Abstract
Purpose of Review
Fungal extracellular vesicles (EVs) were first characterized a decade ago. Knowledge in this field expanded rapidly and although several questions remain unanswered, it is now clear that EVs play fundamental biological roles in fungi. In this manuscript, we update the reader with the most recent information on the participation of EVs in fungal physiology and pathogenesis.
Recent Findings
Fungal EVs were initially associated with the transport of macromolecules across the cell wall and extracellular delivery of virulence factors. However, recent findings indicate that they might also participate in prion transmission, response to nutrient availability, RNA export, morphological transition, and stimulation of different phagocytes.
Summary
The recent literature on fungal EVs reinforces the perception that these compartments are involved in intercellular communication and may represent interesting targets for antifungal development and tools for the generation of fungal vaccines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular vesicles (EVs) are membranous structures that participate in the release of hundreds of molecules to the cellular outer space [1]. EV release is ubiquitous in nature, and all organisms use this mechanism for exporting molecules of very different chemical nature [1,2,3,4,5]. Physical chemical and biological properties of EVs and their mechanisms of biogenesis in both eukaryotes and prokaryotes have been extensively reviewed [1, 6,7,8]. EVs play fundamental roles in a number of pathophysiological conditions, including cancer and microbial infections [6, 9,10,11].
Cell wall-containing organisms, including plantae, fungi, and bacteria, produce EVs that traverse polysaccharide or peptidoglycan matrices to reach the extracellular space [7, 12]. Fungi were the first cell wall-containing eukaryotes demonstrated to produce EVs, which were in fact suggested to represent a eukaryotic solution to the problem of trans-cell wall passage [13]. Initial studies with the yeast-like pathogen Cryptococcus neoformans were rapidly followed by research on other fungal species and Gram-positive bacteria [4, 5, 14]. In fungi, after the characterization of the external membrane structures of C. neoformans as EVs, similar compartments were identified in Histoplasma capsulatum [15], Candida parapsilosis [15], Sporothrix schenckii [15], Saccharomyces cerevisiae [16], Malassezia sympodialis [17], Paracoccidioides brasiliensis [18,19,20], C. albicans [21,22,23], Alternaria infectoria [24], and Pichia fermentans [25•]. It is now assumed that the phenomenon of EV production by fungal cells is a universal mechanism of transport of macromolecules to the extracellular space [8, 26]. Major questions that remain still unanswered are the focus of a number comprehensive reviews [7, 8, 27,28,29], including those focused on methodological approaches [30]. In this article, we aim to update the reader with the most recent and less explored findings originating from fungal EV research.
Historic Overview of Fungal EVs: They Have Always Been There
As mentioned above, fungal EVs were first described in 2007 in studies using the yeast-like pathogen C. neoformans. However, microscopic evidence reporting the existence of fungal EVs became available decades before their first complete description. In 1972, Gibson and Peberdy demonstrated by transmission electron microscopy (TEM) vesicle-like structures budding from the cell surface of protoplast preparations of Aspergillus nidulans [31]. One year later, Takeo and colleagues used freeze-fracture electron microscopy to describe “spherical invaginations which secrete vesicles outside the membrane” of C. neoformans [32]. The same group used freeze-fracture microscopy to characterize “baglike paramural bodies showing multivesicles” in fusion with the plasma membrane [33]. These structures showed striking similarities with the currently well-known multivesicular bodies (MVBs). These MVBs fuse with the plasma membrane and originate EVs called exosomes [34]. In the early 90s, Anderson and colleagues observed vesicles associated with outer layers of the cell wall of C. albicans in TEM preparations [35]. In Schizosaccharomyces pombe protoplasts, multiple membrane blebbing originating EVs were observed by TEM [36]. Finally, 7 years before the characterization of fungal EVs, glucosylceramide-containing vesicles moving from the plasma membrane to the cell wall were observed in cryopreserved TEM preparations of C. neoformans [37]. Glucosylceramide was further confirmed as a major lipid component of C. neoformans EVs [13]. This information is in accordance with early studies describing lipids as cell wall components in fungi [38]. Cell wall lipids are now understood as transitory surface components associated with passage of EVs through the cell wall [8].
