Abstract
Triple-negative breast cancer (TNBC) is defined as a group of breast carcinomas that are negative for expression of hormone receptors and HER2. Although patients with TNBC tend to have a poor prognosis, only chemotherapy is expected to be effective because no therapeutic targets have yet been established. DNA microarray analyses have proved that TNBCs are composed of the basal-like subtype and normal breast (or unclassified) subtype, the former being correlated with an aggressive clinical course. Histological types of TNBCs are reported to be common with those of basal-like subtype, comprising high-grade invasive ductal carcinoma, no special type [solid-tubular carcinoma (or atypical medullary carcinoma), invasive ductal carcinoma with a large central acellular zone], typical medullary carcinoma, and metaplastic carcinomas. The basal-like subtype is characterized by the expression of myoepithelial/basal markers and molecular changes including TP53 gene mutations, BRCA1 inactivation, and many chromosomal alterations. New target molecules for the treatment of TNBCs are under extensive investigation, and their clinical application is awaited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Definition of triple-negative breast cancer
Primary breast cancers have been classified into subgroups according to the extent of tumor spread (pT, pN, clinical stage), histological types, and histological/nuclear grades for the purpose of risk estimation and treatment decision. In parallel with recent advances in drug therapies and in their individualization, the classification of breast cancers has come to be made in terms of the expression status of target molecules. Triple-negative breast cancers (TNBCs) have been defined as a subgroup of breast cancers that are negative for all estrogen receptors (ER), progesterone receptors (PgR), and HER2. In the 2007 St. Gallen consensus meeting for making a decision about adjuvant therapies (chemotherapy, endocrine therapy, and trastuzumab), operable primary breast cancers were recommended to be categorized based on the status of ER, PgR, and HER2 [1].
By using DNA microarray techniques, it has been shown that breast cancers can be classified into biologically distinct groups based on their gene expression profiles. These groups comprise luminal A (ER-positive and HER2-negative), luminal B (ER- and HER2-positive), ERBB2 (ER-negative and HER2-positive), and triple-negative (ER- and HER2-negative) subtypes [2–5]. The TNBC is a heterogeneous group and is further categorized into the basal-like and the normal breast subtypes, which are positive and negative, respectively, for myoepithelial/basal markers such as basal cytokeratins (CKs) (i.e., CK5/6, CK14, and CK17), α-smooth muscle actin (SMA), and epidermal growth factor receptor (EGFR).
For the identification of a TNBC, the threshold of positivity for ER, PgR, and HER2 may differ among institutions. In Japan, both ER and PgR are assessed by the system (J score) advocated by the Japanese Breast Cancer Society or by the Allred score [6–8]. HER2 positivity has been considered present when either strong membrane staining (3+) is evident in >10% of the carcinoma cells by immunohistochemistry or when gene amplification is demonstrated [the ratio of the number of copies of the HER2 gene to the number of copies of chromosome 17 centromere (CEP17) is larger than 2.0 on average in 20 cancer cell nuclei] by fluorescence in situ hybridization (FISH) [9]. It may be appropriate to consider TNBC when the J score is 0 and 1 (percentage of immunopositive cancer cells <1%) or the Allred score is 0 or 2 for both ER and PgR, and the HER2 score is 0 or 1+, or when the HER2 score is 2+ without HER2 gene amplification.
Although TNBCs account for only 10–17% of all breast carcinomas, this subgroup is regarded as important clinically because of the aggressive clinical behavior, poorer patient prognosis, and lack of an established therapeutic target.
Clinicopathological characteristics
TNBCs were characterized by younger patient age, prevalence in African-American women, and clinical aggressiveness with the majority of deaths occurring within the first 5 years following therapy [10]. On the other hand, the rates of late recurrences and deaths became reduced. In node-negative T1 patients, the disease-specific survival rate of patients with TNBC was lower than for those with ER-positive breast cancer but higher than for those with ER-negative, HER2-positive breast cancer [11].
In the clinical studies to examine the efficacy of taxane-based adjuvant chemotherapy to node-positive breast cancers, taxanes (paclitaxel and docetaxel) were shown to significantly improve the prognosis of patients with TNBC [12, 13]. Liedtke et al. compared response to neoadjuvant therapy and long-term survival in 255 patients with stage I–III TNBC and 863 patients with stage I–III non-TNBC [14]. They concluded that TNBC compared with non-TNBC had significantly higher rates of pathologic complete response (pCR) (22% vs. 11%, p = 0.034). Three-year disease-free survival rates (63% vs. 76%) and overall survival rates (74% vs. 89%) were also significantly lower in patients with TNBC than in those with non-TNBC. Patients with TNBC with residual disease had significantly decreased overall survival compared with those with non-TNBC with residual disease. No such significance was observed among patients who achieved pCR. TNBCs were also correlated with visceral metastases, lower incidence of bone metastasis, and shorter post-recurrence survival [14].
