Abstract
Purpose of Review
Extracellular vesicles (EVs) have been implicated in the pathogenesis of several infectious diseases, but their role in fungal virulence has only recently started to emerge. Here we summarize recent discoveries in this field and highlight areas where more research is urgently needed.
Recent Findings
In fungi, EVs carry a heterogenous molecular content including polysaccharides, lipids, allergens, heat-shock proteins (HSPs), enzymes, pigments, and RNAs. Many of these components have been shown to modulate the host immune system and to participate in biofilm production, drug resistance, cell communication, and virulence enhacement. However, the influence of EVs on the course of fungal disease remains elusive, primarily due to technical limitations. Nevertheless, there are many potential medical applications yet to be investigated, including the development of diagnostic tools, vaccine adjuvants, and design of antifungal agents.
Summary
Although at a very early stage, the field of fungal EVs is rapidly expanding and holds exciting potential to strongly impact on our understanding and treatment of this group of diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The release of extracellular vesicles (EVs) is a conserved phenomenon among all forms of life [1]. EV play important roles in different diseases, including cancer metastasis [2], inflammatory disorders [3], prion horizontal transmission [4], spread of bacterial antibiotic resistance and toxin release [5], as well as exchange of genetic material between host cells and protozoans [6]. In fungi, EV structures are composed of bilayered membranes and a heterogeneous cargo that includes lipids, polysacharides, proteins, and nucleic acids. Many of these components are virulence-related molecules that are delivered to the extracellular space [7•, 8•]. Recently, EVs have been implicated in intercellular communication processes that involve the transfer of virulence traits from a pathogenic yeast to an environmental strain [9••].
Fungal EVs were first described in the supernatant cultures of the yeast pathogen Cryptococcus neoformans [10•]. Subsequently, EVs were reported in other fungal species of medical and/or biotechnology importance, including Histoplasma capsulatum [11], Candida albicans [11, 12], Saccharomyces cerevisiae [11, 13], Malassezia sympodialis [14, 15], Paracoccidioides brasiliensis [16], Alternaria infectoria [17], Pichia fermentans [18], Sporothrix schenckii [11], S. brasiliensis [19], C. gattii [9••], and Trichophyton interdigitale [20].
Several different mechanisms are used by fungi to produce EVs. Some EVs are generated from the conventional secretory pathway with the participation of the endoplasmic reticulum, involving the fusion of vesicles derived from the Golgi apparatus with the plasma membrane [21]. Alternatively, via the unconventional pathway, EV formation occurs through plasma membrane invagination, creating endosomes that may develop internal vesicles and result in multivesicular bodies (MVBs) [22]. Although MVBs generally fuse with lysosomes, they may also merge with the plasma membrane, delivering their internal content (exosomes) into the extracellular space [12, 23]. EVs can also be formed from the evagination of the plasma membrane (inverted macropinocytosis), followed by release of microvesicles, also known as ectosomes [23, 24]. For consistency, all vesicle populations will be refered as vesicles or EVs in this review.
EVs originating from fungal cells need to cross the cell wall in order to reach the extracellular environment. There are three hypotheses to explain how EVs overcome this physical barrier: (i) by protein channels formed by the rearrangement of the cytoskeleton; (ii) with the participation of enzymes that would temporarily remodel the cell wall; or (iii) forcing their passage through pores [23, 25]. Most recently, images of intact liposomes reaching the fungal membrane demonstrated the dynamic properties of the fungal cell wall [26]. Therefore, it has been speculated that this mechanism could be reversed to deliver vesicles from the membrane to the outer cell wall. Furthermore, the size of pores in fungal cell wall could enlarge up to 400 nm, aiming to increase nutrient uptake as a response to environmental stress [27].
EVs become unstable and lyse almost immediately in the presence of serum proteins [8•]. Albumin might mediate EV rupture by binding to ergosterol, destabilizing vesicle membranes. This finding suggests that, in the course of infection, EV integrity is short-lived following their release from fungal cells [8•]. Recently, it was shown that mammalian β-galactoside-binding protein galectin-3 (Gal-3) produced a direct lytic effect on C. neoformans EVs [28]. This lectin presents an extensive distribution on the surface of host cells as well as intracellularly [29], and has influenced host response against different fungal infections [30,31,32]. For instance, Gal-3 levels were increased in tissues and serum of mice infected with C. neoformans and contributed to a higher killing rate in Gal-3-deficient mice [28].
The participation of EVs on fungal biology and pathogenesis remains elusive [33, 34]. However, previous studies have correlated fungal EVs with protein metabolism [35], morphological differentiation [18], immune modulation of the host [36•], participation in cell communication [9••], and fungal dissemination [37•]. In this context, this review will focus on findings related to EVs from the main fungal human pathogens, the techinical limitations that currently prevent the development of this field, and its ultimately promising clinical applications.
Cryptococcus spp.
