproductive stage, respectively. The interactions of temperature × genotype and water stress × genotype were significant indicating that response of soybean genotypes to growing temperatures and water stress treatments was differentially influenced. The average 100 seed weight of JS 97-52 when grown at 30/22 °C was 7.5 g which was reduced by 13, 30 and 44% in plants grown at 34/24, 38/26 and 42/28 °C, respectively. As against this, the average 100 seed weight of EC 538828 at 30/22 °C was 24.5 g which was reduced by 12, 18 and 36% in plants grown at 34/24, 38/26 and 42/28 °C. Among the water stress treatments, the average 100 seed weight of JS 97-52 in unstressed plants was 7.7 g, which was reduced by 22% (6.0 g) and 49% (3.9 g) in plants stressed at vegetative and reproductive stages, respectively. In case of genotype EC 538828, the observed 100 seed weight in unstressed plants was 23.3 g, which was reduced by 5% (22.2 g) and 31% (16.0 g), in plants water stressed at vegetative and reproductive stages, respectively. The reduction in seed size was relatively more in JS 97-52 as compared to EC 538828 at all the temperatures and water stress treatments. A non-significant interaction of temperature, water stress and genotype was observed for 100 seed weight (Fig. 7).
Empty and 1, 2, 3 seeded pods
In general, soybean pods are formed with one, two, three or rarely four seeds and their ratio differs between the genotypes. In the present study, out of total number of pods, the maximum contribution was from two seeded pods (~ 65%) and contribution of one and three seeded pods was about ~ 15% each and rest were empty pods (~ 3%) (Table 5). However, the ratio of one, two seeded and three seeded pods was influenced by temperature, water stress and their interaction. Among the temperature treatments, the average percentage of 2 seeded pods did not differ much and ranged between 62 to 66%. However, the variation in 1 and 3 seeded pods was opposite. The average percentage of one seeded pods increased with increasing temperature from 11.5 at 30/22 °C to 21% at 42/28 °C, while it declined in case of 3 seeded pods from 23 at 30/22 °C to 12% at 42/28 °C. Similarly, the percentage of empty pods also significantly increased as the temperatures increased from 30/22 °C (1.4%) to 42/28 °C (5.3%) (Table 5). Among the water stress treatments, there were little differences in two seeded pods (63%) in unstressed plants and in plants water stressed at vegetative stage while it significantly increased to 67% in plants stressed at reproductive stage. Similar to temperature stress, the major variation in response to water stress was observed for 1 and 3 seeded pods. The percentage of 3 seeded pods in unstressed plants was 20% and interestingly a greater decline in these pods was observed in plants stressed at vegetative stage (14%) as compared to plants stressed at reproductive stage (18%). As against this, the greater percentage of 1 seeded pods were observed in plants stressed at vegetative stage (20%) as compared to unstressed plants (14%) and plants stressed at reproductive stage (12%) (Table 5). More or less, not much variation in empty pods was observed among the unstressed plants, and plants water stressed at vegetative and reproductive stages (~ 3%). Between genotypes, average percentage of 2 and 3 seeded pods was significantly high in JS 97-52 (70 and 22%) as compared to EC 538828 (59 and 12%). On the other hand the percentage of 1 and 0 seeded was significantly higher in EC 538828 (25 and 4%) as compared to JS 97-52 (5 and 2%), respectively. The interaction of temperature and water stress treatment was significant, which indicated that these ratios were differentially influenced by temperature when plants were water stressed at vegetative and reproductive stages. In general, with increasing temperatures percentage of empty pods increased in unstressed plants and plants subjected to water stress at vegetative and reproductive stages and the differences among stress treatments were negligible (Table 5). The interaction of temperature × genotype and water stress × genotype was significant for 0, 1, 2 and 3 seeded pods which indicated that ratio of these pods was differentially affected by temperature and water stress treatments in two soybean genotypes (Table 5). Interaction of temperature, water stress and genotype was significant only for empty and 2 seeded pods as shown in Fig. 8.