Novel Roles of EVs
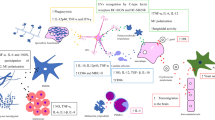
As widely reported, fungal EVs were associated with secretory mechanisms resulting in the extracellular export of pigments, nucleic acids, and proteins, but the physiological mechanisms behind these findings are still not clear. Fungal EVs were also linked to glycan export and surface architecture in C. neoformans [13] and P. brasiliensis [39•, 40]. Other physiological functions of fungal EVs are not clearly understood. On the other hand, EVs are apparently important for immunopathogenesis of mycoses [6]. In the topics below and in Fig. 1, we summarize the most recent findings directly linking EV production with pathophysiological events in fungi.
Overview of the most recent findings related to the functions of fungal EVs. Vesicle preparations of different fungi have been shown to participate in small RNA export, regulation of extracellular proteins, phagocyte responses, and prion transmission. Due to properties that were associated with yeast to hyphae transitions in P. fermentans, fungal EVs are also supposed to participate in morphological switch. EVs are represented as extracellular spherical compartments. CW cell wall. For details, see topics 1 to 5 in the text
Prion Transmission
In S. cerevisiae, diverse cytoplasmic proteins can form self-perpetuating protein aggregates (e.g., prions and prion-like proteins) which are the vectors of heritable non-Mendelian phenotypic traits [41]. These structures correspond to infectious epigenetic elements of inheritance that replicate by templating their aggregated state onto soluble proteins of the same type [42]. As described for their human counterparts, yeast prions induce phenotypic changes in their host cells [43]. The prototype yeast prion is the translation termination factor Sup35. Prions composed of Sup35 are heritable and are transmitted vertically to progeny or horizontally during cell division upon cytoplasmic mixing during mating [44••, 45••].
In mammalian cells, EVs have been proposed as vehicles by which aggregated proteins are transferred between cells, on the basis of the observation that neurodegeneration-associated proteins are secreted as cargo of EVs [46]. In S. cerevisiae, EVs contained Sup35 in its infectious prion conformation, suggesting the possibility of EV-mediated cell-to-cell transfer of fungal prions [45••]. This hypothesis was further confirmed by the observation that Sup35-containing EVs were taken up by recipient cells, which self-sustained Sup35 protein aggregation [44••]. It is still unclear whether prions other than Sup35 are exported via EVs, but these studies suggest a role of EVs in yeast prion clearance and/or propagation [47] and validate the hypothesis that fungal EVs could mediate cell-to-cell communication [48••].
Morphological Transition and Biofilm Formation
The vast majority of the studies characterizing fungal EVs used yeast cells. More recently, EVs were described in the filamentous fungi A. infectoria [24]. Although the roles of EVs in fungal morphological transitions are still unclear, it has been hypothesized that they could have an active role when single cells start to differentiate into multicellular structures [25•]. EVs from pseudohyphal and unicellular cultures of P. fermentans differed in size and composition, suggesting stage-specific changes during EV formation. Importantly, EV production was linked to biofilm formation in P. fermentans cultures under hypoxia [25•], reinforcing the possibility that fungal EVs participate in populational communication. In fact, EV release was associated with development and stability of bacterial biofilms, suggesting a role in extracellular matrix formation [49, 50].
RNA Export
Based on evidence that mammalian EVs consist of vehicles exporting nucleic acids, fungal vesicles were assessed for the presence of RNA. A seminal study indicated that C. neoformans, P. brasiliensis, S. cerevisiae, and C. albicans use EVs to transport RNA to the outer space [48••]. Most of the EV RNA consisted of small (< 250 nt long) molecules distributed into small nucleolar (snoRNA), small nuclear (snRNA), ribosomal (rRNA), transfer (tRNA), and messenger (mRNA) RNAs. More recently, small RNAs were characterized in EVs from M. sympodialis, a fungus associated with atopic eczema [51]. M. sympodialis EVs contained a set of reads with well-defined start and stop positions, in a length range of 16 to 22 nucleotides. RNA composition was not influenced by conditions simulating normal human skin or atopic eczema, suggesting the existence of a constitutive mechanism of EV-mediated export RNA in this fungus [51]. The roles of EV-associated RNA in fungi are still unknown, but the potential of these structures to mediate cell-to-cell communication and pathogenesis is immense.