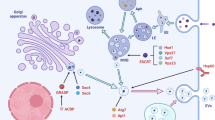
Cryptococcus is the fungal model in which fungal EVs have been most intensively studied to date. The first description of fungal EVs was in fractionated culture supernatants of C. neoformans [10•]. These vesicular compartments were identified as spherical bilayered membranes enriched in lipids including glucosylceramide, ergosterol, and sterol. The presence of virulence-related molecules, including the major capsular polysaccharide glucuronoxylomannan (GXM), was the basis for the classification of these compartments as virulence bags [7•]. GXM molecules are synthesized intracellularly, transported within vesicles from the Golgi complex, and secreted via exocytosis [38]. The Golgi reassembly and stacking protein (GRASP) may be involved in the process of loading GXM into vesicles and fusion with the plasma membrane as well as with RNA export [39, 40]. A mutant strain lacking GRASP in C. neoformans showed alterations in Golgi morphology, hypocapsular phenotype, and reduced GXM secretion. In a mouse model, graspΔ cells were easily phagocytosed and presented attenued virulence, causing late lethality in infected animals. In contrast, levels of urease activity and melanin biosynthesis were not affected in these cells [39].
Melanin production increases C. neoformans resistance towards host-derived reactive oxygen molecules [41]. EVs from C. neoformans were able to melanize after incubation with L-3,4-dihydroxyphenylalanine (L-DOPA) [42], suggesting that partitioning of melanin within these compartments may allow Cryptococcus cells avoid the toxic effects of the intermediates generated by oxidation of L-DOPA [42].
Proteomic analysis of C. neoformans vesicles identified proteins related to protection against oxidative stress, including HSPs, superoxide dismutase, thiol-specific antioxidants, and catalases [7•]. However, EV cargoes are complex and not restricted to virulence factors. Molecules related to cell biology were also detected in EVs, including proteins associated with carbohydrate metabolism, cell organization, molecular transporters, protein degradation, histones, and ribosomal proteins [7•, 13].
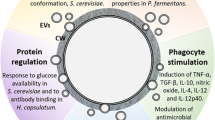
EVs present immunological properties (Table 1). The stimulation of macrophages by C. neoformans EVs increased the levels of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-10, transforming growth factor-beta (TGF-β), and nitric oxide, enhancing phagocytosis index and macrophage antimicrobial activity [36•]. EVs released by C. neoformans were also progressively internalized into the cytoplasm of human brain microvascular endothelial cells, leading to increased yeast transmigration through the blood-brain barrier. This process relied on the redistribution of β-actin, caveolin-1, and vimentin, which induced fusion with host cells [37•]. As with mammalian host cells, the soil amoeba Acanthamoeba castellanii, which is an environmental predator of Cryptococcus cells, is able to internalize C. neoformans EVs and this internalization subsequently enhances yeast survival (Fig. 1) [44].
Genes involved on secretion mechanisms are evolutionary conserved among fungal species. In yeast, the protein Sec6 participates in EV fusion with the plasma membrane [46] and reduction of Sec6 expression by RNA interference leads to intracellular accumulation of vesicles and a lack of laccase or urease secretion. Mutants lacking the SAV1 gene in C. neoformans do not secrete acid phosphatase and accumulate cytoplasmic vesicles containing GXM [38]. In contrast, a Sec14 homologue is essential for the secretion of phospholipase B1 (Plb1) but not for the secretion of GXM or laccase [21, 47]. Modulating traditional secretory genes such as SEC1, SEC4, and SEC6, as well as the vacuolar protein sorting (VPS), affects EV composition and their release kinetics [13, 48, 49]—an effect which, in some cases, leads to an impact on virulence [50, 51]. Genes and molecules that influence the fungal secretory system are summarized in Table 2.
Proteins involved in lipid distribution on the fungal plasma membrane, such as the aminophospholipid translocase 1 (Apt1) and scramblases [52, 53, 59••], have also an important impact on membrane fusion events and therefore fungal secretion. Mutant cells lacking Apt1 produced EVs of varying sizes and reduced GXM content when compared to wild-type strain. Apt1 mutants show decreased macrophage infection and organ colonization, but whether that is directly associated with an altered EV profile remains unknown [52, 53]. On the other hand, a mutant lacking scramblase in C. gattii produced vesicles with unaltered GXM amounts, although EVs from the mutant had larger size and altered RNA profiles [59••].
Interestingly, the absence of any of these proteins was associated with complete elimination of EV release [13, 49], presumably reflecting a requirement for a “wild-type” membrane composition to generate EVs [22,23,24]. In contrast, higher release of EVs was observed in the absence of a putative G1/S cyclin in C. neoformans. However, rather than driving increased virulence, this phenotype in fact reduced virulence in murine macrophages and G. mellonella infection [55].
Candida spp.
