Abstract
Mesenchymal stem cells are multipotent cells capable of replicating as undifferentiated cells, and have the potential of differentiating into mesenchymal tissue lineages such as osteocytes, adipocytes and chondrocytes. Such lineages can then be used in cell therapy. The aim of present study was to characterize bone marrow derived mesenchymal stem cells in four different species, including: sheep, goat, human and mouse. Human bone-marrow mesenchymal stem cells were purchased, those of sheep and goat were isolated from fetal bone marrow, and those of mouse were collected by washing bone cavity of femur and tibia with DMEM/F12. Using flow-cytometry, they were characterized by CD surface antigens. Furthermore, cells of third passage were examined for their osteogenic and adipogenic differentiation potential by oil red and alizarin red staining respectively. According to the results, CD markers studied in the four groups of mesenchymal stem cells showed a different expression. Goat and sheep expressed CD44 and CD166, and weakly expressed CD34, CD45, CD105 and CD90. Similarly, human and mouse mesenchymal cells expressed CD44, CD166, CD105 and CD90 whereas the expression of CD34 and CD45 was negative. In conclusion, although all mesenchymal stem cells display plastic adherence and tri-lineage differentiation, not all express the same panel of surface antigens described for human mesenchymal stem cells. Additional panel of CD markers are necessary to characterize regenerative potential and possible application of these stem cells in regenerative medicine and implantology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cells are generically defined as undifferentiated cells capable of self-renewal through replication as well as differentiation into specific cell lineages [1]. They can be broadly classified as embryonic or adult, depending on the developmental stage from which they are obtained [2]. Embryonic stem cells (ESCs) have the potential of differentiating into various kinds of embryonic and extra embryonic tissues [3]. The other class of stem cells i.e. the adult stem cells are able to differentiate into different cell types [4]. Moreover, mature cells can be reprogrammed through transfect ion by Oct4, Sox2, Klf4 and c-Myc genes culminating into induced pluripotent stem cells [5].
Mesenchymal stem cells (MSCs) were first isolated and characterized by Friedenstein et al. [6] in 1974. Now, they can be isolated from different species and tissues [6], and can be used in clinical and preclinical studies [7, 8]. They can be harvested from connective tissues such as bone marrow, adipose tissue, fetal liver and others, and can be successfully expanded in vitro [9].
Also, compared to ESCs and induced pluripotent stem cells (iPSCs), MSCs are safe for transplantation, because they have Immunomodulatory properties, low immunogenicity and non-tumorigenic characteristics that can decrease the immune rejection of transplanted cells [10].
The common method used for recognition and determination of cell types including MSCs is the cluster of differentiation (CD) markers protocol [11]. Positive expression of CD105, CD73 and CD90 in human MSCs is ≥95% as measured by flow cytometry, while negative expression (≤2%) of CD45, CD14, CD34, CD19, and HLA class II.
Despite the widespread utilization of MSCs from non-humans, there is no established panel of CD markers for their identification in non-human animals. In this study, we evaluated and compared immunophenotyping of CD markers in human, mouse, ovine and caprine bone marrow-mesenchymal stem cells. Such examination and characterization of regenerative potential and possible widespread application of MSCs in regenerative medicine and implantology may lead to the generation of novel cell therapies and provide fresh insight into the therapeutic tackling of complex disorders.
Materials and Methods
Chemicals
Except where otherwise indicated, all chemicals were obtained from Sigma-Aldrich (USA).
BM-MSCs Isolation and Culture
This study was approved by the ethical committee Faculty of Medical Sciences of the Tarbiat Modares University. Ovine and caprine fetuses (30–35 days) were obtained from slaughterhouse, and transported to laboratory in Dulbecco’s phosphate buffered saline (DPBS) on ice. The BM-MSCs were extracted from the ovine and caprine fetuses by aspiration of femurs and tibias with Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 Ham (DMEM/F12) 3:1 medium supplemented with 100 IU/ml penicillin and 100 μg/ml streptomycin. The bone marrow sample was layered on top of an equal volume of ficoll solution, and centrifuged at 1900g for 30 min at room temperature. The cloudy layer was collected into a new tube and washed twice with DPBS. The obtained cells with concentration of 5 × 106 cells/ml were cultured in DMEM/F12 supplemented with 10% FBS (fetal bovine serum), 2 mM l-glutamine and penicillin/streptomycin. The medium was changed every three days and subcultured at the confluency of 80%.