In 92 patients who had received neoadjuvant chemotherapy against invasive TNBC in the National Cancer Center Hospital (NCCH), pCR and near pCR (grade 2b) were acquired in 29 (32%) and 6 (6.5%) patients, respectively, and these rates in TNBCs were higher than those in non-TNBCs [15].
The ratio of basal-like subtype in TNBC was estimated to be up to 56–84% [10, 16]. Therefore, characteristic histopathological features of TNBCs are similar with those of the basal-like subtype. Characteristic histopathological types that constitute TNBCs and basal-like subtype are high-grade invasive ductal carcinoma of no special type, typical medullary carcinoma, metaplastic carcinomas, and adenoid cystic carcinoma (Fig. 1).
Characteristic microscopic features of triple-negative breast cancers or basal-like subtype. a High-grade invasive ductal carcinoma, no special type, with a large central acellular zone. The central acellular zone appears to arise from extensive tissue infarction. b High-grade invasive ductal carcinoma, no special type (solid-tubular carcinoma or atypical medullary carcinoma), which is similar to typical medullary carcinoma but does not completely meet its criteria. c Matrix-producing carcinoma, a component of metaplastic carcinomas, which is composed of overt invasive carcinoma and a cartilaginous/osseous matrix with absence of an intervening zone of spindle cells or an osteoclast component between the carcinoma and the matrix. d Spindle-cell carcinoma, another component of metaplastic carcinomas, which is composed of both spindle cells mimicking mesenchymal cells and poorly differentiated carcinoma cells. These carcinomas are high-grade, lack tubule formation, and mostly have a scant stromal content and a pushing border of invasion
Among the high-grade invasive ductal carcinomas, a large part of cases is made up of high-grade solid-tubular carcinoma (also called as atypical medullary carcinoma). Typical medullary carcinoma is defined as a well-circumscribed carcinoma composed of poorly differentiated cells arranged in large sheets (syncytial architecture) with scant stroma, with no glandular structures, and with a prominent lymphoplasmacytic infiltrate, and with a pushing border of invasion. Central geographic or comedo-type necrosis may also be seen [17, 18]. Tumors showing the association of a predominantly syncytial architecture with only two or three of the other above-mentioned criteria are designated as atypical medullary carcinoma [17, 18]. High-grade invasive ductal carcinoma with a large central acellular zone is also found relatively frequently in TNBCs [19, 20].
Metaplastic carcinomas are known to be a rare but characteristic subgroup of TNBC and basal-like subtype. They are aggressive, chemoresistant tumors characterized by concurrence of a high-grade carcinoma component (poorly differentiated ductal carcinoma) and extensive metaplastic component comprising squamous and/or mesenchymal (spindle-cell, cartilaginous, and/or osseous) metaplasia. In the classification by the Japanese Breast Cancer Society, they are classified into squamous cell carcinoma, spindle cell carcinoma, carcinoma with cartilaginous and/or osseous metaplasia, and matrix-producing carcinoma [21].
In the 92 patients who received neoadjuvant chemotherapy against TNBCs in the NCCH, 85 had invasive ductal carcinoma of no special type (48 solid-tubular carcinomas, 35 scirrhous carcinomas, and 2 papillotubular carcinomas), 4 had metaplastic carcinomas (spindle cell carcinoma, squamous cell carcinoma, matrix-producing carcinoma, and carcinoma with cartilaginous and/or osseous metaplasia), 2 had invasive lobular carcinomas, and 1 had mucinous carcinoma [15]. There was no case of typical medullary carcinoma or adenoid cystic carcinoma. Eighty-three tumors were nuclear grade 3, 8 were grade 2, and 1 was grade 1. In these 85 invasive ductal carcinomas, atypical medullary features, central necrosis, and central acellularity were common [15].
Immunohistochemical and molecular features
Luminal epithelial cells in the lactiferous ducts are thought to differentiate from putative terminal-end-bud stem cells. These stem cells differentiate into early transit cells and subsequently to later transit cells in the terminal duct lobular unit, and thereafter to the luminal epithelial cells or myoepithelial cells in an alternating manner [22]. Because myoepithelial cells are located in the basal layer, they are also called basal cells. Immunohistochemically, the luminal epithelial cells are positive for CK8/18, CK19, epithelial membrane antigen (EMA), and ER. On the other hand, the myoepithelial/basal cells are positive for CK 14, CK5/6, CK17, α-SMA, S100, p63, CD10, and EGFR.