Transmission electron microscopy of vesicles from Candida albicans and C. parapsilosis were described by Albuquerque et al. [11]. Overall, the structural morphology of the vesicles were similar to other fungal EVs [10•, 11]. As in C. neoformans, vesicles from C. albicans also can be internalized by host immune cells, for instance macrophages and dendritic cells (DCs) leading to increased cytokine production [43••] (Fig. 1).
Major components of both C. neoformans and C. albicans EVs are miRNA-like and iRNA sequences, which has led to the suggestion that these molecules could play a role during fungal infection by mimicking endogenous miRNA and regulating gene expression in host cells [60••]. The converse situation, in which host cells secrete vesicles containing small RNAs to silence pathogenesis-related genes in pathogens, has already been documented for Arabidopsis thaliana and the fungal pathogen Botrytis cinerea [61], suggesting that such a mechanism could theoretically occur.
As with C. neoformans, phospholipid biosynthesis in C. albicans affected EV morphology, composition, and immunogenicity. Mutants lacking the gene for phosphatidylserine synthase (CHO1) or for phosphatidylserine decarboxylase (PSD1 and PSD2) produced EVs with increased PC and significantly altered protein content. Interestingly, EVs from psd1Δpsd2Δ were larger, whereas cho1Δ EVs were unable to activate NF-ƙB in bone marrow-derived macrophages (BMDMs) [54].
A comparative study between the proteome of C. albicans EVs and EV-free secretions showed that most proteins in the latter were secreted by the classical pathway, including hydrolases, adhesins, and proteins involved in biofilm formation [62]. This latter finding aligns with recent data showing that Candida biofilms release EVs with a different content from planktonic cells [57••].
Other Fungal Species
Studies describing EVs from filamentous fungi are rare [34]. Alternaria infectoria was the first filamentous fungus to be described secreting EVs [17]. Analysis of their content identified 20 proteins, seven of which had previously been reported in other fungal vesicles. However, a higher number of proteins related to DNA repair and replication were observed. Although A. infectoria contains melanin on their cell wall, laccase was not found. Nevertheless, the enzyme polyketide synthase was detected, which catalyzes a melanin intermediate and is associated with pathogenesis in various fungi, including the melanin producer C. neoformans [17, 42]. Molecules related to cell adhesion, transport, metabolism, and plasma membrane were also found. Interestingly, the authors detected Hsp60, which was also found in EVs from the fatal human pathogen Histoplasma capsulatum [11, 17]. In addition to numerous other proteins involved in pathogenesis and host immune response [11, 63], Histoplasma EVs also show interesting strain-specific differences in their RNA content, the impact of which remains unknown [63].
EVs have also been purified and partially characterized from Saccharomyces cerevisiae, Sporothrix schenckii and S. brasiliensis [11, 19] as well as the human skin commensal yeast, Malassezia sympodialis [14]. As in other fungal species, small RNAs were identified within M. sympodialis vesicles [64]. Since EVs from M. sympodialis are actively internalized by keratinocytes and monocytes (Fig. 1), it has been suggested that these EVs could participate during the skin inflammation process by delivering allergens to the host cells in atopic eczema patients [15].
Vesicles have also been identified from the dermatophyte Trichophyton interdigitale, another skin fungal pathogen, and shown to induce a dose-dependent proinflammatory response in keratinocytes and BMDMs [20]. Similarly in the thermodimorphic fungus Paracoccidioides brasiliensis, EVs promoted the release of proinflammatory cytokines, such as TNF-α, IL-6, and IL-12, and favored M1-polarization (Fig. 1) [45]. Previously, P. brasiliensis EVs were reported to carry α-linked galactosyl epitopes—highly immunogenic molecules that are likely to be stored inside vacuoles resembling multivesicular bodies [65]. Proteomic [66], lipidomic [16], and glycobiome [67] analysis of P. brasiliensis EVs have been performed. One interesting outcome of these studies was the finding that oligosaccharides exposed on EVs surface are mainly composed of high amounts of mannose and lower amounts of N-acetylglucosamine and can be recognized by the mammalian C-type lectin receptors DC-SIGN and DC-SIGNR [67] (Fig. 1).
Limitations and Challenges
Despite the considerable progress made in recent years, the fungal EV field suffers from a number of significant technical limitations at present. Foremost among these is that methods for isolation and quantification of the fungal vesicles are laborious and inefficient [59••, 68]. Ultracentrifugation is the most traditional method, but it is time-consuming [18], requires large samples [69], and results in a final product that can be contaminated with media residue or cell debris [59••]. Some studies have developed novel methodologies in order to get higher yields of EVs. Leone et al. [18] have isolated EVs from P. fermetans biofilm using a mammalian serum exosome kit, reporting isolations that are around 50% as effective as the ultracentrifugation method [18]. Most recently, a new protocol using solid media was proposed, taking into account the fungal colonization over solid surfaces, such as soil and plant or animal tissue. To date, this technique has been exploited for C. neoformans, C. gattii, Candida albicans, H. capsulatum, and S. cerevisae [59••].