Regulation of Extracellular Proteins
Protein composition was first determined in C. neoformans EVs by using a proteomic approach [52]. Proteomic analyses were further performed in EVs obtained from cultures of H. capsulatum [15, 53••], S. cerevisiae [16, 54], P. brasiliensis [19], C. albicans [22, 23], A. infectoria [24], and M. sympodialis [51]. In all cases, EV protein composition was highly diverse and included a high content of cytoplasmic proteins lacking conventional secretory tags. This diversity was associated to the hypothesis that EV formation was a general mechanism of cytoplasmic subtraction for putative regulation of cell volume [26, 55]. However, changes in the protein composition of EVs in S. cerevisiae and H. capsulatum in response to variations in culture conditions and antibody binding, respectively, suggested a higher specificity in EV-mediated protein.
Gluconeogenic enzymes are secreted when S. cerevisiae is cultivated in low glucose conditions [56]. However, when cells are transferred to media containing high glucose, they are internalized [57]. EV analysis in this model revealed that under glucose starvation, small vesicles (30–50 nm) largely predominated in comparison to larger structures (100–300 nm) [54]. This predominance was reverted upon addition of glucose in a process that depended on the expression of END3, a gene that is generally involved in the internalization of extracellular molecules [54, 58]. In fact, the gluconeogenic enzymes fructose-1,6-bisphosphatase, malate dehydrogenase, isocitrate lyase, and phosphoenolpyruvate carboxykinase, as well as non-gluconeogenic enzymes glyceraldehyde-3-phosphate dehydrogenase and cyclophilin A, were distributed in the vesicle-enriched fraction in total extracts prepared from cells grown in low glucose [54, 58, 59]. When this sugar was added to glucose-starved wild-type cells, levels of extracellular fructose-1,6-bisphosphatase, malate dehydrogenase, isocitrate lyase, phosphoenolpyruvate carboxykinase, glyceraldehyde-3-phosphate dehydrogenase, cyclophilin A, superoxide dismutase, thioredoxin, and heat shock proteins were reduced. In cells lacking the END3 gene, levels of these proteins in the extracellular fraction were still high [54, 58, 59].
Further studies confirmed that vesicles carrying metabolic enzymes were endocytosed at a fast rate, whereas vesicles carrying the heat shock protein Ssa1p were endocytosed at a slow rate [60]. The phosphoinositide 3-kinase (PI3K) regulator, Vps15p, affected the distribution of 100–300nm EVs containing Pil1, a major eisosome protein. Lack of Vps15 or Pil1 resulted in the absence of the 100–300nm EVs, and the rapid internalization of vesicles was impaired [60].
In pathogenic fungi, the impact of the host response on EV protein composition remained largely unknown until very recently. Matos-Baltazar and colleagues [53••] demonstrated that antibodies recognizing H. capsulatum heat shock protein 60 (Hsp60) bind to fungal cells and promote changes in EV size and composition, as well as in the activity of the pathogenesis-related enzymes laccase and phosphatase. This was the first demonstration that host’s defense mechanisms can directly impact protein loading in vesicles and fungal metabolism.
Stimulation of Phagocytes
Fungal EVs were proven to be immunologically active [22, 61]. In vivo models of infection with C. albicans or C. neoformans indicated that they could impact infection both in favor of disease control [22] or infection progress [62], respectively. The mechanisms of immunological stimulation by fungal EVs in vivo remain largely unknown, although they were shown to stimulate cytokine responses in models using phagocytes in vitro. Studies with C. neoformans [61], C. albicans [22], and M. sympodialis [17] revealed that EVs from the three species commonly induced tumor necrosis factor alpha (TNF-α). EVs from C. neoformans and C. albicans also shared the ability to induce transforming growth factor beta (TGF-β), IL-10, and nitric oxide. Particularly, M. sympodialis EVs stimulated IL-4 and similar preparations obtained from C. albicans cultures also induced IL-12 and IL-12p40. Of note, Candida vesicles caused upregulation of CD86 and major histocompatibility complex class-II (MHC-II). It is important to highlight that it is still not known whether fungal EVs are produced during infection. Therefore, it is uncertain if vesicle densities used in experimental models of phagocyte stimulation simulate in vivo conditions.
Immunological roles for P. brasiliensis EVs were explored more recently. Carbohydrate ligands for DC-SIGN receptors were initially identified in Paracoccidioides EVs [39•]. Further studies by Da Silva and colleagues demonstrated that EVs from P. brasiliensis promoted transcription of the M1-polarization marker iNOs and diminished that of the M2 markers Arginase-1, Ym-1, and FIZZ-1 in macrophages [63]. EV-induced M1 polarization of macrophages resulted in enhanced fungicidal activity, as concluded from reduced recovery of viable internalized yeast cells. Finally, increased expression of M2-polarization markers, stimulated by IL-4 plus IL-10, was reverted toward an M1 phenotype in response to secondary stimulation with P. brasiliensis vesicles [63].
