Abstract
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic and multifactorial diseases that affect the intestinal tract, both characterized by recurrent inflammation of the intestinal mucosa, resulting in abdominal pain, diarrhea, vomiting and, rectal bleeding. Inflammatory bowel diseases (IBD) regroup these two disorders. The exact pathological mechanism of IBD remains ambiguous and poorly known. In genetically predisposed patients, defects in intestinal mucosal barrier are due to an uncontrolled inflammatory response to normal flora. In addition to the genetic predisposition, these defects could be triggered by environmental factors or by a specific lifestyle which is widely accepted as etiological hypothesis. The involvement of the CD40/CD40L platelet complex in the development of IBD has been overwhelmingly demonstrated. CD40L is climacteric in cell signalling in innate and adaptive immunity, the CD40L expression on the platelet cell surface gives them an immunological competence. The IL-1, a major inflammation mediator could be involved in different ways in the development of IBD. Here, we provide a comprehensive review regarding the role of platelet CD40/CD40L in the pathophysiological effect of IL-1 in the development of Crohn’s disease (CD). This review could potentially help future approaches aiming to target these two pathways for therapeutic purposes and elucidate the immunological mechanisms driving gut inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Crohn's disease: IBD model and generalities
Crohn's disease (CD) is an inflammatory, chronic, and multifactorial disease that affects the digestive system, mainly characterized by recurrent inflammation of the intestinal mucosa causing the loss of physiological functions of the intestine (Voudoukis et al. 2014). Crohn's disease manifests an alternation between acute episodes of abdominal pain, diarrhea and rectal bleeding interspersed with periods of remission with variable durations depending on the clinical state of the patient. In this review we will focus on Crohn's disease (CD) as a model of IBD. The cause of this pathology is still poorly known, the studies explain the appearance of this pathological phenotype by the interaction of multiple parameters: The genetic predisposition, the disruption of intestinal flora, immune dysfunction, and environmental promotion (Senhaji et al. 2015). Researchers have classified the various phenotypes of CD, clinically, molecularly, and serologically in order to facilitate diagnosis of the disease and especially to target the choice of treatment for each disease subtype (Cotton 1971). This, is illustrated through the Vienna classification which was established in 1998, and relies on three important parameters: age of onset, location and behavior of the disease. This classification was reviewed during the world gastroenterology congress held at Montreal in 2005 (Cotton 1971; Gasche et al. 2000; Silverberg et al. 2005).
The genetic contribution was clearly demonstrated (Rachakonda et al. 2018; Ogrinc Wagner et al. 2019). The GWAS (Genome Wide Association Studies) established the association of 163 loci (with more than 300 genes) susceptibility to IBD (Jostins et al. 2012). More recently, a first cross-ethnic GWAS identified 38 new loci associated with IBD, increasing the number of loci at risk to 200 loci (Liu et al. 2015).
CARD15 (Caspase recruitment domain-containing protein 15) or NOD2 was the first gene associated with IBD (Gaya et al. 2006). The latter is located on chromosome 16 and allows (with NOD1) recognition and destruction of intracellular bacteria (Philpott et al. 2014), thus promoting the immune response of the body by activation of the NF‐κB pathway and the overexpression of multiple pro-inflammatory molecules (Lappas 2013; Dos Santos et al. 2017). Mutations of NOD2 have been associated with CD (Girardin et al. 2003; Schaefer et al. 2017), but are not sufficient and do not automatically induce the sick phenotype. Studies have shown that patients with CD have an activation of the Th1, Th17 cytokines such as IL-12, IL-17, IL-23, IL-27 and INF-γ, and also a very important cellular infiltration such as macrophages, B and T lymphocytes (Cho 2008; Cosnes et al. 2011). A recent study demonstrated that macrophages from CD40L-deficient patients lack fungicidal activity with decreased oxidative burst in vitro. Additionally, macrophages have reduced cytokine production, which could be reversed with addition of exogenous recombinant INF-gamma. Transcriptome analysis revealed differential regulation of genes in macrophages from CD40L-deficient patients, with 48 downregulated and 61 upregulated genes versus macrophages from healthy volunteers (Cabral-Marques et al. 2017). The defect of the intestinal immune system induces an imbalance in the physiological functions of the intestine or their loss (Senhaji et al. 2015).
In IBD, there is an increased risk of thromboembolic events due to inflammation, nutritional deficiencies, hospitalizations, surgery and inherited prothrombotic factors (Magro et al. 2014). Based on previous studies that have clearly demonstrated the involvement of platelet CD40/CD40L complex in inflammatory mechanisms, and that have identified IL-1 as a biomarker—among others—for the detection of intestinal inflammation (Senhaji et al. 2015; Kim et al. 2017), we provide a short review over these observations. The present review summarizes the pathological role of IL-1 in the development of Crohn's disease and then focus on the contribution of CD40/CD40L complex in this inflammatory disease.
Interleukin 1: pro-inflammatory cytokine prototype
Interleukin 1 (IL-1) is a soluble regulatory factor of the immune system considered as the prototype of pro-inflammatory cytokines (Dinarello 1997). In 1972 Horton et al. discovered this low molecular weight (17 kD) cytokine by dint of its proliferative action on murine thymocytes as well as its stimulation of bone reabsorption in vitro, hence its former name: Osteoclast-activating-factor (OAF) (Jandinski 1988). Later in 1984, the cloning of the IL-1 gene allowed the assignment of several functions to this cytokine and the identification of the IL-1 receptor ‘natural antagonist’ (IL-1Ra) which inhibits the activity of both active forms IL-1α and IL-1β (Dinarello 1997). Eleven members of the (IL-1) family have been identified, there are 7 agonist molecules (IL-1α, IL-1β, IL-18, IL-33, IL-36α, β and γ), and three receptor antagonists (IL-1Ra, IL-36Ra and IL-38) (Garlanda et al. 2013). The most studied are: IL-1α, IL-1β, IL-1ra and IL-18 and are secreted mainly by monocytes, macrophages and dendritic cells (Garlanda et al. 2013; Corrigendum 2015), in addition to platelets, fibroblasts, and epithelial cells (Dinarello et al. 2012; Garlanda et al. 2013; Zaid et al. 2020). Several mechanisms of secretion of the IL-1 by macrophages have been described (Hu et al. 1988; Monteleone et al. 2015). For instance, IL-1β is produced as an inactive 13 kD precursor within the cytosol where it undergoes cleavage in position Asp116 by IL-1beta conversion enzyme (ICE) recently called cysteine protease or caspase-1 (Delaleu and Bickel 2004; Dinarello et al. 2012). The IL-1β activation requires prior activation by the inflammasome following stimulation of the immune system by infections (Garlanda et al. 2013).