A second major issue is the ongoing need to distinguish between genuine vesicles and cell artifacts. Several pieces of data indicate that fungal EVs are not cell artifacts, including TEM validation [18] and evidence that cellular debris should be eliminated from preparations by centrifugation and filtration [70]. “Mock” purifications on supernatants from heat-killed fungal cells or from media alone do not yield EVs, which can be identified only from live cells [20, 43••], and EVs show a characteristic double membrane that is unlike cell fragments [10•, 11, 54, 71]. Likewise, the striking similarity between proteomic analysis of EVs from different fungal species [7•, 11, 43••, 66] suggests that vesicle secretion is a well-conserved mechanism in fungi. Lastly, mutants of the conventional and unconventional secretion pathways produce EVs with different cargo, size, and quantitity, in comparasion with wild-type strains [53, 59••], indicating the biological origin of EVs.
One of the biggest challenges yet to be exploired is if fungal EVs are released inside host cells. Linked to this point, it is still unknown which receptors play a role on fungal EV uptake by the host cells. Antibodies against the fungal EV surface [72] or the identification of a fungal molecule as an “EV-specific marker” would help solve this question, but to date neither reagent is available.
Lastly, the field is significantly hampered by the lack of a gene modification that results in a mutant unable to secrete vesicles [72] or even a reliable way to discriminate between different vesicle populations. It is still unclear whether the biogenesis of EVs influences their cargo and function [35]. In this context, the enhancement of EVs isolation and analysis methodologies is critical for the development of the field and for the potential applications for fungal EVs, which will be discussed in the next session.
Applications and Future Perspectives
Clinically, host-derived EVs are already under development as biomarkers [73]. An exciting next step would be to use a similar approach to diagnose fungal diseases. Proteins from C. neoformans EVs were recognized by human serum from patients diagnosed with cryptococcosis, but not from healthy individuals [7•]. Similarly, protein extracts of H. capsulatum EVs reacted with serum from patients with histoplasmosis [11], and EVs from P. brasiliensis reacted strongly against anti-α-Gal IgG from patients with paracoccidioidomycosis [65]. A reliable EV-based diagnostic would dramatically reduce the time to diagnosis several fungal pathogens and would be particularly helpful for those organisms for which there is no available molecular test currently on the market [74].
EVs from fungal cells could potentially be also used as vaccine antigens or adjuvants, since several components (such as Bgl2 in C. albicans) stimulate an immune response and can confer protection against invasive candidiasis infection in mice [62]. In bacteria, outer membrane vesicles (OMV) have been highlighted as part of a vaccine strategy [75]. OMV meningococcal vaccine has been approved by regulatory agencies [76], and shown to also protect against gonorrhea [77]. A similar approach may be extremely worthwhile for fungal pathogens.
Lastly, vesicle mechanisms of secretion could be used as a potential antifungal target. Mutants of the unconventional secretion pathway, such as apt1Δ, were reported to be hypovirulent and unable to disseminate to the brain in mice models [53, 78]. Additionaly, EVs could be used as vehicle to deliver drug molecules to specific target tissues [79].
Summary
Despite huge recent advances in fungal EV biology, there are many important aspects that remain to be explored, such as the production of EV markers and the development of more accurate techniques for EV isolation and characterization. However, given that the fungal EV field is less than 15 years old, this is undoubtedly an area with many more exciting discoveries to come.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect Immun. American Society for Microbiology. 2012;80:1948–57. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22409932. Accessed 23 Jan 2019.
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Curry Jr WT, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. Nature Publishing Group. 2008;10:1470. Available from: https://doi.org/10.1038/ncb1800.
Burbano C, Rojas M, Muñoz-Vahos C, Vanegas-García A, Correa LA, Vásquez G, et al. Extracellular vesicles are associated with the systemic inflammation of patients with seropositive rheumatoid arthritis. Sci Rep. 2018;8:17917. Available from:. https://doi.org/10.1038/s41598-018-36335-x.
Liu S, Hossinger A, Hofmann JP, Denner P, Vorberg IM. Horizontal transmission of cytosolic Sup35 prions by extracellular vesicles. MBio. 2016;7:1–12 Available from: http://mbio.asm.org/content/7/4/e00915-16. Accessed 16 Nov 2016.
Coelho C, Brown L, Maryam M, Vij R, Smith DFQ, Burnet MC, et al. Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J Biol Chem United States. 2019;294:1202–17.
Sisquella X, Ofir-Birin Y, Pimentel MA, Cheng L, Abou Karam P, Sampaio NG, et al. Malaria parasite DNA-harbouring vesicles activate cytosolic immune sensors. Nat Commun England. 2017;8:1985.
• Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, et al. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot Cell. 2008;7:58–67 First report about fungal EV content.
