Abstract
Background
Nanoparticle albumin-bound (nab)-paclitaxel is a solvent-free formulation of paclitaxel that is bound to albumin and has demonstrated improved progression free survival in previous studies of breast cancer. However, it is difficult to treat Japanese patients with metastatic or recurrent breast cancer with the recommended dose of 260 mg/m2 of (nab)-paclitaxel for more than six cycles due to the occurrence of adverse events. To evaluate the treatment continuity and safety of low-dose nab-paclitaxel, we conducted a phase II study of nab-paclitaxel in patients with metastatic or recurrent breast cancer who had received up to one prior chemotherapy.
Patients and methods
Treatment included low doses of 180 mg/m2 nab-paclitaxel that were administered on day 1 of each 3-week cycle to 35 patients. The primary endpoint was the completion rate of six cycles of treatment.
Results
A total of 35 eligible patients were enrolled and received a median of eight (range 2–24) cycles of low-dose nab-paclitaxel therapy. The completion rate of six cycles of treatment was 66%. ORR and clinical benefit rate was 23 and 71%, respectively. Median PFS was 6.5 months and median OS was 44 months. Adverse events were relatively mild. Commonly observed grade 3/4 adverse events were neutropenia (46%), leukopenia (9%), and hypertension (3%). No grade 3-4 peripheral sensory neuropathy occurred.
Conclusion
Treatment with a low dose of nab-paclitaxel once every 3 weeks was tolerable and of acceptable safety and might be beneficial for patients with metastatic or recurrent breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignancy worldwide; it has the highest morbidity rate and the fifth highest mortality rate for disease in Japanese women [1]. Breast cancer is sensitive to systemic chemotherapy, which has been used in an attempt to control cancer-related symptoms and prolong survival. Nanoparticle albumin-bound (nab)-paclitaxel is one of the most active anticancer drugs for the treatment of solid tumors, effectively blocking cancer cells in the G2/M phase through the inhibition of microtubular de-polymerization [2, 3]. Nab-paclitaxel is a solvent-free formulation of paclitaxel that is bound to human serum albumin. Solvent-based paclitaxel is formulated in the polyethoxylated castor oil derivative Kolliphor EL (formerly Cremophor EL) and dehydrated ethanol. These solvents have been associated with hypersensitivity reactions and peripheral neuropathy [4, 5]. An administration schedule of nab-paclitaxel at a dose of 260 mg/m2 by intravenous infusion every 3 weeks has been widely accepted in breast cancer patients with metastatic breast cancer [6, 7]. An additional merit of nab-paclitaxel is that, because it does not contain Kolliphor EL, pre-treatment with an agent such as steroid and antihistamine is unnecessary, which allows shortening of the time of administration [8].
In a phase III randomized trial (CA012 study), nab-paclitaxel (260 mg/m2) showed a significant and clinically meaningful improvement in time to tumor progression compared to the standard treatment of tri-weekly solvent-based paclitaxel (175 mg/m2) in patients with metastatic or recurrent breast cancer. However, Grade 3 sensory neuropathy was more common in the nab-paclitaxel arm than in the solvent-based paclitaxel arm (10 vs. 2%, respectively) [8].
In a phase I study of nab-paclitaxel in Japan, the number of cases of Japanese patients was extremely limited with only 12 cases. Based on these data, the recommended dose of nab-paclitaxel was set at 260 mg/m2. Careful observation of the safety of the recommended dose of 260 mg/m2 is considered to be necessary, because neutropenia of Grade 3 or more developed in eight of the 12 enrolled patients (66.7%). Peripheral sensory neuropathy, which is one of the severe adverse events of nab-paclitaxel, greatly influences the quality of life, and can become such a big problem that further treatment is refused. Thus, a 260 mg/m2 dose regimen of nab-paclitaxel is accompanied by an increase in toxicity. Its toxicity profiles are acceptable only with appropriately selected patients and comprehensive toxicity management strategies. In addition, it is necessary to confirm the efficacy of this regimen, because the only study that has been conducted in Japan is this phase I study [9].