The ability of fungal EVs to stimulate environmental phagocytes remained unknown until very recently, when C. neoformans vesicles were demonstrated to modulate the antimicrobial potential of the amoeboid predator Acanthamoeba. castellanii [64•]. The amoebae efficiently internalized cryptococcal EVs and their components, which resulted in increased survival of C. neoformans during interaction with A. castellanii. This observation strongly suggests that fungal EVs can participate in environmental interactions of fungi with their predators.
Conclusions and Perspectives
A continuous flow of generation of knowledge was established in the field of fungal EVs. The literature clearly validates this perception, which is reinforced by unpublished work presented in scientific meetings and discussion between colleagues and/or collaborators. Efforts are specially welcome to address questions related to the potential roles of fungal EVs during infection, assuming that, as suggested by serological studies [15, 20, 22, 52, 53••], they are indeed produced in vivo. Regulators of the biogenesis of fungal EVs are still unknown, as genetic studies systematically failed to identify genes consistently impacting EV production. Studies on the mechanisms by which fungal EVs are formed, therefore, are also desirable. The field, however, progressed fast; and after a decade of knowledge, it is now clear that EV production is an important mechanism of extracellular release in the Fungi. Venues for exploring the use of EVs as vaccines and targets for antifungals are open and clearly promising.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Choi H, Lee DS. Illuminating the physiology of extracellular vesicles. Stem Cell Res Ther. 2016;7(1):55. https://doi.org/10.1186/s13287-016-0316-1.
Zamanian M, Fraser LM, Agbedanu PN, Harischandra H, Moorhead AR, Day TA, et al. Release of small RNA-containing exosome-like vesicles from the human filarial parasite Brugia malayi. PLoS Negl Trop Dis. 2015;9(9):e0004069. https://doi.org/10.1371/journal.pntd.0004069.
Silverman JM, Clos J, de'Oliveira CC, Shirvani O, Fang Y, Wang C, et al. An exosome-based secretion pathway is responsible for protein export from Leishmania and communication with macrophages. J Cell Sci. 2010;123(Pt 6):842–52. https://doi.org/10.1242/jcs.056465.
Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest. 2011;121(4):1471–83. https://doi.org/10.1172/JCI44261.
Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O'Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009;5(4):e1000382. https://doi.org/10.1371/journal.ppat.1000382.
Joffe LS, Nimrichter L, Rodrigues ML, Del Poeta M. Potential roles of fungal extracellular vesicles during infection. mSphere. 2016;1(4) https://doi.org/10.1128/mSphere.00099-16.
Rodrigues ML, Godinho RM, Zamith-Miranda D, Nimrichter L. Traveling into outer space: unanswered questions about fungal extracellular vesicles. PLoS Pathog. 2015;11(12):e1005240. https://doi.org/10.1371/journal.ppat.1005240.
Nimrichter L, de Souza MM, Del Poeta M, Nosanchuk JD, Joffe L, Tavares Pde M, et al. Extracellular vesicle-associated transitory cell wall components and their impact on the interaction of fungi with host cells. Front Microbiol. 2016;7:1034. https://doi.org/10.3389/fmicb.2016.01034.
Raab-Traub N, Dittmer DP. Viral effects on the content and function of extracellular vesicles. Nat Rev Microbiol. 2017; https://doi.org/10.1038/nrmicro.2017.60.
Gopal SK, Greening DW, Rai A, Chen M, Xu R, Shafiq A, et al. Extracellular vesicles: their role in cancer biology and epithelial-mesenchymal transition. Biochem J. 2017;474(1):21–45. https://doi.org/10.1042/BCJ20160006.
Fuhrmann G, Neuer AL, Herrmann IK. Extracellular vesicles—a promising avenue for the detection and treatment of infectious diseases? Eur J Pharm Biopharm: Off J Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2017;118:56–61. https://doi.org/10.1016/j.ejpb.2017.04.005.
Samuel M, Bleackley M, Anderson M, Mathivanan S. Extracellular vesicles including exosomes in cross kingdom regulation: a viewpoint from plant-fungal interactions. Front Plant Sci. 2015;6:766. https://doi.org/10.3389/fpls.2015.00766.
Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6(1):48–59. https://doi.org/10.1128/EC.00318-06.
Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Natl Acad Sci U S A. 2010;107(44):19002–7. https://doi.org/10.1073/pnas.1008843107.
Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10(8):1695–710. https://doi.org/10.1111/j.1462-5822.2008.01160.x.
Oliveira DL, Nakayasu ES, Joffe LS, Guimaraes AJ, Sobreira TJ, Nosanchuk JD, et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One. 2010;5(6):e11113. https://doi.org/10.1371/journal.pone.0011113.
Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, et al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses—novel mechanisms for host-microbe interactions in atopic eczema. PLoS One. 2011;6(7):e21480. https://doi.org/10.1371/journal.pone.0021480.
Vallejo MC, Nakayasu ES, Longo LV, Ganiko L, Lopes FG, Matsuo AL, et al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One. 2012;7(6):e39463. https://doi.org/10.1371/journal.pone.0039463.
Vallejo MC, Nakayasu ES, Matsuo AL, Sobreira TJ, Longo LV, Ganiko L, et al. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J Proteome Res. 2012;11(3):1676–85. https://doi.org/10.1021/pr200872s.
Vallejo MC, Matsuo AL, Ganiko L, Medeiros LC, Miranda K, Silva LS, et al. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-Galactosyl epitopes. Eukaryot Cell. 2011;10(3):343–51. https://doi.org/10.1128/EC.00227-10.
Wolf JM, Espadas J, Luque-Garcia J, Reynolds T, Casadevall A. Lipid biosynthetic genes affect Candida albicans extracellular vesicle morphology, cargo, and immunostimulatory properties. Eukaryot Cell. 2015;14(8):745–54. https://doi.org/10.1128/EC.00054-15.
Vargas G, Rocha JD, Oliveira DL, Albuquerque PC, Frases S, Santos SS, et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol. 2015;17(3):389–407. https://doi.org/10.1111/cmi.12374.
Gil-Bona A, Llama-Palacios A, Parra CM, Vivanco F, Nombela C, Monteoliva L, et al. Proteomics unravels extracellular vesicles as carriers of classical cytoplasmic proteins in Candida albicans. J Proteome Res. 2015;14(1):142–53. https://doi.org/10.1021/pr5007944.
Silva BM, Prados-Rosales R, Espadas-Moreno J, Wolf JM, Luque-Garcia JL, Goncalves T, et al. Characterization of Alternaria infectoria extracellular vesicles. Med Mycol. 2014;52(2):202–10. https://doi.org/10.1093/mmy/myt003.
• Leone F, Bellani L, Mucciflora S, Giorgetti L, Bongioanni P, Simili M, et al. Analysis of extracellular vesicles produced in the biofilm by the dimorphic yeast Pichia fermentans. J Cell Physiol. 2017; https://doi.org/10.1002/jcp.25885. First association between fungal EVs, biofilms and morphological transition
Rodrigues ML, Nakayasu ES, Almeida IC, Nimrichter L. The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J Proteome. 2014;97:177–86. https://doi.org/10.1016/j.jprot.2013.04.001.
Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. 2015;13(10):620–30. https://doi.org/10.1038/nrmicro3480.
Wolf JM, Casadevall A. Challenges posed by extracellular vesicles from eukaryotic microbes. Curr Opin Microbiol. 2014;22:73–8. https://doi.org/10.1016/j.mib.2014.09.012.
Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends Microbiol. 2009;17(4):158–62. https://doi.org/10.1016/j.tim.2008.12.005.
Rodrigues ML, Oliveira DL, Vargas G, Girard-Dias W, Franzen AJ, Frases S, et al. Analysis of yeast extracellular vesicles. Methods Mol Biol. 2016;1459:175–90. https://doi.org/10.1007/978-1-4939-3804-9_12.
Gibson RK, Peberdy JF. Fine structure of protoplasts of Aspergillus nidulans. J Gen Microbiol. 1972;72(3):529–38. https://doi.org/10.1099/00221287-72-3-529.
Takeo K, Uesaka I, Uehira K, Nishiura M. Fine structure of Cryptococcus neoformans grown in vivo as observed by freeze-etching. J Bacteriol. 1973;113(3):1449–54.