• Wolf JM, Rivera J, Casadevall A. Serum albumin disrupts Cryptococcus neoformans and Bacillus anthracis extracellular vesicles. Cell Microbiol. 2012;14:762–73 First evidence that fungal EVs have a short live in vivo and could be disrupted by serum albumin.
•• Bielska E, Sisquella MA, Aldeieg M, Birch C, O’Donoghue EJ, May RC. Pathogen-derived extracellular vesicles mediate virulence in the fatal human pathogen Cryptococcus gattii. Nat Commun. 2018;9:1556. Available from: https://doi.org/10.1038/s41467-018-03991-6. First report associating fungal EVs and enhancement of virulence.
• Rodrigues ML, Nimrichter L, Oliveira DL, Frases S, Miranda K, Zaragoza O, et al. Vesicular polysaccharide export in Cryptococcus neoformans is a eukaryotic solution to the problem of fungal trans-cell wall transport. Eukaryot Cell. 2007;6:48–59 First report describing fungal extracellular vesicles.
Albuquerque PC, Nakayasu ES, Rodrigues ML, Frases S, Casadevall A, Zancope-Oliveira RM, et al. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell MicrobiolEngland. 2008;10:1695–710.
Nosanchuk JD, Nimrichter L, Casadevall A, Rodrigues ML. A role for vesicular transport of macromolecules across cell walls in fungal pathogenesis. Commun Integr Biol. 2008;1:37–9 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2629580&tool=pmcentrez&rendertype=abstract. Accessed 13 Feb 2015.
Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJP, Nosanchuk JD, et al. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One. 2010;5:e11113.
Gehrmann U, Qazi KR, Johansson C, Hultenby K, Karlsson M, Lundeberg L, et al. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses—novel mechanisms for host-microbe interactions in atopic eczema. PLoS One. Public Library of Science. 2011;6:e21480–e21480. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21799736. Accessed 22 Feb 2019.
Johansson HJ, Vallhov H, Holm T, Gehrmann U, Andersson A, Johansson C, et al. Extracellular nanovesicles released from the commensal yeast Malassezia sympodialis are enriched in allergens and interact with cells in human skin. Sci Rep. Nature Publishing Group UK. 2018;8:9182. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29907748. Accessed 22 Feb 2019.
Vallejo MC, Nakayasu ES, Longo LVG, Ganiko L, Lopes FG, Matsuo AL, et al. Lipidomic analysis of extracellular vesicles from the pathogenic phase of Paracoccidioides brasiliensis. PLoS One. United States. 2012;7:e39463.
Silva BMA, Prados-Rosales R, Espadas-Moreno J, Wolf JM, Luque-Garcia JL, Goncalves T, et al. Characterization of Alternaria infectoria extracellular vesicles. Med Mycol England. 2014;52:202–10.
Leone F, Bellani L, Muccifora S, Giorgetti L, Bongioanni P, Simili M, et al. Analysis of extracellular vesicles produced in the biofilm by the dimorphic yeast Pichia fermentans. J Cell Physiol United States. 2018;233:2759–67.
Ikeda MAK, de Almeida JRF, Jannuzzi GP, Cronemberger-Andrade A, Torrecilhas ACT, Moretti NS, et al. Extracellular vesicles from Sporothrix brasiliensis are an important virulence factor that induce an increase in fungal burden in experimental sporotrichosis. Front Microbiol. Switzerland. 2018;9:2286.
Bitencourt TA, Rezende CP, Quaresemin NR, Moreno P, Hatanaka O, Rossi A, et al. Extracellular vesicles from the dermatophyte Trichophyton interdigitale modulate macrophage and keratinocyte functions. Front Immunol. Switzerland. 2018;9:2343.
Rodrigues ML, Djordjevic JT. Unravelling secretion in Cryptococcus neoformans: more than one way to skin a cat. Mycopathologia. 2012;173:407–18.
Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJ, Nosanchuk JD, et al. Biogenesis of extracellular vesicles in yeast: many questions with few answers. Commun Integr Biol. 2010;3:533–5.
Wolf JM, Casadevall A. Challenges posed by extracellular vesicles from eukaryotic microbes. Curr Opin Microbiol. Elsevier Ltd. 2014;22:73–8. Available from: https://doi.org/10.1016/j.mib.2014.09.012
Rodrigues ML, Franzen AJ, Nimrichter L, Miranda K. Vesicular mechanisms of traffic of fungal molecules to the extracellular space. Curr Opin Microbiol. Elsevier Ltd; 2013;16:414–20. Available from: https://doi.org/10.1016/j.mib.2013.04.002
Casadevall A, Nosanchuk JD, Williamson P, Rodrigues ML. Vesicular transport across the fungal cell wall. Trends Microbiol England. 2009;17:158–62.