The mice used for the experiments were male 8–10 week old inbred Balb/c (Pasteur Institute, Iran). They were sacrificed by cervical dislocation according to the guidelines of the Institutional Animal Ethics Committee (IAEC). The BM-MSCs were extracted from femoral and tibial bone marrow of five mice by inserting a 26-gauge syringe at bone cavity, washing it with 10 ml DMEM/F12 3:1 medium supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin. Following centrifugation of the flush out at 1500 rpm for 10 min, most of the blood cells remained in the supernatant. These cells were suspended in DMEM/F12 containing 10% FBS, 2 mM l-glutamine and 1% penicillin/streptomycin, and the suspension was filtered through a 70 µm mesh to eliminate tissue debris. The obtained cells were cultured in DMEM/F12 supplemented with 10% FBS, and 1% penicillin/streptomycin in a flask at 37 °C in a 5% CO2 humidified incubator. After 48 h, non-adherent cells were removed and adherent -MSCs cells were cultured for 7 more days.
Flow-Cytometric Analysis
Fetal BM-MSCs of ovine and caprine, and human and mouse BM-MSCs were examined for expression of specific surface markers by flow-cytometry. Briefly, the cultured cells were suspended in cold DPBS at the dilution of 106 cells/ml. Incubation with primary antibodies (CD34, CD44, CD45, CD90, CD105 and CD166) was followed by exposure to secondary conjugated antibody at 4 °C for 30 min, and the complex was analyzed by flow-cytometry.
Multilineage Cell Differentiation
To examine adipogenic and osteogenic differentiation potential, the ovine and caprine BM-MSCs, human and mouse BM-MSCs of third passage were induced with DMEM medium supplemented with 50 μg/ml ascorbic acid 3-phosphate, 100 nM dexamethasone and 50 μg/ml indomethacin and DMEM medium containing 10% FBS, 50 mg/ml ascorbic 2-phosphate, 10 nM dexamethasone and 10 mM β-glycerol phosphate respectively. After 21 days, the cells were studied by Oil red O staining for lipid droplets, and osteoblast differentiation was evaluated by Alizarin red S for mineralized matrix.
Results
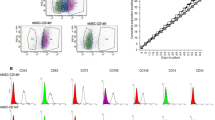
MSCs isolated from four different species showed the potential of differentiating into osteogenic and adipogenic cell lines (Fig. 1). The obtained data indicates that isolated cells of ovine and goat BM-MSCs strongly express CD166 and CD44 surface markers, whereas their ability for expression of CD90, CD105 as well as CD45 and CD34 is negative (Fig. 2a, b). In addition, Immunophenotypic analysis of surface markers in mouse BM-MSCs showed that they express CD44, CD90 and CD105, however, percent expression of CD166 is weak, and that of CD34 is negative (Fig. 3). In regard to human BM-MSCs, we found that isolated cells express CD44, CD90, CD105 and CD166, while they are negative for CD45 and CD34 expression (Fig. 4).
Multi-lineage differentiation of the isolated mesenchymal stem cells from: 1 ovine, 2 caprine, 3 mouse and 4 human. A Adipogenic control cultured in regular expansion medium with normal morphology and negative for Oil Red O staining; B Osteogenic control cultured in regular expansion medium with normal morphology and negative for Alizarin Red Staining; C Differentiated adipocytes stained with Oil Red O; D Osteocytes stained with Alizarin Red S (magnification ×200)
Discussion
The physiological characteristics such as high proliferative capacity, multi-lineage differentiation potential, as well as transdifferentiation into ectodermal and endodermal cell lineages, facile manipulation, immunomodulation and lack of forming teratoma, make MSCs an appropriate candidate for use in regenerative medicine and in vitro or in vivo study of cellular differentiation as well [12]. Although large animals such as ovine and caprine are good models for preclinical experiments (i.e., orthopedic injuries or transmissible spongiform encephalopathies), their MSCs properties are not well known [13].