A number of studies providing data on the effect of IL-1β deficiency on the development of IBD have focused on the relation of the NLRP3 inflammasome to intestinal inflammation. or the effect of IL-1β deletion or inactivation on the development of intestinal inflammation. One of the first studies exploring the role of NLRP3 in intestinal inflammation were those of Bauer et al. who found that mice lacking NLRP3 (and thus exhibiting decreased IL-1β secretion) were characterized by decreased DSS-colitis and TNBS-colitis compared to control mice (Bauer et al. 2010, 2012).
In addition, it has been shown that DSS-colitis was more severe in the absence of inflammasome function and IL-1β production (Hirota et al. 2011). In this study, NLRP3 deficiency was associated with reduced levels of regulatory cytokines, IL-10 and TGF-β in contrast to the study of Bauer et al. described above in which NLRP3 deficiency was associated with reduced inflammation and increase numbers of tolerogenic dendritic cells (Bauer et al. 2010; Mao et al. 2018).
The pro-inflammatory cytokines IL-1α and IL-1β have similar structures, and are encoded by distinct genes located on chromosome 2. They bind to the same receptors (Sims and Smith 2010; Garlanda et al. 2013), inducing the activation of different signaling pathways such as JNK, p38-MAPK and NF‐κB as the major activated pathway (Dinarello 2011; Garlanda et al. 2013). The activation of these signaling pathways, mainly generates induction and regulation of several genes involved in inflammatory response by increasing the expression of adhesion molecules, thus, IL-1 is involved in the development of inflammatory diseases including CD (Dinarello 2011), and considered to be a major mediator of inflammation (Gabay et al. 2010). The IL-1 pathophysiological mechanism will be detailed in the fourth chapter of this review. In addition to its pro-inflammatory role, studies have associated IL-1 with other biological processes such as embryonic development, bone reabsorption, angiogenesis in colon cancer cells, as well as its association with several pathologies, mainly of inflammatory nature (Dinarello 1997, 2004; Delaleu and Bickel 2004; Dinarello et al. 2012).
Platelet CD40/CD40L complex association
Impaired platelet activation may cause persistent mucosal inflammation through P-selectin, CD40-CD40L and other systems influencing granulocytes, macrophages or endothelial cells (Chen et al. 2015). Due to its involvement in various physiological and pathological processes, its easy detection in plasma, and its abundance and variable expression, the platelet CD40/CD40L complex association has been of particular interest (Callard et al. 1993; Grewal and Flavell 1998; Lee and Koretzky 1998; van Kooten and Banchereau 2000; Urbich et al. 2002; Stokes et al. 2009; Elgueta et al. 2009; Lievens et al. 2009; Zhang et al. 2013).
Recently, the studies have multiplied to understand the type of interaction with its CD40 membrane receptor and the different consequences of this interaction. In this work, we highlighted the effect of this interaction in the onset and evolution of CD, we also present the therapeutic possibilities that target this point of cellular communication (CD40/CD40L).
Formerly called CD154, gp39, TBAM or TRAP, CD40L is an immunomodulatory 33 kDa type II transmembrane glycoprotein, member of the tumor necrosis factor superfamily (TNF). Its coding gene (CD40G) is located on the long arm of the chromosome X in position q26.3-q27.1 (van Kooten and Banchereau 2000; Alaaeddine et al. 2012). CD40G transcript consists of 261 amino acids: 22 of which form the cytoplasmic component, 24 are transmembrane amino acids, while the extracellular fraction consists of 215 amino acids (van Kooten and Banchereau 2000; Schonbeck et al. 2000). Ion exchange between the ligand basic chain loaded residues and the receptor acid chains stabilizes the CD40/CD40L interaction (An et al. 2011).
It has been shown that CD40L binds to αIIbβ3 and stabilizes arterial thrombi in mice (Andre et al. 2002). Additionally, other studies have shown that CD40L can induce platelet activation and secretion of reactive oxygen species and the chemokine RANTES through binding to CD40 (Inwald et al. 2003; Danese et al. 2004; Chakrabarti et al. 2005; Bou Khzam et al. 2013b). The CD40L can also stimulate the pro-angiogenic function of peripheral blood angiogenic outgrowth cells via increased release of matrix metalloproteinase-9 (Bou Khzam et al. 2013a). Another study investigating the role of CD40L in platelets revealed that sCD40L primes and enhances agonist-induced activation and aggregation of human platelets, through a CD40-mediated tumor necrosis factor receptor–associated factor (TRAF)-2/Rac1/p38 mitogen-activated protein kinase (MAPK)–dependent pathway, which ultimately leads to platelet shape change and actin polymerization (Yacoub et al. 2010). The authors of the same study showed that enhanced levels of sCD40L exacerbate thrombus formation and leukocyte infiltration in response to vascular injury, in a CD40-dependent manner (Yacoub et al. 2010). Recently, Kojok et al. have shown that CD40L primes platelets via signaling pathways involving CD40/transforming growth factor‐β‐activated kinase 1/NF‐κB, which predisposes platelets to enhanced activation and aggregation in response to thrombotic stimuli (Kojok et al. 2018).
The cellular expression of CD40L is variable depending on the cell type and condition, while being highly expressed on the surface of T-cells and activated platelets, CD40L is moderately expressed on B-cells and dendritic cells, weakly expressed on the membranes of inactive macrophages, neutrophils and endothelial cells, and most likely to be silenced or undetectable in and inactivated platelets (Hibi and Ogata 2006; Lievens et al. 2009). Significant variability in the clinical evolution and phenotype of CD40L deficiency was also shown in a case study (Gunaydin et al. 2014). Similarly to its ligand, the CD40 receptor is a membrane glycoprotein (48 kDa) of type I, belonging to the TNFR receptor superfamily (Locksley et al. 2001). The encoding gene is located at the long arm of chromosome 20 in position 2q12-q13.2 (Lafage-Pochitaloff et al. 1994). The CD40 phosphoprotein counts 277 amino acids with a well-defined conformation playing an important role in the fixation of CD40L (Braesch-Andersen et al. 1989; Naismith and Sprang 1998; Singh et al. 1998). CD40 consists of an extracellular domain of 22 cysteine residues, a signal peptide, two potential N-linked glycosylation sites, and cytoplasmic fraction with a central region, which allows its membrane anchorage (Braesch-Andersen et al. 1989; Naismith and Sprang 1998; Singh et al. 1998). The results of this study showed that vitamin D administration in mild-to-moderate UC patients led to the downregulation of the CD40L gene (Sharifi et al. 2020). The activation of CD40L after binding to its receptor may induce its cleavage at methionine 113 of the extracellular domain (Zirlik et al. 2007) and release of another form of CD40L: soluble CD40L (sCD40L) (Zirlik et al. 2007; Zhang et al. 2013). It is a truncated protein of 240 amino acids with two possible isoforms (Aloui et al. 2014) whose functionality is still unknown (Naismith and Sprang 1998). sCD40L has a cytokine activity and binds to CD40 in trimeric form thus inducing biological responses (Anand et al. 2003; Zhang et al. 2013). It should be taking in consideration that the soluble form of CD40L in blood is mainly produced by activated platelets and in turn activates its producer cells (activated platelets), across an auto-amplification platelet activation loop (Aloui et al. 2014).