Walker L, Sood P, Lenardon MD, Milne G, Olson J, Jensen G, et al. The viscoelastic properties of the fungal cell wall allow traffic of ambisome as intact liposome vesicles. Heitman Axel White, Theodore JB, editor. MBio. 2018;9:e02383–17 Available from: http://mbio.asm.org/content/9/1/e02383-17.abstract. Accessed 16 Mar 2019.
de Souza Pereira R, Geibel J. Direct observation of oxidative stress on the cell wall of Saccharomyces cerevisiae strains with atomic force microscopy. Mol Cell Biochem. 1999;201:17–24. Available from:. https://doi.org/10.1023/A:1007007704657.
Almeida F, Wolf JM, da Silva TA, DeLeon-Rodriguez CM, Rezende CP, Pessoni AM, et al. Galectin-3 impacts Cryptococcus neoformans infection through direct antifungal effects. Nat Commun. England. 2017;8:1968.
Diaz-Alvarez L, Ortega E. The many roles of galectin-3, a multifaceted molecule, in innate immune responses against pathogens. Mediat Inflamm. United States. 2017;2017:9247574.
Ruas LP, Bernardes ES, Fermino ML, de Oliveira LL, Hsu DK, Liu F-T, et al. Lack of galectin-3 drives response to Paracoccidioides brasiliensis toward a Th2-biased immunity. PLoS One. United States. 2009;4:e4519.
Linden JR, De Paepe ME, Laforce-Nesbitt SS, Bliss JM. Galectin-3 plays an important role in protection against disseminated candidiasis. Med Mycol. England. 2013;51:641–51.
Wu S-Y, Yu J-S, Liu F-T, Miaw S-C, Wu-Hsieh BA. Galectin-3 negatively regulates dendritic cell production of IL-23/IL-17-axis cytokines in infection by Histoplasma capsulatum. J Immunol United States. 2013;190:3427–37.
Joffe LS, Nimrichter L, Rodrigues ML, Del Poeta M. Potential roles of fungal extracellular vesicles during infection. mSphere. American Society for Microbiology. 2016;1:e00099–16. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27390779. Accessed 9 Nov 2016.
Rizzo J, Nimrichter L, Rodrigues ML. What is new? Recent knowledge on fungal extracellular vesicles. Curr Fungal Infect Rep. 2017;11:141–7.
Rodrigues ML, Nakayasu ES, Almeida IC, Nimrichter L. The impact of proteomics on the understanding of functions and biogenesis of fungal extracellular vesicles. J Proteomics. Elsevier B.V. 2014;97:177–86. Available from: https://doi.org/10.1016/j.jprot.2013.04.001
• Oliveira DL, Freire-de-Lima CG, Nosanchuk JD, Casadevall A, Rodrigues ML, Nimrichter L. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infect Immun. 2010;78:1601–9 First report associating fungal EVs with immune modulation of host cells.
• Huang S-H, Wu C-H, Chang YC, Kwon-Chung KJ, Brown RJ, Jong A. Cryptococcus neoformans-derived microvesicles enhance the pathogenesis of fungal brain infection. PLoS One. United States. 2012;7:e48570. First report associating EVs and fungal dissemination to the brain.
Yoneda A, Doering TL. A eukaryotic capsular polysaccharide is synthesized intracellularly and secreted via exocytosis. Mol Biol Cell United States. 2006;17:5131–40.
Kmetzsch L, Joffe LS, Staats CC, de Oliveira DL, Fonseca FL, Cordero RJB, et al. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol Microbiol. 2011;81:206–18.
Peres da Silva R, Martins S d T, Rizzo J, Dos Reis FCG, Joffe LS, Vainstein M. et al, Golgi reassembly and stacking protein (GRASP) participates in vesicle-mediated RNA export in Cryptococcus neoformans. Genes (Basel). Switzerland. 2018;9.
Coelho C, Bocca AL, Casadevall A. The tools for virulence of Cryptococcus neoformans. Adv Appl Microbiol United States. 2014;87:1–41.
Eisenman HC, Frases S, Nicola AM, Rodrigues ML, Casadevall A. Vesicle-associated melanization in Cryptococcus neoformans. Microbiology. 2009;155:3860–7.
•• Vargas G, Rocha JDB, Oliveira DL, Albuquerque PC, Frases S, Santos SS, et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell Microbiol. 2015;17:389–407. First report suggesting the potential use of fungal EVs as vaccine adjuvants.
Rizzo J, Albuquerque PC, Wolf JM, Nascimento R, Pereira MD, Nosanchuk JD, et al. Analysis of multiple components involved in the interaction between Cryptococcus neoformans and Acanthamoeba castellanii. Fungal Biol Netherlands. 2017;121:602–14.
da Silva TA, Roque-Barreira MC, Casadevall A, Almeida F. Extracellular vesicles from Paracoccidioides brasiliensis induced M1 polarization in vitro. Sci Rep. The Author(s); 2016;6:35867. Available from: https://doi.org/10.1038/srep35867.
Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell United States. 1980;21:205–15.