Discussion
Growth and yield under environmental stresses is the manifestation of effect on physiological and biochemical processes associated with growth and yield formation. Under field conditions, plant performance in terms of biomass accumulation, development, partioning and yield mainly depends on its ability to acclimatize to the changes occurring in environment which help the plants to withstand the stress (Wang et al. 2003). Accelerated depletion of soil water, presumably by a combination of evaporation and transpiration, was evident at high temperature in the present study. When plants were subjected to soil moisture deficit at vegetative and reproductive stage, the rate at which leaf water potential declined was significantly faster at reproductive stage and at high temperatures as compared to lower temperatures (Fig. 1a, b). This could be associated with the canopy size which was much smaller at vegetative stage resulting in lesser loss of water due to transpiration as compared to the reproductive stage. High soil temperature can reduce water more rapidly from the soil by increasing evaporation by influencing soil temperatures, rate of transpiration and deficit in vapor pressure. Temperature obviously interacted with water stress by affecting the availability of soil water. This suggested that though drought is the only stress that directly controls the water status of the plant, but the severity of drought is also dependent on prevailing temperature (Machado and Paulsen 2001). Between the two genotypes, the water stress developed much faster in JS 97-52 as compared to EC 538828 at reproductive stage. This difference could be associated with the canopy size, which was smaller for EC 538828 as compared to JS 97-52.
Adjustments in phenology play a critical role in adaptation of plants to adverse environmental factors (Setiyono et al. 2007). Drought and heat stress not only effects the change of one phenological stage to other but also the duration of each stage. Increase in temperature resulted in a significant reduction in each phenological stage in soybean. Imposing the water stress at two growth stages also significantly affected the phenology. As compared to unstressed plants, each phenological stage was significantly delayed in plants subjected to water stress at vegetative stage. The delay was conspicuously more in genotype JS 97-52 as compared to EC 538828. However, a reverse trend was observed when water stress was imposed at reproductive stage where the maturity was almost more than 20 days in JS 97-52 at temperature 38/26 and 42/28 °C as compared to unstressed plants. The difference in EC 538828 was only 2 days (Table 1). This could be associated with plant plasticity, as plants stressed at vegetative phase had a capacity to recover after the water stress was over. On the other hand, plants subjected to water stress at reproductive stage, had a no chance of recovering and plants completed their life cycle early.
Leaf area is often reduced under heat and drought stress due to which transpiration surface of leaf is drastically decreased (Alves and Setter 2004). Under the influence of high temperature, rate of leaf elongation is increased, while its duration is decreased (Bos et al. 2000). In the present study dry matter in terms of leaf area and above ground biomass declined with the increase in growing temperatures both in unstressed and water stressed plants. However, the magnitude of reduction at higher temperature was more in water stressed plants as compared to unstressed plants. Also, the reduction in dry matter production was more in plants which were water stress at reproductive stage as compared to vegetative stage (Tables 2, 3). Hence, the results indicated that when the soybean plants face the water stress conditions at vegetative phase, they could recover the biomass once the stress is released while occurrence of water stress at reproductive stage, the recovery was not possible. Also the degree of loss of dry matter due to water stress increased with the increase in growing temperatures. Between two genotypes, JS 97-52 gave higher above ground biomass and leaf area as compared to EC 538828. However, the reduction in above ground biomass and leaf area in response to temperature and water stress was also very high in JS 97-52 as compared to EC 538828.
Yield is mainly a function of number of plants, dry matter production, seed numbers and seed size. High temperature (Egli and Wardlaw 1980) and water stress (Harris et al. 1984) have been reported to reduce the seed yield in soybean. However, there is limited information available on interactive effects of high temperature and water stress on crop plants including soybean and therefore needs investigation (Prasad et al. 2008). The combined effect of both high temperature and water stress on yield of many crops is stronger than the effects of each stress alone (Dreesen et al. 2012). In India, most of the soybean growing regions fall under semiarid region, thus high temperature and drought are the severe restraint for soybean production. Therefore, understanding of combined effects of high temperature and water stress on soybean is important for improving its productivity. Temperature tolerance is an important factor of drought resistance for semiarid region because large spill in the rains that cause drought are always accompanied by high temperature conditions (Ntare and Williams 1998).