The involvement of sCD40L in pathology has been well documented (Fanslow et al. 1994; Aloui et al. 2014), it has been extensively studied in inflammatory and autoimmune responses (van Kooten and Banchereau 2000; Dejica and Manea 2006; Antoniades et al. 2009; Alaaeddine et al. 2012). In addition to a pivotal role in humoral immunity, the importance of this pathway is evident in cell-mediated immunity with several molecules in the clinic aimed at modulating this pathway (Karnell et al. 2019). Both forms (membrane and soluble) bind to several receptors other than the CD40 such as the integrins αIIbβ3, αMβ2, and α5β1 (Andre et al. 2002; Zirlik et al. 2007).
The platelet’s CD40L expression was demonstrated for the first time in 1998 (Elgueta et al. 2009), which impressed immunologists, ever since CD40L was known to be expressed only in immunoregulatory cells. The platelets were considered as cells responsible for coagulation and maintenance of hemostasis without any immune role (Delmas et al. 2005; Aloui et al. 2014). On the other hand, the platelet count could be used as a predictor of relapse in UC patients while increased activation status of platelets occur in the pathogenesis of several immune mediated inflammatory diseases (Pankratz et al. 2016; Nakarai et al. 2018). Patients with IBD show enhanced in vivo thromboxane-dependent platelet activation and lipid peroxidation (Di Sabatino et al. 2016). Several studies have been devoted to understanding this unexpected platelet expression, which identified the presence of CD40L in the alpha granules of platelets (Charafeddine et al. 2012). However, the biosynthesis mechanisms were problematic, because platelets are anucleate cells and do not express CD40L mRNAs in their cytoplasm (Gnatenko et al. 2003; Nagalla et al. 2011; Rowley et al. 2011; Simon et al. 2014). The later eliminates the hypothesis of immunologists who suggested that platelet CD40L production is most likely due to a retrotranscription mechanism as is the case for other platelet cytokines (production in novo by activated platelets) (Denis et al. 2005; Nurden et al. 2008; Garraud et al. 2011; Ple et al. 2012). platelet CD40L was finally considered a preformed protein prior to platelet fragmentation, synthesized by megakaryocytes and stored in platelet alpha granules (Rendu and Brohard-Bohn 2001; Flaumenhaft 2003; Blair and Flaumenhaft 2009). Besides, a study had reported the presence of elevated levels of sCD40L in the circulation of CD and UC patients, and had demonstrated that sCD40L originates primarily from platelets likely activated in the IBD microvascular bed (Danese et al. 2003). Furthermore, serum sCD40 could potentially be investigated as a marker in UC (Lampinen et al. 2019). The importance of these observations resides with the potent and far reaching biological activities triggered by activation of the CD40 pathway in a wide panel of immune and non-immune cells types (van Kooten and Banchereau 2000).
In response to a given external stimulus, platelets get activated by well-studied mechanisms, they then release the granule content (platelet degranulation) and secrete alpha granules in soluble form or export them to the membrane in fixed form which leads to expression of platelet CD40L by fusion of these alpha granules (containing CD40L) to the membrane (Aloui et al. 2014). Finally when disabled, the platelets will no longer display this ligand; then the CD40L will be cleaved and released in its soluble form in the blood (Leroyer et al. 2008), making the platelets the major source of plasma sCD40L. The identification of the platelet’s CD40L has contributed significantly to the understanding of platelet biology, first from a hematological aspect especially through their role in platelet thrombus stabilisation. The progress in the immunological understanding of CD40L has attributed immunological competence to platelets, these cells intervene in the induction of an inflammatory reaction by their interaction with cells expressing CD40 on their surface (Alaaeddine et al. 2012), making a strong link between platelets and other cells of the immune system. This cellular interaction promotes the maturation of dendritic cells known as the main antigen presenting cell (APC) and contributes to isotopic switching, LB maturation and antibody production (Henn et al. 2001; Elzey et al. 2003, 2005; Cognasse et al. 2007), other studies (in vivo and in vitro) have shown the orientation of the immune system towards a CD8 response in several models following the interaction of platelets with immunoreactive cells (Henn et al. 2001; Elzey et al. 2003, 2005; Cognasse et al. 2007). In addition, the CD40L/CD40 interaction induces the expression of several pro-inflammatory and pro-thrombotic genes (IL-1, IL-6, MCP-1, IL-8, IL-12, INF γ, TNFα) (Zhang et al. 2013). All these data support the idea that platelets intervene strongly during immune responses because of the CD40/CD40L axis in physiological state but also in pathophysiology. Platelets are considered critical factors in the pathological behavior of liver inflammation, and considered as risk factors for venous thrombosis (Chauhan and Adams 2017). The association of their sCD40L product with pathological condition during Behcet's disease has recently been demonstrated (Perazzio et al. 2017) as well as in transfusion risks (Aloui et al. 2014). Several studies have associated these cells with inflammatory and autoimmune states, CD has been chosen in this review to explain the involvement of platelets in inflammation and autoimmunity through the CD40/CD40L axis.
Pathophysiological mechanism of Crohn's disease: intervention of the two major elements (platelet CD40/CD40L and IL-1β)
In 1968, Morowitz et al. evaluated the concentration of circulating thrombocytes in active phase IBD patients (Morowitz et al. 1968), they found that the concentration of these cells was higher when compared to healthy subjects. Although findings raise the possibility of using antiplatelet therapy in humans with ulcerative colitis (Petrovic et al. 2020) supporting other results considering platelet-activating receptor (PAFR) as an important regulator of liver inflammation during colitis (Liu et al. 2020). Other authors consider PAFR is a microbial sensor regulating local inflammasome responses (Liu et al. 2019). Therefore, the thrombocytosis at inflammatory sites coupled along with significant recruitment of macrophages, monocytes, and infiltration of other immune cells was described for the first time (Voudoukis et al. 2014). The question about the platelet’s contribution to the amplification of the inflammatory and autoimmune conditions observed in patients with CD will be meticulously detailed in this chapter.