Takeo K, Uesaka I, Uehira K, Nishiura M. Fine structure of Cryptococcus neoformans grown in vitro as observed by freeze-etching. J Bacteriol. 1973;113(3):1442–8.
Zha QB, Yao YF, Ren ZJ, Li XJ, Tang JH. Extracellular vesicles: an overview of biogenesis, function, and role in breast cancer. Tumour Biol: J Int Soc Oncodevelopmental Biol Med. 2017;39(2):1010428317691182. https://doi.org/10.1177/1010428317691182.
Anderson J, Mihalik R, Soll DR. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J Bacteriol. 1990;172(1):224–35.
Osumi M. The ultrastructure of yeast: cell wall structure and formation. Micron. 1998;29(2–3):207–33.
Rodrigues ML, Travassos LR, Miranda KR, Franzen AJ, Rozental S, de Souza W, et al. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun. 2000;68(12):7049–60.
Cox RA, Best GK. Cell wall composition of two strains of Blastomyces dermatitidis exhibiting differences in virulence for mice. Infect Immun. 1972;5(4):449–53.
• Peres da Silva R, Heiss C, Black I, Azadi P, Gerlach JQ, Travassos LR, et al. Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for DC-SIGN receptors. Sci Rep. 2015;5:14213. https://doi.org/10.1038/srep14213. Description of biologically active glycans in P. brasiliensis EVs
Albuquerque PC, Cordero RJ, Fonseca FL, Peres da Silva R, Ramos CL, Miranda KR, et al. A Paracoccidioides brasiliensis glycan shares serologic and functional properties with cryptococcal glucuronoxylomannan. Fungal Genet Biol: FG & B. 2012;49(11):943–54. https://doi.org/10.1016/j.fgb.2012.09.002.
Liebman SW, Chernoff YO. Prions in yeast. Genetics. 2012;191(4):1041–72. https://doi.org/10.1534/genetics.111.137760.
Wickner RB, Shewmaker FP, Bateman DA, Edskes HK, Gorkovskiy A, Dayani Y, et al. Yeast prions: structure, biology, and prion-handling systems. Microbiology and molecular biology reviews : MMBR. 2015;79(1):1–17. https://doi.org/10.1128/MMBR.00041-14.
Liu S, Hossinger A, Gobbels S, Vorberg IM. Prions on the run: how extracellular vesicles serve as delivery vehicles for self-templating protein aggregates. Prion. 2017;11(2):98–112. https://doi.org/10.1080/19336896.2017.1306162.
•• Liu S, Hossinger A, Hofmann JP, Denner P, Vorberg IM. Horizontal transmission of cytosolic Sup35 prions by extracellular vesicles. MBio. 2016;7(4) https://doi.org/10.1128/mBio.00915-16. Demonstration that biologically active prions are exported in fungal EVs
•• Kabani M, Melki R. Sup35p in its soluble and prion states is packaged inside extracellular vesicles. MBio. 2015;6(4) https://doi.org/10.1128/mBio.01017-15. First association between fungal prions and EVs
Thompson AG, Gray E, Heman-Ackah SM, Mager I, Talbot K, Andaloussi SE, et al. Extracellular vesicles in neurodegenerative disease–pathogenesis to biomarkers. Nat Rev Neurol. 2016;12(6):346–57. https://doi.org/10.1038/nrneurol.2016.68.
Kabani M, Melki R. More than just trash bins? Potential roles for extracellular vesicles in the vertical and horizontal transmission of yeast prions. Curr Genet. 2016;62(2):265–70. https://doi.org/10.1007/s00294-015-0534-6.
•• Peres da Silva R, Puccia R, Rodrigues ML, Oliveira DL, Joffe LS, Cesar GV, et al. Extracellular vesicle-mediated export of fungal RNA. Sci Rep. 2015;5:7763. https://doi.org/10.1038/srep07763. First demonstration of RNA export in fungal EVs
Yonezawa H, Osaki T, Kurata S, Fukuda M, Kawakami H, Ochiai K, et al. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 2009;9:197. https://doi.org/10.1186/1471-2180-9-197.
Manning AJ, Kuehn MJ. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J Mol Microbiol Biotechnol. 2013;23(1–2):131–41. https://doi.org/10.1159/000346548.