Chayakulkeeree M, Johnston SA, Oei JB, Lev S, Williamson PR, Wilson CF, et al. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol Microbiol. 2011;80:1088–101 Available from: https://www.ncbi.nlm.nih.gov/pubmed/21453402. Accesed 13 Mar 2019.
Panepinto J, Komperda K, Frases S, Park YD, Djordjevic JT, Casadevall A, et al. Sec6-dependent sorting of fungal extracellular exosomes and laccase of Cryptococcus neoformans. Mol Microbiol. 2009;71:1165–76.
Oliveira DL, Rizzo J, Joffe LS, Godinho RMC, Rodrigues ML. Where do they come from and where do they go: candidates for regulating extracellular vesicle formation in fungi. Int J Mol Sci. 2013;14:9581–603.
Hu G, Caza M, Cadieux B, Chan V, Liu V, Kronstad J. Cryptococcus neoformans requires the ESCRT protein Vps23 for iron acquisition from heme, for capsule formation, and for virulence. Infect Immun. 2013;81:292–302.
Godinho RMDC, Crestani J, Kmetzsch L, Araujo GDS, Frases S, Staats CC, et al. The vacuolar-sorting protein Snf7 is required for export of virulence determinants in members of the Cryptococcus neoformans complex. Sci Rep. 2014;4:6198. Available from: https://www.nature.com/articles/srep06198.
Hu G, Kronstad JW. A putative P-type ATPase, Apt1, is involved in stress tolerance and virulence in Cryptococcus neoformans. Eukaryot Cell. 2010;9:74–83.
Rizzo J, Oliveira DL, Joffe LS, Hu G, Gazos-Lopes F, Fonseca FL, et al. Role of the Apt1 protein in polysaccharide secretion by Cryptococcus neoformans. Eukaryot Cell. 2014;13:715–26.
Wolf JM, Espadas J, Luque-Garcia J, Reynolds T, Casadevall A. Lipid biosynthetic genes affect Candida albicans extracellular vesicle morphology, cargo, and immunostimulatory properties. Eukaryot Cell. 2015;14:745–54. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4519749&tool=pmcentrez&rendertype=abstract. Accessed 27 Feb 2019.
García-Rodas R, Cordero RJB, Trevijano-Contador N, Janbon G, Moyrand F, Casadevall A, et al. Capsule growth in <span class="named-content genus-species" id="named-content-1">Cryptococcus neoformans</span> is coordinated with cell cycle progression. Heitman J, editor. MBio. 2014;5:e00945–14 Available from: http://mbio.asm.org/content/5/3/e00945-14.abstract. Accessed 26 Feb 2015.
Robertson EJ, Wolf JM, Casadevall A. EDTA inhibits biofilm formation, extracellular vesicular secretion, and shedding of the capsular polysaccharide glucuronoxylomannan by Cryptococcus neoformans. Appl Environ Microbiol United States. 2012;78:7977–84.
•• Zarnowski R, Sanchez H, Covelli AS, Dominguez E, Jaromin A, Bernhardt J, et al. Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis. PLoS Biol. United States. 2018;16:e2006872. First report associating fungal EVs with biofilm formation and antifungal resistance.
Kmetzsch L, Joffe LS, Staats CC, de Oliveira DL, Fonseca FL, Cordero RJB, et al. Role for Golgi reassembly and stacking protein (GRASP) in polysaccharide secretion and fungal virulence. Mol Microbiol England. 2011;81:206–18.
•• Reis FCG, Borges BS, Jozefowicz LJ, Sena BAG, Garcia AWA, Medeiros LC, et al. A novel protocol for the isolation of fungal extracellular vesicles reveals the participation of a putative scramblase in polysaccharide export and capsule construction in <em>Cryptococcus gattii</em> Mitchell AP, editor. mSphere. 2019;4:e00080–19 Available from: http://msphere.asm.org/content/4/2/e00080-19.abstract. Accessed 06 Apr 2019. Novel and more efficient protocol for fungal EV isolation.
•• Peres da Silva R, Puccia R, Rodrigues ML, Oliveira DL, Joffe LS, César GV, et al. Extracellular vesicle-mediated export of fungal RNA. Sci Rep. 2015;5:7763. Available from: http://www.nature.com/srep/2015/150114/srep07763/full/srep07763.html. Accessed 13 Feb 2015. First report describing the presence of RNA within fungal EVs.
Cai Q, Qiao L, Wang M, He B, Lin F-M, Palmquist J, et al. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science United States. 2018;360:1126–9.
Gil-Bona A, Llama-Palacios A, Parra CM, Vivanco F, Nombela C, Monteoliva L, et al. Proteomics unravels extracellular vesicles as carriers of classical cytoplasmic proteins in Candida albicans. J Proteome Res. 2015;14:142–53.
Alves LR, da Silva RP, Sanchez DA, Zamith-Miranda D, Rodrigues ML, Goldenberg S, et al. Extracellular vesicle-mediated RNA release in <em>Histoplasma capsulatum</em> bioRxiv. 2019;570291. https://doi.org/10.1128/mSphere.00176-19.