In this regard, the development of a less toxic chemotherapy regimen using nab-paclitaxel is necessary to properly treat patients with breast cancer. To investigate the potential beneficial effects of low-dose (180 mg/m2) nab-paclitaxel treatment for patients with metastatic or recurrent breast cancer, we conducted a phase II study to assess treatment continuity and the efficacy of administration of this low dose of nab-paclitaxel once every 3 weeks. To evaluate the feasibility of low-dose nab-paclitaxel exploratory, we calculated the 6 month completion rate. Moreover, this study may provide useful information regarding the safety and the efficacy of a low dose of nab-paclitaxel that might be used in combination with other drugs in the future.
Patients and methods
The present study was conducted in accordance with the Declaration of Helsinki and was approved by the review board of each participating institution. All participants provided written informed consent prior to study entry. The study protocol was registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (protocol ID UMIN000007166). This was an investigator-initiated clinical trial that was not supported by any industry funding.
Eligibility criteria
All eligible patients had to fulfill the following eligibility criteria: Histologically-confirmed metastatic or recurrent breast cancer; over 20 years of age; Eastern Cooperative Oncology Group performance status (PS) 0–2; at least one measurable lesion according to the response evaluation criteria in solid tumors [RECIST version 1.1 [10]]; at least one prior chemotherapy for metastatic setting in patients under 64 years; regardless of prior chemotherapy in patients more than 65 years; life expectancy ≥3 months; adequate bone marrow function (hemoglobin >9 g/dl, absolute neutrophil count ≥1500/mm3 and platelet count ≥100,000/mm3); adequate liver function (total bilirubin ≤1.5 mg/dl and serum transaminase ≤2.5 × upper normal limit (UNL)); adequate renal function (serum creatinine ≤1.5 × UNL); no other severe medical conditions; no other active malignancies; no history of nab-paclitaxel; and provision of written informed consent.
Treatment schedule and evaluation of toxicity
Nab-paclitaxel (Abraxane; Taiho Pharmaceutical Company, Tokyo, Japan) was administered at a dose of 180 mg/m2 over 30 min by intravenous infusion on day 1 of every 3-week cycle (Fig. 1). Patients with HER2-positive tumors received concurrent trastuzumab at a dose of 6 mg/kg (loading dose, 8 mg/kg) on day 1 of every 3-week cycle. This treatment was repeated every 3 weeks until disease progression or unacceptable toxicity was observed. Toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE version 4.0 [11]). Nab-paclitaxel could be administered if the neutrophil count was ≥1500/mm3, the platelet count was ≥100,000/mm3 and all relevant non-hematological toxicities were grade 2 or lower. Treatment dose reductions were planned for hematological grade 3-4 or non-hematological grade 3 toxicities, in which nab-paclitaxel was to be re-introduced at a dose of 150 mg/m2. Treatment could be withheld for up to 3 weeks. If hematological adverse events did not improve to grade 1 after 3 weeks and non-hematological toxicities did not improve to grade 2 or lower, patients were excluded from the study.
Endpoints and evaluation of treatment
The primary endpoint was the completion rate of six cycles of treatment. We defined the patients who could not complete six cycles of nab-paclitaxel by all causes including progressive disease as non-completion case. Tumor response was evaluated every 12 weeks by means of computed tomography. Measurable lesions were assessed according to RECIST version 1.1 [6] and were classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease. Secondary endpoints were progression free survival (PFS), overall response rate (ORR), and incidence of adverse events. PFS was defined as the time from initiation of nab-paclitaxel to disease progression or death from any cause. Intention-to-treatment (ITT) analysis was used to evaluate patients.
Statistical analysis
In the phase III CA012 trial, the completion rate of six cycle treatment was 55.9%.
We hypothesized that our regimen would be effective if the completion rate of six cycle treatment reached 50%. Assuming that the completion rate of six cycle treatment is 50% and the 90% Confidence interval (90% CI) range is 15%, the estimated sample size for the present trial was 30 cases and the total sample size was 35 patients allowing for a follow-up loss rate of 10%. PFS was estimated using the Kaplan–Meier method. Data were analyzed statistically using the IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., NY, USA).
Results
Patient characteristics
Thirty-five patients were enrolled in the trial between January 2012 and March 2015. The major clinicopathological characteristics of the patients are listed in Table 1. The median age was 59 years (range 44–73 years) and all cases were female. All patients had a good performance status (ECOG 0). Four patients had received no prior chemotherapy and 20 had received one prior course of chemotherapy. All had measurable disease according to the RECIST criteria (visceral metastasis in 22 and others in 13), and all were determined to be eligible for the present study. Of these 35 patients, 37% had HER-2 positive tumors and 57% had hormone-receptor positive tumors.