Rayner S, Bruhn S, Vallhov H, Andersson A, Billmyre RB, Scheynius A. Identification of small RNAs in extracellular vesicles from the commensal yeast Malassezia sympodialis. Sci Rep. 2017;7:39742. https://doi.org/10.1038/srep39742.
Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7(1):58–67. https://doi.org/10.1128/EC.00370-07.
•• Matos Baltazar L, Nakayasu ES, Sobreira TJ, Choi H, Casadevall A, Nimrichter L, et al. Antibody binding alters the characteristics and contents of extracellular vesicles released by Histoplasma capsulatum. mSphere. 2016;1(2) https://doi.org/10.1128/mSphere.00085-15. Proof of concept that humoral responses can affect the composition of fungal EVs
Giardina BJ, Stein K, Chiang HL. The endocytosis gene END3 is essential for the glucose-induced rapid decline of small vesicles in the extracellular fraction in Saccharomyces cerevisiae. J Extracellular Vesicles. 2014;3 https://doi.org/10.3402/jev.v3.23497.
Rodrigues ML, Franzen AJ, Nimrichter L, Miranda K. Vesicular mechanisms of traffic of fungal molecules to the extracellular space. Curr Opin Microbiol. 2013;16(4):414–20. https://doi.org/10.1016/j.mib.2013.04.002.
Giardina BJ, Chiang HL. The key gluconeogenic enzyme fructose-1,6-bisphosphatase is secreted during prolonged glucose starvation and is internalized following glucose re-feeding via the non-classical secretory and internalizing pathways in Saccharomyces cerevisiae. Plant Signal Behav. 2013;8(8) https://doi.org/10.4161/psb.24936.
Alibhoy AA, Giardina BJ, Dunton DD, Chiang HL. Vps34p is required for the decline of extracellular fructose-1,6-bisphosphatase in the vacuole import and degradation pathway. J Biol Chem. 2012;287(39):33080–93. https://doi.org/10.1074/jbc.M112.360412.
Stein K, Chiang HL. Exocytosis and endocytosis of small vesicles across the plasma membrane in Saccharomyces cerevisiae. Membranes. 2014;4(3):608–29. https://doi.org/10.3390/membranes4030608.
Giardina BJ, Stanley BA, Chiang HL. Glucose induces rapid changes in the secretome of Saccharomyces cerevisiae. Proteome Sci. 2014;12(1):9. https://doi.org/10.1186/1477-5956-12-9.
Stein K, Winters C, Chiang HL. Vps15p regulates the distribution of cup-shaped organelles containing the major eisosome protein Pil1p to the extracellular fraction required for endocytosis of extracellular vesicles carrying metabolic enzymes. Biol Cell. 2017;109(5):190–209. https://doi.org/10.1111/boc.201600060.
Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun. 2010;78(4):1601–9. https://doi.org/10.1128/IAI.01171-09.
Huang SH, CH W, Chang YC, Kwon-Chung KJ, Brown RJ, Jong A. Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PLoS One. 2012;7(11):e48570. https://doi.org/10.1371/journal.pone.0048570.
da Silva TA, Roque-Barreira MC, Casadevall A, Almeida F. Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro. Sci Rep. 2016;6:35867. https://doi.org/10.1038/srep35867.
• Rizzo J, Albuquerque PC, Wolf JM, Nascimento R, Pereira MD, Nosanchuk JD, et al. Analysis of multiple components involved in the interaction between Cryptococcus neoformans and Acanthamoeba castellanii. Fungal Biol. 2017;121(6–7):602–14. https://doi.org/10.1016/j.funbio.2017.04.002. First association between fungal EVs and antimicrobial activity of environmental predators
Acknowledgements
Work on fungal extracellular vesicles has been supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
The authors also acknowledge support from the Instituto Nacional de Ciência e Tecnologia de Inovação em Doenças Negligenciadas (INCT-IDN). J.R. was a Ph.D. student affiliated to the Graduate Program in Chemical Biology of the Federal University of Rio de Janeiro.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Marcio L. Rodrigues has received a Pathfinder Award from the Wellcome Trust (GB) Grant Number: WT103212MF.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Fungal Genomics and Pathogenesis
Rights and permissions
About this article
Cite this article
Rizzo, J., Nimrichter, L. & Rodrigues, M.L. What Is New? Recent Knowledge on Fungal Extracellular Vesicles. Curr Fungal Infect Rep 11, 141–147 (2017). https://doi.org/10.1007/s12281-017-0293-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-017-0293-6