During inflammation, the over expression of the CD40L molecule on the platelet surface is detectable by flow cytometry (Delmas et al. 2005), while it remains weak in platelets at rest (1–5 ng of CD40L in 108 activated platelets, about 600 to 1000 molecules of CD40L per activated platelet) (Delmas et al. 2005). The ligand (CD40L) is therefore released only by activated platelets in patients with Crohn's disease (Ripoche 2011; Vatn and Sandvik 2015). Plasma levels of sCD40L were also reported to be significantly increased in CD patients when compared to normal controls (Ludwiczek et al. 2003). It is now established that this pro-inflammatory signaling molecule is largely expressed and excreted by activated platelets during inflammation, especially in the case of IBD. The platelet activation is rapidly induced by contact with an injured vascular wall, thrombocytes then change their shape, develop receptors for different cytokines (Gachet 2013; Tekelioglu et al. 2014), degranulate and release their rich content in cytokines, growth factors, coagulation factors, and biologically active molecules inducing hemostasis and enhancing inflammation at the site of the endothelial lesion (Gachet 2013; Voudoukis et al. 2014; Saluk et al. 2014). Although a recent study reported that the serum levels of sCD40L should not be considered as clinical markers of IBD activity (Cibor et al. 2020), other studies have suggested that they could be considered as potential indicators of several IBD-related conditions (Danese et al. 2004; Koutroubakis et al. 2004). Moreover, both CD40L and CD40 have been reported to be increased in both the circulation and the gut mucosa (Karnell et al. 2019). Furthermore, in the inflamed bowel, CD40 is strongly over expressed in both endothelial and mesenchymal cells within the mucosa and submucosa. It has been demonstrated that knocking out CD40 attenuates the effects of microcystin-leucine arginine in mice with pre-existing colitis by decreasing the severity of weight loss, allowing a full recovery in bloody stools, preventing the exacerbation of colonic shortening, colonic ulceration, and preventing the upregulation of the pro-inflammatory and pro-fibrotic cytokines IL-1β, MCP-1, and PAI-1 (Su et al. 2020). In addition, CD40 expression has been shown to be associated with UC clinical activity (Kaminska et al. 2015; Li et al. 2015; Karnell et al. 2019). According to Tekelioglu et al., the strong expression of P-selectin (CD62P) could be an important criterion for CD onset trough thrombocytes by stimulating the secretion of various inflammatory substances including Thromboglobulin, Fibrinogen, Platelet Factor 4, IL-1β, and CD40L (Tekelioglu et al. 2014). Studies have shown that all the analyzed endothelial cells significantly expressed CD40 on their surface following various inflammatory signals, including TNF alpha, INF gamma, and IL-1 (Delmas et al. 2005). Furthermore, constitutive activation of CD40 in DCs results in inflammation of the gastrointestinal tract, thereby impairing lipid uptake, which consequently results in attenuated atherosclerosis (Kusters et al. 2017).
Figure 1 shows some platelet modifications after their activation. The sudden increase in the expression level of CD40 or its ligand CD154 in the intestinal mucosa (Liu et al. 1999; Polese et al. 2002) induces abnormal signaling that contributes to the initiation of the autoimmune and inflammatory condition in patients with CD (Yacoub et al. 2010). Activated platelet’s CD40L interacts with its receptor on the surface of endothelial cells (Henn et al. 1998; Senhaji et al. 2015). This platelet-endothelial cell interaction results in increased expression of different adhesion molecules: ICAM-1, VCAM-1, E-selectins, P-selectins and stimulates the secretion of pro-inflammatory cytokines IL-1, IL-6, IL -8, and MCP -1 (Bavendiek et al. 2002; Rizvi et al. 2008; Voudoukis et al. 2014; Saluk et al. 2014), which promotes the increased recruitment of monocytes, cytotoxic T lymphocytes, NK, and other immune cells at inflammatory sites. There are also results that support a possibility that IL-35 could be used to suppress active CD in a clinical setting (Zhao et al. 2020). In the presence of a favorable cytokine environment, recruited T lymphocytes differentiate into Th17 and exert their abrasion effect by releasing IL-17 in the inflammatory site (Bavendiek et al. 2002; Hibi and Ogata 2006; Rizvi et al. 2008; Saluk et al. 2014). Earlier this year, the modulation of T cell signaling pathway by the SAA was suggested to be an attractive target for anti-inflammatory therapies (Lee et al. 2020). On the other hand, a cohort study by Britton et al. on 30 human microbiotas from healthy donors and patients with IBD highlighted the impact on intestinal Th17 and RORγt + regulatory T cell compartments as a unifying feature of IBD microbiotas, suggesting a general mechanism for microbial contribution to IBD pathogenesis (Britton et al. 2019). The Th1 type response in these patients leads to an intestinal mucosa tissue rupture (Yacoub et al. 2010; Senhaji et al. 2015), in addition to an increased NK activation in the inflamed mucosa (Hibi and Ogata 2006). All these observations explain the role of the platelet axis CD40/CD40L and its amplification by platelet IL-β in the inflammatory and autoimmunity responses.
Schematic representation of a wafer at rest (A) and an activated platelet (B) showing two biological phenomena triggered by platelet activation (a/b) and the platelet activation loop induced by the CD40 axis/CD40L (1,2,3) a: Membrane expression of CD40L at an activated platelet. a Membrane externalization of CD40L during platelet activation and exocytosis of the granular content stored at rest in α granules (1). The platelets also release a soluble form of CD40L by cleaving mCD40L at the end of platelet activation (2) capable of binding to the CD40 receptor and initiating the series of looped biological responses (3). The sCD40L can also be fixed on other receivers (αiibβ3 among others) b synthesis of active IL-1β by an activated platelet. From an inactive precursor located in the cytoplasm, the active platelet synthesizes active IL-1β using caspase 1. IL-1β secreted activates the looped platelet
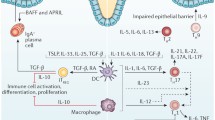
Figure 2 illustrates the interaction between the CD40/CD40L platelet axis and platelet IL-1β in the development of Crohn's disease. In a clinical study, most patients with CD responded positively to treatment with a specific CD40L antagonist (ch5D12), while the remaining patients entered remission phase (Kasran et al. 2005).