Anti-tumor activity
In the 35 eligible patients, 23 patients completed the six cycle treatment, so the completion rate of six cycles of treatment was 66% [90% CI was 53–79%], and 90% CI range was 13%, respectively. The reasons of the study treatment less than six cycles (12 patients) were nine progressive disease, one adverse event, one other disease, and one patient choice.
According to ITT analysis, RR was 23% (8/35), including CR in one and PR in seven patients. Seventeen patients showed SD, and disease in 10 patients progressed. The clinical benefit rate disease [CR + PR + SD (>6mo)] was 51% (18/35) (Table 2). The response rate by the subtype was 15% (2/13), 29% (2/7), 17% (1/6), 25% (2/8) in HR+/HER2− type, HR+/HER2+ type, HR−/HER2+ type, and HR−/HER2− type, respectively.
The median PFS was 6.5 months (95% CI 4.3–8.7 months) (Fig. 2) and the mean PFS was 9.5 months (95% CI 6.6–12.3 months). The median OS was 44.7 months (95% CI 34.8–54.6 months) and the mean OS was 36.4 months (95% CI 29.9–42.8 months) (Fig. 3). The median follow-up period was 20.8 months (range 4.4–52.3 months). Information from the off-treatment forms at the failure of this regimen indicated that 8 out of 31 patients (26%) received eribulin regimens, and seven out of 31 patients (23%) received bevacizumab plus paclitaxel regimens (Table 3).
Dose reduction of nab-paclitaxel 150 mg/m2 was not needed in all patients.
Toxicity
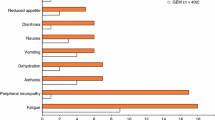
The median number of treatment cycles was eight (range 2–24). All patients were evaluable for toxicity (Table 4). No toxic deaths were observed. Hematological toxicity was mainly represented by neutropenia that was recorded in 26 patients (74%); grade 3-4 neutropenia was recorded in 16 cases (46%). Grade 3-4 leukopenia was observed in 9% of patients. No patient experienced febrile neutropenia. Anemia was observed in 23 patients. Non-hematological toxicity was mainly represented by peripheral sensory neuropathy that was recorded in 32 patients (91%); however, grade 3-4 peripheral sensory neuropathy was not reported. We did not use antidepressant medications, such as amitriptyline and nortriptyline, may help relieve the pain of peripheral neuropathy. Nineteen patients (54%) experienced fatigue. Eleven patients experienced loss of appetite. Only one patient experienced grade 3 hypertension. All patients received chemotherapy in an outpatient clinic.
Discussion
In this study, we showed that a low dose of nab-paclitaxel (180 mg/m2 q3 W) chemotherapy that was administered once every 3 weeks might be a promising regimen with good tolerability for patients with metastatic or recurrent breast cancer. The majority of adverse events were mild in severity. In particular, no patient had grade 3-4 peripheral sensory neuropathy. Several clinical trials of nab-paclitaxel (260 mg/m2) have been carried out in patients with breast cancer in a neoadjuvant and metastatic setting, which resulted in adverse events, in particular the occurrence of grade 3-4 peripheral sensory neuropathy [8, 12,13,14,15,16]. Surprisingly, in a phase II neoadjuvant setting of nab-paclitaxel regimens (260 mg/m2 q3 W, four cycles), the rate of grade 3-4 peripheral sensory neuropathy was 84% [15]. An investigation of treatment of Japanese patients with breast cancer with doses of 260 mg/m2 nab-paclitaxel was conducted in accordance with the conditions for approval of the use of this drug. In that study, a total of 934 Japanese patients were evaluated to determine the safety of nab-paclitaxel. Major adverse drug reactions included myelosuppression and peripheral sensory neuropathy, which were primary causes for discontinuation. The rate of completion of six cycles of treatment was very low (37%, 349 out of 934 patients). The cancellation percentage for every course was 12.8–30.2%, and the adverse event that became the main cancellation reason was peripheral sensory neuropathy, muscle ache, arthralgia, leukopenia or neutropenia. Most of the adverse events that become the cancellation reason after four courses were peripheral sensory nerve disorders [16]. Based on retrospective results of nab-paclitaxel treatment of elderly people (>65 years), there was a much higher development of Grade3 peripheral sensory neuropathy in nab-paclitaxel (260 mg/m2 q3 W) as compared with conventional paclitaxel (175 mg/m2 q3 W) treatment. Thus, in nab-paclitaxel administration, it is also necessary to consider the age of patients without a history of chemotherapy treatment [17]. For Japanese patients, treatment of nab-paclitaxel 260 mg/m 2 might be intolerable, so we tried to confirm the feasibility of low-dose nab-paclitaxel. A 6-month completion rate was set as the primary endpoint to demonstrate that the low-dose nab-paclitaxel is similar in the feasibility of global Phase III trial. In the global Phase III trial, the 6-month completion rate of nab-paclitaxel was 55.9%. In our study, the completion rate for 6 months is 66%, and this result demonstrates good tolerability of low-dose nab-paclitaxel chemotherapy.