The extensive possible clinical applications of MSCs have attracted detailed studies of CD cell surface markers [7, 8]. Indeed, CD90 and CD105 are two well-known specific markers of mesenchymal cell lineages, and CD34 and CD45 are known as haematopoietic markers [14]. Also, the expression of CD44 and CD166 have been demonstrated in human MSCs [15]. In this study, we compared the expression of these and other CD markers in MSCs isolated from four species (Human, mouse, ovine and caprine). We found that like human MSCs, ovine and caprine MSCs express CD44 and CD166 (Fig. 2a, b). However, unlike human MSCs, ovine and caprine MSCs express low percentage of CD90 and CD105 (Fig. 2a, b). Similarly, in the study of Lyahyai et al. [16]; CD29, CD73 and CD90 were detected, but CD105 was undetectable. Also, isolated MSCs from three different sources (bone marrow, synovial membrane and adipose tissue) expressed CD44 and MHC-I [17], while using mouse antibodies, no expression of CD73, CD90 and CD105 was seen in Ovine endometrial MSCs [18]. In a study by Grzesiak et al. [19] in which human antibodies were used, ovine adipose derived MSCs showed low expression of CD90 and CD105; isolated bone-marrow and adipose tissue MSCs from Balb/c, C3H and C5BL/6 mice were positive for CD29, CD44, CD105 and Sca-1, but negative for CD34, TER-119, CD45, and CD11b [20]; mouse BM-MSCs were positive for CD44, CD29, CD31, CD44, CD41, CD105 and SCA-1,and negative for CD45, CD3e, CD34, CD45 and CD117 [21]. Additionally, immunophenotypic analysis demonstrated that murine epiphysis derived mesenchymal stem cells were positive for CD29, CD44, CD73, CD105, CD166, Sca-1 and SSEA-4, and negative for CD31, CD34, CD11b and CD45 [22]. These findings are confirmed by our study, since mouse BM-MSCs were positive for CD105, CD90, CD44 and CD166, but they weakly expressed CD34 and CD45 (Fig. 3).
One negative CD markers in human panel is CD34; however, several studies have shown that MSCs derived from adipose tissue express CD34 at the time of isolation, but they gradually lose expression during culturing process [23]. Nonetheless, some researchers [24] doubt negative expression of CD34, because this marker can vary on the MSC tissue source.
It has been known that expression of CD90, CD105 and CD73 can vary in different species and strains [25]. CD90 or Thy1 acts in the cell to cell matrix interactions and in wound repair [26]. CD166 or ALCAM mediates intercellular adhesion in concert with hemophilic or heterothallic interactions; this CD marker is a member of the immunoglobulin super family of cell adhesion molecules that is distributed widely in tissues [27]. CD44 acts as a receptor for hyaluronic acid, and interacts with other ligand such as osteopontin, matrix metalloproteinases and collagens, fibronectin and laminin [28]. Similar to other studies [29, 30], we detected high expression of CD105, CD90, CD44 and CD166 in human MSCs, but the expression of CD34 and CD45 were negative (Fig. 4).
In conclusion, Although MSCs of different species are widely used in many different types of research studies; there are no established minimal criteria for their identification in non-human animals. In this study, we found that unlike human and mouse, isolated BM-MSCs of large animals such as ovine and caprine, have low expression of CD90 and CD105, but similar to them, they show high expression of CD44 and CD166; Also, the expression of CD34 and CD45 in BM-MSCs isolated from all four species are negative. Further studies are needed to complete the panel of CD markers for detection and characterization of isolated animal MSCs. These studies offer the potential to enhance our understanding of the entire system of CD markers, and may be used for the optimal application of MSCs of large animals in human regenerative preclinical experiments.
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- CD:
-
Cluster of differentiation
- ESCs:
-
Embryonic stem cells
- iPSCs:
-
Induced pluripotent stem cells
- BM-MSCs:
-
Bone marrow mesenchymal stem cells
- DPBS:
-
Dulbecco’s phosphate buffered saline
- FBS:
-
Fetal bovine serum
References
Fortier LA. Stem cells: classifications, controversies, and clinical applications. Vet Surg. 2005;34(5):415–23.
Blau HM, Brazelton T, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105(7):829–41.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7.
Ferrari M, Corradi A, Lazzaretti M, De’Cillà M, Losi C, Villa R, et al. Adult stem cells: perspectives for therapeutic applications. Vet Res Commun. 2007;31(1):1–8.