In the present study, results clearly indicated that temperature alone affected the seed yield and beyond 34/24 °C there was severe decline in yield. The combined effect of temperature and water stress further declined the yield particularly so when water stress was given at reproductive stage. Though the JS 97-52 gave very high yield at lower temperatures but the effect of high temperature and water stress at vegetative and reproductive stages resulted in severe yield losses as compared to EC 538828. The temperatures affected the seed yield as all the yield attributes were affected in similar way as seed yield. In control plants, the total biomass was reduced by 6, 43 and 64% in JS 97-52 and in EC 538828 the reduction was 7, 18 and 38% at temperatures 34/24, 38/26 and 42/28 °C respectively. The effect on number of pods was very high in JS 97-52 which showed 3, 11 and 27% reduction while there was no change in number of pods in EC 538828 at temperatures 34/24, 38/26 and 42/28 °C as compared to 30/22 °C. Hundred seed weight, seeds/pl and seeds/pod was reduced in similar trend in two genotypes in response to temperature. Seed size mainly depends on the availability of photosynthates that are either currently available or those that are translocated from other parts of plants into the seeds. High temperature (Prasad et al. 2006) and water stress (Frederick et al. 1991) leads to reduction in photosynthesis, nitrogen supplies, rapid senescence of leaves, forced maturity and reduced seed filling and ultimately leads to severe yield loss. Under drought, the rapid senescence of leaves and reduced photosynthesis obstruct the supply of assimilates available to seed ultimately affecting the seed size while, high temperature mainly affects yield through seed number by influencing pollen or ovule function, resulting in lower seed-set. Our results were similar with other reports which showed that legumes are very sensitive to water stress during the pod and seed fill stage (Krishnamurthy et al. 2010). The impact of water stress when combined with the temperature was more severe to soybean yield as compared to temperature stress alone. Also, the stress given at reproductive stage was more detrimental to seed yield and its attributes as compared to water stress at vegetative stage. In the present study, when the water stress was imposed at vegetative stage, number of pods and seeds were not affected significantly in both the genotypes at all the temperatures. When water stress was imposed at reproductive stage, there was severe reduction in JS 97-52 while in EC 538828, it was not affected significantly as compared to control plants. The 100 seed weight was significantly influenced by water stress at vegetative and reproductive stage and the degree of reduction was much higher when the stress was given at reproductive stage in both the genotypes (Table 4). There were large differences for 0, 1, 2 and 3 seeded pods under the influence of temperature and water stress conditions When plants were grown at higher temperature, proportion of 0 and 1 seeded pods was more as compared to low temperature due to less availability of photosynthates at higher temperatures. As against these, at low temperature the 2 and 3 seeded pods proportion was more. Water stress also influenced the seeds/pod as compared to unstressed plants. The proportion of 2 and 3 seeded pods was less while proportion of 0 and 1 seeded pods was more in water stressed plants (Table 5). Beside the number of pods and seeds, the other factors such as phenology, dry matter and leaf area were less affected in EC 538828, which could have played key role in its better performance under temperature and water stress as compared to JS 97-52.
Both temperature and water stress affected the growth and yield but the effect was more severe when water stress was imposed at higher temperatures. Also, when water stress was imposed at reproductive stage, the decline in these parameters was more severe as compared to when it was imposed at vegetative stage. The data thus indicated that when plants faced stress at vegetative stage, due to plant plasticity and consequent recovery, the dry matter, seed yield and its attributes was less affected. On the contrary, when plants faced water stress at reproductive stage, the plants did not have such plasticity to recover resulting in much severe reduction in seed yield and its attributes as compared to the plants, which did not undergo any water stress. Between the two genotypes, JS 97-52 was more severely influenced by the temperature and water stress as compared to EC 538828 indicating that genotypic differences do exist in the temperature and water stress tolerance. Variations existed between the two contrasting genotypes, which were attributed to differential sensitivity to stress. Thus, the heat-tolerant genotypes should be able to tolerate drought conditions and drought tolerant genotype should be able to resist high temperature conditions as these two stresses often occur simultaneously under field conditions.
Conclusions
Water stress is often accompanied with high temperature, so the most important role of plant breeders is to develop cultivars which can tolerate both temperature and water stress. Understanding the combined effects of high temperature and drought on physiological traits and yield in breeding programme will help to predict the effect of future climate change on soybean production and improve its productivity. Thus, there is need to develop dedicated research programs aimed at identifying soybean genotypes which can tolerate both drought and high temperature. Tolerance mechanisms for drought and high temperature may be different; therefore, an integrated approach should be taken for identification of variety.