Representative schema of the cooperative role between platelet IL-1β and platelet CD40L in the development of Crohn's disease. a The TIR domain of each cytosolic tail of IL-1R recruits a set of proteases present in the cytoplasm (TRAF6 IKK IRAKS MyD88 …), which triggers a complex sequence of platelet cytosolic reactions, phosphorylation and combinatorial ubiquitination inducing the activation of different signaling pathways (NF‐κB, MAPK..) and the expression of several inflammatory molecules, including IL-1β. IL-1β is inactive as a 13 kD precursor in the lysosomes, in which it undergoes cleavage in the Asp116 position by the IL-1beta (ICE) converting enzyme or caspase-1, which is in turn activated by inflammasome. IL-1β formed is secreted into the extracellular medium by exocytosis, and in turn stimulates the platelet loop. Endothelial cells stimulated by IL-1β (among others) on expresses CD40 at their surface thus promoting interaction with platelet CD40L. b Following the CD40/CD40L interaction, several signaling pathways are triggered at the epithelial cells (dependent and independent of TRAF). Which allows the recruitment of specific molecules to the cytoplasmic tail of the CD40 epithelial receptor. Activation of the endothelium by already activated platelets (CD40L) induces specific cellular responses, mainly the release of inflammatory cytokines (IL-1, CCL5, TNF, …) and promotes the recruitment of immune cells to the inflammatory site. stimulating a strong expression of adhesion molecules on endothelial cells, hence the contribution of the CD40/CD40L platelet axis in the induction of inflammation in CD patients C During inflammation in CD patients, the level of chemokines increases at the inflammatory site, which promotes cellular infiltration at this level. Recruited LTs proliferate and differentiate into Th17 and Th1 in response to citokinic media signals, with remarkable activation of NK (natural killer), thus explaining the tissue destruction seen in CD patients, hence the autoimmune function of platelet axis CD40/CD40L
Therapeutic targets: new data
The multifactorial pathology of CD requires a deep understanding of its immunopathogenesis in the development of new therapies development of new therapies requires a good understanding of the CD (Shih and Targan 2008; Zhang and Li 2014; Senhaji et al. 2015). Researchers are increasingly interested in the study of the components involved in this disease (Torres and Rios 2008; Fava and Danese 2011; Zhang and Li 2014) to expand the therapeutic repertoire and develop effective and beneficial treatment strategies (Torres and Rios 2008; Kurti et al. 2018; Katsanos et al. 2018; Louis 2018). The increased inflammation and excessive immune responses in patient’s intestinal mucosa (Shih and Targan 2008; Zhang and Li 2014), were widely studied and very well documented (Xu et al. 2014; Kaistha and Levine 2014). The current therapeutic strategies mainly target modulation of the intestinal immune response to reduce the inflammatory state and regulate the balance of different immune responses at affected areas (Moss 2015; Ahluwalia et al. 2018). On the other hand, healthy lifestyle intervenes remarkably in patient’s improvement during treatment period (Tuvlin et al. 2007; Swanson et al. 2010; de Souza and Fiocchi 2016). During the flare phases, the patient's body absorbs less nutrients and the intestinal mucosa is strongly irritated (Zhang and Li 2014; Cohen et al. 2014), hence contributes to the apparent symptoms in patients. For this reason, researchers and nutritionists suggest an adequate diet during the active phases (Riordan et al. 1998; Zallot et al. 2013; de Silva et al. 2014; Cohen et al. 2014; Owczarek et al. 2016) in order to prevent the inflamed mucous membranes from becoming more irritated and relief the painful symptoms (de Silva et al. 2014; Cohen et al. 2014). In addition to therapeutics a supportive diet including injecting intravenously nutritional solutions (supplements of vitamin complexes, minerals, etc.), is advised to avoid undernutrition (Jorgensen et al. 2013; Farrukh and Mayberry 2014; Cohen et al. 2014; Owczarek et al. 2016).
The conventional principle for treating IBD is based on its development modality, which is characterized by the alternation between activation or crisis phases and remission phases (Wallace et al. 2014; Senhaji et al. 2015). To have a remarkable therapeutic efficiency and ensure good quality of life for patients, current treatments aim to induce and maintain the remission phase, to heal the irritated mucosa and to prevent long-term complications (Sandborn 2016; Hanauer 2017). Treatment should be carefully selected because of the wide variety of existing therapeutic strategies. Treatments are prescribed according to the state of each patient, the intensity and severity of the symptoms, the location of the inflammation, and also taking into account the side effects already manifested (Rosen et al. 1982; Ursing et al. 1982). Surgery remains the last resort to "cure" these patients through the upkeep of remission phase (Rosen et al. 1982; Ursing et al. 1982; Peppercorn 1993; Lal and Steinhart 2006; Ahluwalia et al. 2018). Far from conventional treatment methods, research is currently focused on platelets receptors as target of future therapeutic. In fact, in addition to the platelets function in primary hemostasis, there is a rising number of studies supporting their significant role as amplifying agents in inflammatory processes/disorders (e.g. Crohn’s disease) and immune response (Weyrich et al. 2003; Semple et al. 2011; Tariket et al. 2019; Aouiss et al. 2019) by releasing sCD40L, IL-1 and other inflammatory mediators (Semple and Freedman 2010; Jenne et al. 2013). In a recent study, Kojok et al. demonstrated that the modulation of the CD40/CD40L axis by aspirin, reduces the potentiating effect of CD40L on platelet aggregation via inhibition of myosin light chain (Kojok et al. 2020).