Recently, in a phase III neoadjuvant setting compared with solvent-based paclitaxel, nab-paclitaxel was administered weekly for three consecutive weeks followed by 1 week of rest (150 mg/m2 qW, 3/4 cycles). However, grade 3/4 peripheral sensory neuropathy occurred in 15% of the patients treated with the starting dose of 150 mg/m2 nab-paclitaxel. The median time to resolve grade 3/4 peripheral sensory neuropathy to grade 1 was 17.0 weeks. In addition, the taxane dose had to be reduced in 30% of the patients in the nab-paclitaxel group [18]. In a CA002-0LD study (this study only reported in abstract form and never published) that examined the effect of administration of low doses of nab-paclitaxel (175 mg/m2 q3 W) every 3 weeks, grade 3-4 peripheral sensory neuropathy was not reported [19]. In a phase III trial (CA012) of patients with metastatic breast cancer, nab-paclitaxel (260 mg/m2 q3 W) demonstrated a superior anti-tumor effect compared with the standard (solvent-based) paclitaxel (175 mg/m2 q3 W). The ORR was 33% and median TTP was 23.0 weeks in the nab-paclitaxel arm [8]. In a CA002-0LD study that examined treatment with a low dose of nab-paclitaxel (175 mg/m2 q3 W) every 3 weeks, ORR was 39.5%. Median TTP and OS were 23.3 and 59.6 weeks, respectively. In other words, the study showed that even if the doses of nab-paclitaxel were reduced, it might still be possible to maintain efficacy [19]. Many investigators have reported that the ORR ranged from 33 to 54%, the median PFS or TTP ranged from 5.3 to 7.6 months, and the median OS ranged from 15.0 to 17.8 months in patients with metastatic breast cancer which were treated with 260 mg/m2 nab-paclitaxel [8, 20]. The ORR in our study was comparable to those obtained in previous studies, and the PFS and OS in our study were equivalent or longer than those reported in these previous studies. Furthermore, nab-paclitaxel is expected in triple negative breast cancer. In the present study, low doses of nab-paclitaxel showed high response rate of 25%.
Low doses of nab-paclitaxel (180 mg/m2 q3 W) had very tolerable toxicities, as well as active anti-tumor effects. Mild neurotoxicity could be attributed to the low dosage of nab-paclitaxel. The final aim of cancer chemotherapy is to prolong the survival of patients, with good QOL. In particular, non-hematological toxicities substantially reduce patient QOL. Our study showed a low incidence of severe non-hematological toxicities, such as Grade 3-4 peripheral sensory neuropathy, which may have contributed to enhancement of the OS without reducing QOL. In the analysis from health claims date in United States, weekly administration of nab-paclitaxel is selected more frequently than tri-weekly administration. [21]. However, weekly administration is high frequency and non-convenience for outpatients. Tri-weekly administration of nab-paclitaxel is convenience, and decreases the outpatient frequency. Adverse events can be mitigated as much as possible using nab-paclitaxel at a low dose and thus continued administration of nab-paclitaxel is possible. Ongoing trials will reveal the optimal dose of the low doses of nab-paclitaxel, which are used for unselected Japanese patient populations [22].