Rayner S, Bruhn S, Vallhov H, Andersson A, Billmyre RB, Scheynius A. Identification of small RNAs in extracellular vesicles from the commensal yeast Malassezia sympodialis. Sci Rep. The Author(s). 2017;7:39742. Available from: https://doi.org/10.1038/srep39742.
Vallejo MC, Matsuo AL, Ganiko L, Medeiros LCS, Miranda K, Silva LS, et al. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic alpha-galactosyl epitopes. Eukaryot Cell United States. 2011;10:343–51.
Vallejo MC, Nakayasu ES, Matsuo AL, Sobreira TJP, Longo LVG, Ganiko L, et al. Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J Proteome Res. American Chemical Society. 2012;11:1676–85. Available from: https://doi.org/10.1021/pr200872s.
da Silva RP, Heiss C, Black I, Azadi P, Gerlach JQ, Travassos LR, et al. Extracellular vesicles from Paracoccidioides pathogenic species transport polysaccharide and expose ligands for DC-SIGN receptors. Sci Rep. The Author(s). 2015;5:14213. Available from: https://doi.org/10.1038/srep14213.
Brown L, Wolf JM, Prados-Rosales R, Casadevall A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat Rev Microbiol. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved. 2015;13:620. Available from: https://doi.org/10.1038/nrmicro3480.
Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev United States. 2018;118:1917–50.
Rodrigues ML, Oliveira DL, Vargas G, Girard-Dias W, Franzen AJ, Frases S, et al. Analysis of yeast extracellular vesicles. Methods Mol Biol United States. 2016;1459:175–90.
Wolf JM, Espadas-Moreno J, Luque-Garcia JL, Casadevall A. Interaction of cryptococcus neoformans extracellular vesicles with the Cell Wall. Eukaryot Cell. 2014;13:1484–93.
Zamith-Miranda D, Nimrichter L, Rodrigues ML, Nosanchuk JD. Fungal extracellular vesicles: modulating host-pathogen interactions by both the fungus and the host. Microbes Infect France. 2018;20:501–4.
Westphal M, Lamszus K. Circulating biomarkers for gliomas. Nat Rev Neurol. Nature Publishing Group. 2015;11:556–66. Available from: https://doi.org/10.1038/nrneurol.2015.171.
Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev United States. 2014;27:490–526.
Tan K, Li R, Huang X, Liu Q. Outer membrane vesicles: current status and future direction of these novel vaccine adjuvants. Front Microbiol. Frontiers Media S.A. 2018;9:783. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29755431. Accessed 14 Mar 2019.
van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015/11/11. WILEY-VCH Verlag; 2015;10:1689–706. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26912077. Accessed 14 Mar 2019.
Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. Elsevier. 2017;390:1603–10. Available from: https://doi.org/10.1016/S0140-6736(17)31449-6.
Rizzo J, Colombo AC, Zamith-Miranda D, Silva VKA, Allegood JC, Casadevall A, et al. The putative flippase Apt1 is required for intracellular membrane architecture and biosynthesis of polysaccharide and lipids in Cryptococcus neoformans. Biochim Biophys Acta, Mol Cell Res Netherlands. 1865;2018:532–41.
Gangadaran P, Hong CM, Ahn B-C. An update on in vivo imaging of extracellular vesicles as drug delivery vehicles. Front Pharmacol. Frontiers Media S.A. 2018;9:169. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29541030. Accessed 23 Jan 2019.
Funding
VKAS is supported by Instituto Oswaldo Cruz (IOC)/Fundação Oswaldo Cruz (FIOCRUZ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, grant: 88881.188560/2018-01). MLR is supported by grants from the Brazilian agency Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq (grants 405520/2018-2, 440015/2018-9, and 301304/2017-3) and Fiocruz (grants VPPCB-007-FIO-18 and VPPIS-001-FIO18). The authors also acknowledge support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Finance Code 001) and the Instituto Nacional de Ciência e Tecnologia de Inovação em Doenças de Populações Negligenciadas (INCT-IDPN). MLR is currently on leave from the position of Associate Professor at the Microbiology Institute of the Federal University of Rio de Janeiro, Brazil. RCM is supported by Lister Institute for Preventive Medicine and the European Research Council under the European Union’s Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement No. 614562 and from the Biotechnology and Biological Sciences Research Council (BBSRC) via grant BB/R008485/1. RCM is additionally supported by a Wolfson Royal Society Research Merit Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Mycology
Rights and permissions
About this article
Cite this article
Silva, V.K.A., Rodrigues, M.L. & May, R.C. Deciphering Fungal Extracellular Vesicles: From Cell Biology to Pathogenesis. Curr Clin Micro Rpt 6, 89–97 (2019). https://doi.org/10.1007/s40588-019-00128-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-019-00128-1