Figure 3 represents a classification of the currently available treatments according to the symptoms of IBD patients. The therapeutic strategies and medical management of IBD have evolved from classical treatments (aminosalicates, corticosteroids …) to more targeted treatment (immunomodulation) using inhibitors of tumor necrosis factor (Ahluwalia et al. 2018; Katsanos et al. 2018). TNF is a cytokine that induces the transcription of other inflammatory cytokines and is therefore responsible for amplifying and maintaining chronic inflammation of IBD (Ahluwalia et al. 2018; Katsanos et al. 2018). Current therapies of IBD are increasingly targeted and oriented to allow an effective response without corticosteroids administration (Ahluwalia et al. 2018; Katsanos et al. 2018; Louis 2018). The anti-TNF treatment uses monoclonal antibodies (mAb) to neutralize the two forms of TNF (free and transmembrane) (Ahluwalia et al. 2018). According to several blocking strategies evaluated in patients with Crohn's disease (Levin et al. 2016; Paschou et al. 2018), to destroy TNF-producing cells by apoptosis (Levin et al. 2016; Ahluwalia et al. 2018). Anti-TNF can achieve and maintain effective remission with mucosal healing, in the absence of steroids (Ahluwalia et al. 2018; Louis 2018). There are several commercially available anti-TNF antibodies such as adalimumab (ADA), infliximab (IFX), golimumab and certulizumabpegol all having the same therapeutic purpose (Levin et al. 2016). Despite its efficiency, undesirable adverse effect were observed in patients treated with these drugs (Katsanos et al. 2018). The total loss of response to anti-TNF was also noted (Luther et al. 2018; Katsanos et al. 2018). According to a recent study 20 to 30% of patients with no primary response to anti-TNF, stop the use of this treatment, and 30 to 40% lose the therapeutic response of anti-TNF one year after treatment (Katsanos et al. 2018). Recently, researchers examined variation in colonic gene expression in patients treated with anti-TNF and untreated healthy individuals to determine the involvement of TNF-mediated inflammatory pathways in the loss of treatment responses (Luther et al. 2018). The results of this study did not associate the loss of response and the resistance to Anti-TNFα Therapy to the emergence of TNF-induced Inflammatory pathways (Luther et al. 2018). On the other hand, the manipulation of gut microbiota is increasingly used for the development of new therapeutic strategies (Shanahan and Quigley 2014). To predict the effectiveness of the response to anti-TNF treatment in "non-responders" or "resistant", researchers are studying intestinal microbiota and the expression of antimicrobial peptides (AMPs) in sick and in healthy individuals (Shanahan and Quigley 2014; Magnusson et al. 2016). In the presence of this preferential response or non-response to anti-TNF, it is important to think about developing more specific therapeutic strategies. In this sense, personalized medicine oversees the characterization of patients according to their susceptibility to the response to specific therapies (Flamant and Roblin 2018). In order to provide physicians with a wide range of moderately effective treatments for each case pharmacokinetics have been largely focused on targeting a variety of molecules and pathways involved in the pathogenesis of Crohn's disease (Shuai and Liu 2003; Geremia et al. 2011; Babon et al. 2014; Vermeire et al. 2017; Sands et al. 2017; Sandborn et al. 2017). In this sense, CD40 / CD40L and IL-1 are considered important therapeutic targets considering their role in the onset of Crohn's disease. In a clinical study, most patients with CD responded positively to treatment with a specific CD40L antagonist (ch5D12), while the remaining patients entered remission phase (Kasran et al. 2005). On the other hand, blocking the inflammatory action of IL-1 has proved effective response in disease course (Dinarello et al. 2012; Garlanda et al. 2013).
Figure 4 shows some molecular targets in the treatment of Crohn's disease. In an attempt to understand whether the inflammatory environment of CD disrupted the properties of resident intestinal stem cells (ISC), the expression of ISC marker genes under different conditions (active phases and remission phases) was evaluated (Suzuki et al. 2018). Given their important role in maintaining the integrity of the intestinal epithelium and homeostasis, ISC are continue to attract strong interest from the scientific community (Ellins 1985; Ruiz et al. 2017).
Some possible therapeutic targets to alleviate inflammatory symptoms in patients with Crohn's disease, 1–2: Inhibition of the CD40/CD40L interaction, either by monoclonal antibodies against sCD40L (membrane also) or by an antagonist chimeric monoclonal anti-human CD40 antibody ‘’ch5D12′’ 3: Inhibition of the Jack3 protein by the immunomodulator ‘’Tofacitinib ‘’ 4: Inhibition of the transcription factor NF-kb by the administration of IL-10, this makes it possible to stop the transcription of several pro-inflammatory cytokines and to promote the anti-inflammatory response 5-9-10: Neutralization of IL-1, either by a recombinant soluble molecule "Rilonacept" which also binds the natural antagonist of the IL-1 receptor (IL-1ra), or by the monoclonal antibody anti-IL-1 ''Canakinumab ''6: Inhibition of the chemokine CCL5 by its antagonist7: Inhibition of the TNF Cytokine by Anti-TNF Monoclonal Antibodies (adalimumab, infliximab, golimumab and certulizumabpegol) 8: Blocking of the IL-1/IL-1R interaction, by IL-1 receptor antagonist’’anakinra’’
Conclusion
Deep understanding of the immunopathogenesis of Crohn's disease is essential for developing more effective treatments. The importance of CD40/CD40L and IL-1 pathway makes them a significant target in Crohn’s disease therapeutics development. In a recently published review article describing the potential contribution of CD40 signaling on hematopoietic and non-hematopoietic cells to the pathogenesis of autoimmune diseases, the authors conclude that therapeutics targeting the CD40-CD40L axis have the potential to broadly modulate a multitude of responses influenced by this pathway, including cellular immune processes (Kojok et al. 2020). From a general point of view, the biological molecules involved in the pathogenesis of Crohn's disease can therefore be considered as biomarkers that make it possible to choose the right therapeutic pathway for the right patient. The more these molecules are studied, the more effective and targeted the treatment will be.