Conclusions
In conclusion, treatment with a low dose of nab-paclitaxel once every 3 weeks is tolerable and of acceptable safety. Intense regimens of nab-paclitaxel may not be the best treatment approach for unselected patients for personalization. Tri-weekly administration of nab-paclitaxel might be beneficial for patients with metastatic or recurrent breast cancer.
References
Center for Cancer Control and Information Services, National Cancer Center, Japan. http://ganjoho.jp/public/index.html. Accesed 14 April 2017.
Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–7.
Jordan MA, Wendell K, Gardiner S, et al. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56:816–25.
Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44.
Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–53.
Ibrahim NK, Samuels B, Page R, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23:6019–26.
Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–9.
Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803.
Yamada K, Yamamoto N, Yamada Y, et al. Phase I and pharmacokinetic study of ABI-007, albumin-bound paclitaxel, administered every 3 weeks in Japanese patients with solid tumors. Jpn J Clin Oncol. 2010;40:404–11.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
NCI, NIH, DHHS. National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. NIH publication# 09-7473. May 29, 2009. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (Accessed Nov 4, 2015).
McArthur HL, Rugo H, Nulsen B, et al. A feasibility study of bevacizumab plus dose-dense doxorubicin-cyclophosphamide (AC) followed by nanoparticle albumin-bound paclitaxel in early-stage breast cancer. Clin Cancer Res. 2011;17:3398–407.
Pippen J, Paul D, Vukelja S, et al. Dose-dense doxorubicin and cyclophosphamide followed by dose-dense albumin-bound paclitaxel plus bevacizumab is safe as adjuvant therapy in patients with early stage breast cancer. Breast Cancer Res Treat. 2011;130:825–31.
Zelnak AB, Nikolinakos P, Srinivasiah J, et al. Georgia Center for Oncology Research and Education. High pathologic complete response in Her2-positive, early-stage breast cancer to a novel nonanthracycline neoadjuvant chemotherapy. Clin Breast Cancer. 2015;15:31–6.
Tanaka S, Iwamoto M, Kimura K, et al. Phase II study of neoadjuvant anthracycline-based regimens combined with nanoparticle albumin-bound paclitaxel and trastuzumab for human epidermal growth factor receptor 2-positive operable breast cancer. Clin Breast Cancer. 2015;15:191–6.
Nakamura S, Iwata H, Funato Y, et al. Results of a drug use investigation of nanoparticle albumin-bound Paclitaxel for breast cancer. Gan To Kagaku Ryoho. 2015;42:447–55 (in Japanese).
Aapro M, Tjulandin S, Bhar P, et al. Weekly nab-paclitaxel is safe and effective in ≥65 years old patients with metastatic breast cancer: a post hoc analysis. Breast. 2011;20:468–74.
Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17:345–56.
Nuhad K, Brian S, Ray P et al. Nanoparticle paclitaxel (ABI-007) in metastatic breast cancer (MBC): efficacy and evidence of dose-dependent activity in two multicenter phase II studies. Proc Am Soc Clin Oncol 2002;21:53a (abstr 209)
Guan Z, Li QL, Feng F, et al. Superior efficacy of Cremophor-free albumin-bound paclitaxel compared with solvent-based paclitaxel in Chinese patients with metastatic breast cancer. Asia Pac J clin Oncol. 2009;5:165–74.
Liang C, Li L, Fraser CD, et al. The treatment patterns, efficacy, and safety of nab-paclitaxel for the treatment of metastatic breast cancer in the United States: results from health insurance claims analysis. BMC Cancer. 2015;15:1019–30.
UMIN000015516. Randomized, optimal dose finding, Phase II Study of triweekly Abraxane in patients with metastatic breast cancer. Available at:https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000017916&language=J. Accesed 14 April 2017.
Acknowledgements
We gratefully acknowledge the participating patients, their families, and the study investigators for their invaluable contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Yamamoto, S., Maeda, N., Nagashima, Y. et al. A phase II, multicenter, single-arm study of tri-weekly low-dose nanoparticle albumin-bound paclitaxel chemotherapy for patients with metastatic or recurrent breast cancer. Breast Cancer 24, 783–789 (2017). https://doi.org/10.1007/s12282-017-0779-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-017-0779-7