References
Alves AAC, Setter TL (2004) Response of cassava leaf area expansion to water deficit: cell proliferation, cell expansion and delayed development. Ann Bot 94:605–613
Bai Y, Han X, Wu J, Chen Z, Li L (2004) Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 431:181–184
Bhatia VS, Jumrani K (2016) A maximin-minimax approach for classifying soybean genotypes for drought tolerance based on yield potential and loss. Plant Breed 136:691–700
Bhatia VS, Jumrani K, Pandey GP (2014a) Developing drought tolerance in soybean using physiological approaches. Soybean Res 12:1–19
Bhatia VS, Jumrani K, Pandey GP (2014b) Evaluation of the usefulness of senescing agent potassium iodide as a screening tool for tolerance to terminal drought in soybean. Plant Knowl J 3:23–30
Bos HJ, Tijani-Eniola T, Struik PC (2000) Morphological analysis of leaf growth of maize: responses to temperature and light intensity. Neth J Agric Sci 48:181–198
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought-from genes to the whole plant. Funct Plant Biol 30:239–264
Dreesen PE, De Boeck HJ, Janssens IA, Nijs I (2012) Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ Exp Bot 79:21–30
Egli DB, Wardlaw IF (1980) Temperature response of seed growth characteristics of soybean. Agron J 72:560–564
FAO (2014) FAOSTAT database. http://apps.fao.org
Fehr RW, Caviness CE (1977) Stages of soybean development. SREC Lowa Agric Home Econ Exp Stn, Iowa State University, Ames, IA, Rep 80
Frederick JR, Wooley TJ, Hesketh JD, Peters DB (1991) Seed yield and ergonomic traits of old and modern soybean cultivars under irrigation and soil water deficit. Field Crops Res 27:71–82
Harris DS, Schapaugh WT, Kanemasu ET (1984) Genetic diversity in soybean for leaf canopy temperature and yield. Crop Sci 24:839–842
Jumrani K, Bhatia VS, Pandey GP (2017) Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynth Res 131:333–350
Krishnamurthy L, Kashiwagi J, Gaur PM, Upadhyaya HD, Vadez V (2010) Sources of tolerance to terminal drought in the chickpea (Cicer arietinum L.) minicore germplasm. Field Crops Res 119:322–330
Machado S, Paulsen GM (2001) Combined effects of drought and high temperature on water relations of wheat and sorghum. Plant Soil 233:179–187
Ntare BR, Williams JH (1998) Heritability and Genotype × Environment interaction for yield and components of yield. Model in the segregating populations under semi-arid conditions. Afr Crop Sci J 6:119–127
Prasad PVV, Boote KJ, Allen LH (2006) Adverse high temperature effects on pollen viability, seed-set, seed yield and harvest index of grain sorghum (Sorghum bicolor (L.) Moench) are more severe at elevated carbon dioxide due to higher tissue temperatures. Agric For Meteorol 139:237–251
Prasad PVV, Staggenborg SA, Ristic Z (2008) Impacts of drought and/or heat stress on physiological, developmental, growth and yield processes of crop plants. In: Ahuja LH, Saseendran SA (eds) Response of crops to limited water: understanding and modeling water stress effects on plant growth processes. Advances in Agricultural Systems Modeling Series 1. ASA-CSSA, Madison, pp 301–355
Rizhsky L, Liang HJ, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134:1683–1696
Setiyono TD, Weiss A, Specht J, Bastidas AM, Cassman KG, Dobermann A (2007) Understanding and modeling the effect of temperature and daylength on soybean phenology under high-yield conditions. Field Crops Res 100:257–271
Shah NH, Paulsen GM (2003) Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 257:219–226
Wang WX, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Acknowledgements
Kanchan Jumrani would like to acknowledge the Council of Scientific and Industrial Research (CSIR)/University Grants Commission (UGC) (Grant No. 20-06/2010 (i) EU-IV), Government of India for providing the financial support in the form of research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jumrani, K., Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol Mol Biol Plants 24, 37–50 (2018). https://doi.org/10.1007/s12298-017-0480-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-017-0480-5