Most of the breast cancers of luminal subtype and ERBB2 subtype are believed to arise from the luminal epithelial cells in the lactiferous duct. In contrast, basal-like subtypes, including basal-like invasive ductal carcinoma, no special type, metaplastic carcinomas, typical medullary carcinoma, and adenoid cystic carcinoma, were shown to have immunophenotypes of both luminal epithelial and myoepithelial/basal cells [17]. Therefore, these carcinoma types are considered to arise from early transit cells or stem cells that retain their potential for bidirectional differentiation.
Recently, the metaplastic carcinoma was shown to display mRNA profiles different from those of other components of the basal-like subtypes [23]. The expression profiles of metaplastic carcinomas were, similarly to a recently identified claudin-low breast cancer subset, characterized by low expression of GATA3-regulated genes and of genes responsible for cell–cell adhesion with enrichment for markers linked to stem cell function and epithelial-to-mesenchymal transition. In that study, the authors concluded that metaplastic carcinomas had a tumorigenic signature defined using CD44+/CD24− breast tumor-initiating stem cell-like cells [23].
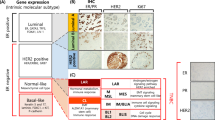
Because DNA microarray technology is expensive and cannot be used routinely, a number of immunohistochemical studies of the TNBCs have been performed. In fact, the consensus criteria for the basal-like subtype have not yet been established. Nielsen et al. suggested four representative surrogate markers for the basal-like subtype: ER, HER2, EGFR, and CK5/6 [4]. They reported that the sensitivity and specificity of the combination of ER negativity, HER2 negativity, and EGFR and/or CK5/6 positivity were 76 and 100%, respectively [4]. Other additional criteria used for the basal-like subtype comprise (1) ER negativity and HER2 negativity, and vimentin, EGFR, CK8/18, and/or CK5/6 positivity, and (2) triple negativity, and CK5/6 and/or EGFR positivity. Other markers that have been included in the panel of myoepithelial/basal biomarkers are laminin [24, 25], c-KIT [26], p63 [27], nestin [28], osteonectin [29], caveolin 1 [30], and NGFR [31] (Fig. 2). However, there are transitional components that are ER-positive or HER2-positive in breast cancers expressing CK5/6, EGFR, and vimentin. These entities complicate the immunohistochemical definition of the basal-like subtype.
The basal-like subtype is associated with aggressive behavior, poor patient prognosis, and a specific pattern of distant metastasis with an increased propensity for visceral metastases to the brain and lung and less likelihood of metastasizing to bone and liver [17]. As mentioned above, the basal-like subtype has common histologic features with TNBC, comprising a high histological tumor grade, marked cellular pleomorphism, lack of tubule formation, frequent lymphocyte infiltration, scant stromal content, a pushing border of invasion, central geographic or comedo-type necrosis, and central acellularity [17].
There are many studies on genetic alterations in TNBCs, especially in basal-like subtypes. Some reports have shown that p53 mutations and inactivation of the Rb pathway occur in the basal-like subtype [5, 32]. Calza et al. have reported that p53 gene mutations occur in 65% of basal-like subtypes, compared with only 3.7% of the normal breast subtype [33]. Another study has reported that either p53 gene mutation or diffuse nuclear p53 immunoreaction is detectable in 85% of basal-like subtypes [10]. The correlations among TNBCs, a basal-like subtype, and p53 alterations are presented schematically in Fig. 3.
Schematic representation of relationships among triple-negative breast cancer (TNBC), basal-like subtype, and p53 gene mutation. TNBC is composed of the basal-like subtype and normal breast (or unclassified) subtype. Of the basal-like components, 56–84% are included among the TNBCs. Breast cancers harboring p53 mutation account for 28% of all TNBCs, while breast cancers harboring p53 mutation or showing immunohistochemical positivity for p53 account for 85% of all basal-like breast cancers. BC, basal-like; p53, p53 gene mutation or p53 protein accumulation in nuclei; TN triple-negative
DNA microarray analysis has shown that breast carcinomas occurring in patients with germ-line BRCA1 gene mutations tend to fall into the basal-like category. In fact, BRCA1-mutation-related breast cancers and basal-like subtypes have similar clinical and pathological characteristics, and are suggested to share similar carcinogenetic pathways. Regardless of the presence of BRCA1 mutations, there is lower BRCA1 mRNA expression in sporadic cases of the basal-like subtype. It is believed that this may be the result of epigenetic mechanisms such as acquired DNA hypermethylation of the BRCA1 gene promoter or dysfunction of the upstream pathways that regulate BRCA1 expression. DNA methylation of the BRCA1 promoter has been found frequently in typical medullary carcinoma and metaplastic carcinomas, while other mechanisms have been considered to account for BRCA downregulation in the basal-like subtype of invasive ductal carcinoma, no special type [16, 17, 34].