Pera MF, Hasegawa K. Simpler and safer cell reprogramming. Nat Biotechnol. 2008;26(1):59–60.
Heidari B, Shirazi A, Akhondi MM, Hassanpour H, Behzadi B, Naderi MM, et al. Comparison of proliferative and multilineage differentiation potential of sheep mesenchymal stem cells derived from bone marrow, liver, and adipose tissue. Avicenna J Med Biotechnol. 2013;5(2):104–17.
Matsumoto R, Omura T, Yoshiyama M, Hayashi T, Inamoto S, Koh K-R, et al. Vascular endothelial growth factor–expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc. 2005;25(6):1168–73.
McCulloch EA, Till JE. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res. 1960;13(1):115–25.
In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–45.
Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer Res. 2002;62(13):3603–8.
Lee K-D. Applications of mesenchymal stem cells: an updated review. Chang Gung Med J. 2008;31(3):228–36.
Kolf CM, Cho E, Tuan RS. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9(1):204.
McCarty RC, Xian CJ, Gronthos S, Zannettino ACW, Foster BK. Application of autologous bone marrow derived mesenchymal stem cells to an ovine model of growth plate cartilage injury. Open Orthop J. 2010;4:204–10.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–6.
Davoodian N, Lotfi AS, Soleimani M, Mowla SJ. MicroRNA-122 overexpression promotes hepatic differentiation of human adipose tissue-derived stem cells. J Cell Biochem. 2014;115(9):1582–93.
Lyahyai J, Mediano DR, Ranera B, Sanz A, Remacha AR, Bolea R, et al. Isolation and characterization of ovine mesenchymal stem cells derived from peripheral blood. BMC Vet Res. 2012;8:169.
Godoy RF, Alves ALG, Gibson AJ, Lima EM, Goodship AE. Do progenitor cells from different tissue have the same phenotype? Res Vet Sci. 2014;96(3):454–9.
Letouzey V, Tan KS, Deane JA, Ulrich D, Gurung S, Ong YR, et al. Isolation and characterisation of mesenchymal stem/stromal cells in the ovine endometrium. PLoS ONE. 2015;10(5):e0127531.
Grzesiak J, Krzysztof M, Karol W, Joanna C. Isolation and morphological characterisation of ovine adipose-derived mesenchymal stem cells in culture. Int J Stem Cells. 2011;4(2):99–104.
Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon C, et al. Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc. 2008;40(8):2649–54.
Dickinson H, Milton P, Jenkin G. The isolation and characterization of putative mesenchymal stem cells from the spiny mouse. Cytotechnology. 2012;64(5):591–9.
Cheng CC, Lian WS, Hsiao FSH, Liu IH, Lin SP, Lee YH, et al. Isolation and characterization of novel murine epiphysis derived mesenchymal stem cells. PLoS ONE. 2012;7(4):e36085.
Pachón-Peña G, Yu G, Tucker A, Wu X, Vendrell J, Bunnell B, et al. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226(3):843–51.
Lin C-S, Ning H, Lin G, Lue TF. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14(10):1159–63.
Anderson P, Carrillo-Gálvez AB, García-Pérez A, Cobo M, Martín F. CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS ONE. 2013;8(10):e76979.
Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006;20(8):1045–54.
Gilsanz A, Sánchez-Martín L, Gutiérrez-López MD, Ovalle S, Machado-Pineda Y, Reyes R, et al. ALCAM/CD166 adhesive function is regulated by the tetraspanin CD9. Cell Mol Life Sci. 2013;70(3):475–93.
Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52(4):189–96.
Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332(2):370–9.
Roche S, Delorme B, Oostendorp RA, Barbet R, Caton D, Noel D, et al. Comparative proteomic analysis of human mesenchymal and embryonic stem cells: towards the definition of a mesenchymal stem cell proteomic signature. Proteomics. 2009;9(2):223–32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghaneialvar, H., Soltani, L., Rahmani, H.R. et al. Characterization and Classification of Mesenchymal Stem Cells in Several Species Using Surface Markers for Cell Therapy Purposes. Ind J Clin Biochem 33, 46–52 (2018). https://doi.org/10.1007/s12291-017-0641-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-017-0641-x