Future prospects
Although taxanes were shown to be effective for TNBCs in clinical trials [12, 13], there are a number of cases resistant to taxanes. It has been suggested that BRCA1 downregulation gives rise to the defects in DNA repair, especially in homologous recombination, and BRCA1 dysfunction confers enhanced sensitivity to certain cross-linking agents, such as platinum salts-based chemotherapeutic agents. There are a number of clinical trials testing the efficacy of platinum salts for the management of TNBCs [10]. A neoadjuvant trial of a single cisplatinum agent for 28 women with TNBC showed a pCR rate of 22% [35]. The poly ADP-ribose polymerase (PARP) directly binds to damaged DNA and is involved in DNA-base excision repair. It has been shown that BRCA2-deficient cells are extremely sensitive to PARP inhibition [36]. Phase I and phase II trials to examine the safety and efficacy of a PARP inhibitor are ongoing for ovarian and breast cancers occurring in BRCA carriers [37]. It was suggested that the EGFR inhibitors or KIT inhibitors might be effective in cases of basal-like breast cancer because of frequent overexpression of EGFR and KIT. However, molecular-targeted therapies to the EGFR or KIT were not very effective.
In the analysis of alterations in chromosome copies using array-comparative genomic hybridization (CGH), Adelaide et al. compared genomic alterations between luminal A and basal-like subtypes, confirming that frequent copy number alterations (>20% of cases) in luminal subtypes were gains of 1q, 8p12-pter, 8q21-qter, 14q13–q14, 14q11, 12p13, and 17q23, and losses of 1p36-pter, 2qter, 3p14, 4q34–q35, 6q, 8p21-pter, 9qter, 16q, 17p13-pter, 19pter, and 22q [38]. On the other hand, frequent copy number alterations in basal-like subtypes were gains of 1q, 3q24–q27, 8p11–p12, 8q21-qter, 10p, 12p13-pter, and 17q25-qter and losses of 4q13–q14, 4q34–q35, 5q, 8p21-pter, and 14q21 [38].
Among the basal-like subtype, two distinct variations, i.e., medullary carcinoma and ductal grade 3 carcinoma, show different profiles of chromosomal copy number alterations. Gains of chromosomal regions 4q34 and 12p13 and loss of 4q32–q33, 11q13, and 15q11 were associated with medullary carcinomas, whereas gains of 1q23–q24, 1q42, 5p13, 5q35, 8p23, 15q13, and 16p12 correlated with ductal carcinoma grade 3. The differences confirm that the basal-like subtype comprises a heterogeneous set of poorly differentiated carcinomas [38]. They discovered several proto-oncogenes on the chromosome loci that showed a distinct increase in copy number.
Chin et al. also studied genomic aberrations linked to the intrinsic phenotype of breast cancers using array-CGH technology [39]. Although high-level amplification at any locus was infrequent in the basal-like tumors, these tumors were relatively enriched for low-level copy number gains involving 3q, 8q, and 10p, and losses involving 3p, 4p, 4q, 5q, 12q, 13q, 14q, and 15q [39]. From these analyses new molecular targets are expected to be identified.
Several problems with TNBCs remain to be solved. With regard to the basal-like subtype, which constitutes a majority of TNBCs, consensus on the standard criteria has not established for staining and scoring of basal markers using immunohistochemistry. Furthermore, it now has been shown that the basal-like subtype comprises heterogeneous groups. At present, >30% of TNBCs are sensitive to standard anthracyclin-taxane-based regimens, but it is impossible to predict efficiently the chemosensitive TNBCs histopathologically or by specific biomarkers. For the identification of new targets of molecular therapies and of chemosensitive TNBCs, further research is necessary.
References
Goldhirsch A, Wood W, Celber R, Coats A, Thurlimann B, Senn HJ. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–44.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jefferey SS, Rees CA, et al. Molecular portraits of human breast tumors. Nature. 2000;406:747–52.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74.
Sorlie T, Tibshirani R, Parker J, Hastit T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23.
Umemura S, Kurosumi M, Moriya T, Oyama T, Arihiro K, Yamashita H, et al. Immunohistochemical evaluation for hormone receptors in breast cancer: a practically useful evaluation system and handling protocol. Breast Cancer. 2006;13:232–5.
Allred DC, Harvey JM, Berardo M, Clarl GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68.
Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81.
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45.
Reis-Filho JS, Tutt ANJ. Triple negative tumors: a critical review. Histopathology. 2008;52:108–18.
Chia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26:5697–704.
Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–506.
Hugh J, Hanson J, Cheang MC, Nielsen TO, Perou CM, Dumontet C, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–76.
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81.
Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Kouno T, et al. Evaluation of tumor-infiltrating lymphocytes (TIL) and tumor cell apoptosis as predictive markers for response to neoadjuvant chemotherapy in triple-negative breast cancer. Proc Am Soc Clin Oncol. 2008;45(Part I):559
Schneider BP, Winer EP, Foulkes WD, Garber J, Perou CM, Richardson A, et al. Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14:8010–8.
Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–81.
Ellis IO, Cornelisse CJ, Schnitt SJ, Sasco AJ, Sastre-Garau X, Kaaks R. Invasive breast carcinoma. In: Tavassoli FA, Devilee P, et al., editors. World health organization classification of tumours. Pathology & genetics. Tumours of the breast and female genital organs. Lyon: IARC Press; 2003. p. 13–59.
Tsuda H, Takarabe T, Hasegawa T, Murata T, Hirohashi S. Myoepithelial differentiation in high-grade invasive ductal carcinomas with large central acellular zones. Hum Pathol. 1999;30:1134–9.
Tsuda H, Takarabe T, Hasegawa F, Fukutomi T, Hirohashi S. Large, central acellular zones indicating myoepithelial tumor differentiation in high-grade invasive ductal carcinomas as markers of predisposition to lung and brain metastases. Am J Surg Pathol. 2000;24:197–202.
The Japanese Breast Cancer Society. General rules for clinical and pathological recording of breast cancer. 16th ed. Tokyo: Kanehara Shuppan; 2008.
Smalley M, Ashworth A. Stem cells and breast cancer: a field in transit. Nat Rev Cancer. 2003;3:832–44.
Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–24.
Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–71.
Rodriguez-Pinilla SM, Sarrio D, Honrado E, Moreno-Bueno G, Hardisson D, Calero F, et al. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. J Clin Pathol. 2007;60:1006–12.
Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and HER-2/neu-overexpressing phenotypes. Hum Pathol. 2006;37:1217–26.
Laakso M, Loman N, Borg A, Isola J. Cytokeratin 5/14-positive breast cancer: true basal phenotype confined to BRCA1 tumors. Mod Pathol. 2005;18:1321–8.
Li H, Cherukuri P, Li N, Cowling V, Spinella M, Cole M, et al. Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res. 2007;67:501–10.
Lakhani SR, Reis-Filho JS, Fulford L, Penault-Llorca F, van der Vijver M, Parry S, et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2006;11:5175–80.
Savage K, Lambros MB, Robertson D, Jones RL, Jones C, Mackay A, et al. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin Cancer Res. 2007;13:90–101.
Reis-Filho JS, Steele D, Di Palma S, Jones RL, Savage K, James M, et al. Distribution and significance of nerve growth factor receptor (NGFR/p75NTR) in normal, benign and malignant breast tissue. Mod Pathol. 2006;19:307–19.
Herschkowitz J, He X, Fan C, Perou C. The functional loss of the retinoblastoma tumor suppressor is a common event in basal-like and luminal B breast cancers. Breast Cancer Res. 2008;105:R75.
Calza S, Hall P, Auer G, Bjohle J, Klaar S, Kronenwett U, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34.
Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–32.
Garber J, Richardson A, Harris L, Miron A, Silver D, Golshan M, et al. Neoadjuvant cisplatin (CDDP) in triple-negative breast cancer (BC). Breast Cancer Res Treat. 2006;100:S149. Abstract 3074.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7.
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:189–91.
Adelaide J, Finetti P, Bekhouche I, Repellini L, Geneiy J, Sircoulomb F, et al. Integrated profiling of basal and luminal breast cancers. Cancer Res. 2007;67:11565–75.
Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–41.
Acknowledgments
This work was supported in part by a grant-in-aid for cancer research from the Ministry of Health, Labor, and Welfare, Japan, and a research grant from the Princess Takamatsu Cancer Research Fund.
Conflict of interest statement
The authors indicate no conflict of interest in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is based on a presentation delivered at Symposium 3, “Triple-negative breast cancer,” held on 27 September 2008 at the 16th Annual Meeting of the Japanese Breast Cancer Society in Osaka.
About this article
Cite this article
Sasaki, Y., Tsuda, H. Clinicopathological characteristics of triple-negative breast cancers. Breast Cancer 16, 254–259 (2009). https://doi.org/10.1007/s12282-009-0153-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-009-0153-5