References
Ahluwalia B, Moraes L, Magnusson MK, Ohman L (2018) Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. https://doi.org/10.1080/00365521.2018.1447597
Alaaeddine N, Hassan GS, Yacoub D, Mourad W (2012) CD154: an immunoinflammatory mediator in systemic lupus erythematosus and rheumatoid arthritis. Clin Dev Immunol 2012:490148. https://doi.org/10.1155/2012/490148
Aloui C, Prigent A, Sut C, Hamzeh-Cognasse H, Pozzetto B, Richard Y, Cognasse F, Laradi S, Garraud O (2014) The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int J Mol Sci 15:22342–22364. https://doi.org/10.3390/ijms151222342
An HJ, Kim YJ, Song DH, Park BS, Kim HM, Lee JD, Paik SG, Lee JO, Lee H (2011) Crystallographic and mutational analysis of the CD40-CD154 complex and its implications for receptor activation. J Biol Chem 286:11226–11235. https://doi.org/10.1074/jbc.M110.208215
Anand SX, Viles-Gonzalez JF, Badimon JJ, Cavusoglu E, Marmur JD (2003) Membrane-associated CD40L and sCD40L in atherothrombotic disease. Thromb Haemost 90:377–384. https://doi.org/10.1160/TH03-05-0268
Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, Phillips DR, Wagner DD (2002) CD40L stabilizes arterial thrombi by a beta3 integrin—dependent mechanism. Nat Med 8:247–252. https://doi.org/10.1038/nm0302-247
Antoniades C, Bakogiannis C, Tousoulis D, Antonopoulos AS, Stefanadis C (2009) The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol 54:669–677. https://doi.org/10.1016/j.jacc.2009.03.076
Aouiss A, Anka Idrissi D, Kabine M, Zaid Y (2019) Update of inflammatory proliferative retinopathy: ischemia, hypoxia and angiogenesis. Curr Res Transl Med 67:62–71. https://doi.org/10.1016/j.retram.2019.01.005
Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN (2014) The molecular regulation of Janus kinase (JAK) activation. Biochem J 462:1–13. https://doi.org/10.1042/BJ20140712
Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, Tschopp J, Endres S, Latz E, Schnurr M (2010) Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 59:1192–1199. https://doi.org/10.1136/gut.2009.197822
Bauer C, Duewell P, Lehr HA, Endres S, Schnurr M (2012) Protective and aggravating effects of Nlrp3 inflammasome activation in IBD models: influence of genetic and environmental factors. Dig Dis 30(Suppl 1):82–90. https://doi.org/10.1159/000341681
Bavendiek U, Libby P, Kilbride M, Reynolds R, Mackman N, Schonbeck U (2002) Induction of tissue factor expression in human endothelial cells by CD40 ligand is mediated via activator protein 1, nuclear factor kappa B, and Egr-1. J Biol Chem 277:25032–25039. https://doi.org/10.1074/jbc.M204003200
Blair P, Flaumenhaft R (2009) Platelet alpha-granules: basic biology and clinical correlates. Blood Rev 23:177–189. https://doi.org/10.1016/j.blre.2009.04.001
Bou Khzam L, Boulahya R, Abou-Saleh H, Hachem A, Zaid Y, Merhi Y (2013) Soluble CD40 ligand stimulates the pro-angiogenic function of peripheral blood angiogenic outgrowth cells via increased release of matrix metalloproteinase-9. PLoS ONE 8:e84289. https://doi.org/10.1371/journal.pone.0084289
Bou Khzam L, Hachem A, Zaid Y, Boulahya R, Mourad W, Merhi Y (2013) Soluble CD40 ligand impairs the anti-platelet function of peripheral blood angiogenic outgrowth cells via increased production of reactive oxygen species. Thromb Haemost 109:940–947. https://doi.org/10.1160/TH12-09-0679
Braesch-Andersen S, Paulie S, Koho H, Nika H, Aspenstrom P, Perlmann P (1989) Biochemical characteristics and partial amino acid sequence of the receptor-like human B cell and carcinoma antigen CDw40. J Immunol 142:562–567
Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, Li Z, Mortha A, Merad M, Das A, Gevers D, McGovern DPB, Singh N, Braun J, Jacobs JP, Clemente JC, Grinspan A, Sands BE, Colombel JF, Dubinsky MC, Faith JJ (2019) Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORgammat(+) regulatory T cells and exacerbate colitis in mice. Immunity 50(212–224):e4. https://doi.org/10.1016/j.immuni.2018.12.015
Cabral-Marques O, Ramos RN, Schimke LF, Khan TA, Amaral EP, Barbosa Bomfim CC, Junior OR, Franca TT, Arslanian C, Carola Correia Lima JD, Weber CW, Ferreira JF, Tavares FS, Sun J, D’Imperio Lima MR, Seelaender M, Garcia Calich VL, Marzagao Barbuto JA, Costa-Carvalho BT, Riemekasten G, Seminario G, Bezrodnik L, Notarangelo L, Torgerson TR, Ochs HD, Condino-Neto A (2017) Human CD40 ligand deficiency dysregulates the macrophage transcriptome causing functional defects that are improved by exogenous IFN-gamma. J Allergy Clin Immunol 139(900–912):e7. https://doi.org/10.1016/j.jaci.2016.07.018
Callard RE, Armitage RJ, Fanslow WC, Spriggs MK (1993) CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today 14:559–564. https://doi.org/10.1016/0167-5699(93)90188-Q
Chakrabarti S, Varghese S, Vitseva O, Tanriverdi K, Freedman JE (2005) CD40 ligand influences platelet release of reactive oxygen intermediates. Arter Thromb Vasc Biol 25:2428–2434. https://doi.org/10.1161/01.ATV.0000184765.59207.f3
Charafeddine AH, Kim EJ, Maynard DM, Yi H, Weaver TA, Gunay-Aygun M, Russell M, Gahl WA, Kirk AD (2012) Platelet-derived CD154: ultrastructural localization and clinical correlation in organ transplantation. Am J Transpl 12:3143–3151. https://doi.org/10.1111/j.1600-6143.2012.04241.x
Chauhan A, Adams DH (2017) Platelets are critical drivers of illness behaviors during liver inflammation. Gastroenterology 153:1188–1190. https://doi.org/10.1053/j.gastro.2017.09.029
Chen C, Li Y, Yu Z, Liu Z, Shi Y, Lewandowska U, Sobczak M, Fichna J, Kreis M (2015) Platelet activity in the pathophysiology of inflammatory bowel diseases. Curr Drug Targets 16:219–225. https://doi.org/10.2174/1389450116666150113122229
Cho JH (2008) Inflammatory bowel disease: genetic and epidemiologic considerations. World J Gastroenterol 14:338–347. https://doi.org/10.3748/wjg.14.338
Cibor D, Szczeklik K, Koziol K, Pocztar H, Mach T, Owczarek D (2020) Serum concentration of selected biochemical markers of endothelial dysfunction and inflammation in patients with the varying activity of inflammatory bowel disease. Pol Arch Intern Med 130:598–606. https://doi.org/10.20452/pamw.15463
Cognasse F, Hamzeh-Cognasse H, Lafarge S, Chavarin P, Cogne M, Richard Y, Garraud O (2007) Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp Hematol 35:1376–1387. https://doi.org/10.1016/j.exphem.2007.05.021
Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch B, Eshee L, Mason D (2014) Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J Pediatr Gastroenterol Nutr 59:516–521. https://doi.org/10.1097/MPG.0000000000000449
Corrigendum, Longhurst HJ, Tarzi MD, Ashworth F, Bethune C, Cale C, Dempster J, Gompels M, Jolles S, Seneviratne S, Symons C, Price A, Edgar D (2015) C1 inhibitor deficiency: 2014 United Kingdom consensus document. Clin Exp Immunol 181:188. https://doi.org/10.1111/cei.12649
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A (2011) Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140:1785–1794. https://doi.org/10.1053/j.gastro.2011.01.055
Cotton JB (1971) Use of solumedrol in pediatrics. Apropos of 45 cases. Lyon Med 225:251–254
Danese S, Katz JA, Saibeni S, Papa A, Gasbarrini A, Vecchi M, Fiocchi C (2003) Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut 52:1435–1441. https://doi.org/10.1136/gut.52.10.1435
Danese S, de la Motte C, Reyes BM, Sans M, Levine AD, Fiocchi C (2004) Cutting edge: T cells trigger CD40-dependent platelet activation and granular RANTES release: a novel pathway for immune response amplification. J Immunol 172:2011–2015. https://doi.org/10.4049/jimmunol.172.4.2011
de Souza HS, Fiocchi C (2016) Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 13:13–27. https://doi.org/10.1038/nrgastro.2015.186
de Silva PS, Luben R, Shrestha SS, Khaw KT, Hart AR (2014) Dietary arachidonic and oleic acid intake in ulcerative colitis etiology: a prospective cohort study using 7-day food diaries. Eur J Gastroenterol Hepatol 26:11–18. https://doi.org/10.1097/MEG.0b013e328365c372
Dejica DI, Manea EM (2006) Costimulatory molecule CD154 in systemic lupus erythematosus and rheumatoid arthritis. Therap Perspect Roum Arch Microbiol Immunol 65:66–74
Delaleu N, Bickel M (2004) Interleukin-1 beta and interleukin-18: regulation and activity in local inflammation. Periodontol 2000 35:42–52. https://doi.org/10.1111/j.0906-6713.2004.003569.x
Delmas Y, Viallard JF, Villeneuve J, Grosset C, Pasquet JM, Dechanet-Merville J, Nurden P, Pellegrin JL, Rosenbaum J, Combe C, Nurden AT, Ripoche J (2005) Platelet-associated CD154: a new interface in haemostasis and in the inflammatory reaction. Med Sci Paris 21:825–831. https://doi.org/10.1051/medsci/20052110825
Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, Fratto CM, Tolley E, Kraiss LW, McIntyre TM, Zimmerman GA, Weyrich AS (2005) Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell 122:379–391. https://doi.org/10.1016/j.cell.2005.06.015
Di Sabatino A, Santilli F, Guerci M, Simeone P, Ardizzone S, Massari A, Giuffrida P, Tripaldi R, Malara A, Liani R, Gurini E, Aronico N, Balduini A, Corazza GR, Davi G (2016) Oxidative stress and thromboxane-dependent platelet activation in inflammatory bowel disease: effects of anti-TNF-alpha treatment. Thromb Haemost 116:486–495. https://doi.org/10.1160/TH16-02-0167
Dinarello CA (1997) Interleukin-1. Cytokine Growth Factor Rev 8:253–265. https://doi.org/10.1016/s1359-6101(97)00023-3
Dinarello CA (2004) Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr Opin Pharmacol 4:378–385. https://doi.org/10.1016/j.coph.2004.03.010
Dinarello CA (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117:3720–3732. https://doi.org/10.1182/blood-2010-07-273417
Dinarello CA, Simon A, van der Meer JW (2012) Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11:633–652. https://doi.org/10.1038/nrd3800
Dos Santos JC, Damen M, Oosting M, de Jong DJ, Heinhuis B, Gomes RS, Araujo CS, Netea MG, Ribeiro-Dias F, Joosten LAB (2017) The NOD2 receptor is crucial for immune responses towards New World Leishmania species. Sci Rep 7:15219. https://doi.org/10.1038/s41598-017-15412-7
Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ (2009) Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 229:152–172. https://doi.org/10.1111/j.1600-065X.2009.00782.x
Ellins SR (1985) Coyote control and taste aversion: a predation problem or a people problem? Appetite 6:272–275. https://doi.org/10.1016/S0195-6663(85)80017-9
Elzey BD, Tian J, Jensen RJ, Swanson AK, Lees JR, Lentz SR, Stein CS, Nieswandt B, Wang Y, Davidson BL, Ratliff TL (2003) Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 19:9–19. https://doi.org/10.1016/s1074-7613(03)00177-8
Elzey BD, Grant JF, Sinn HW, Nieswandt B, Waldschmidt TJ, Ratliff TL (2005) Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol 78:80–84. https://doi.org/10.1189/jlb.1104669
Fanslow WC, Srinivasan S, Paxton R, Gibson MG, Spriggs MK, Armitage RJ (1994) Structural characteristics of CD40 ligand that determine biological function. Semin Immunol 6:267–278. https://doi.org/10.1006/smim.1994.1035
Farrukh A, Mayberry JF (2014) Is there a role for fish oil in inflammatory bowel disease? World J Clin Cases 2:250. https://doi.org/10.12998/wjcc.v2.i7.250
Fava F, Danese S (2011) Intestinal microbiota in inflammatory bowel disease: friend of foe? World J Gastroenterol 17:557–566. https://doi.org/10.3748/wjg.v17.i5.557
Flamant M, Roblin X (2018) Inflammatory bowel disease: towards a personalized medicine. Ther Adv Gastroenterol 11:1756283X17745029. https://doi.org/10.1177/1756283X17745029
Flaumenhaft R (2003) Molecular basis of platelet granule secretion. Arter Thromb Vasc Biol 23:1152–1160. https://doi.org/10.1161/01.ATV.0000075965.88456.48
Gabay C, Lamacchia C, Palmer G (2010) IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol 6:232–241. https://doi.org/10.1038/nrrheum.2010.4
Gachet C (2013) Molecular mechanisms of platelet activation. Bull Acad Natl Med 197:361–373. https://doi.org/10.1152/physrev.1989.69.1.58
Garlanda C, Dinarello CA, Mantovani A (2013) The interleukin-1 family: back to the future. Immunity 39:1003–1018. https://doi.org/10.1016/j.immuni.2013.11.010
Garraud O, Berthet J, Hamzeh-Cognasse H, Cognasse F (2011) Pathogen sensing, subsequent signalling, and signalosome in human platelets. Thromb Res 127:283–286. https://doi.org/10.1016/j.thromres.2010.10.015
Gasche C, Scholmerich J, Brynskov J, D’Haens G, Hanauer SB, Irvine EJ, Jewell DP, Rachmilewitz D, Sachar DB, Sandborn WJ, Sutherland LR (2000) A simple classification of Crohn’s disease: report of the working party for the world congresses of gastroenterology, Vienna 1998. Inflamm Bowel Dis 6:8–15. https://doi.org/10.1097/00054725-200002000-00002
Gaya DR, Russell RK, Nimmo ER, Satsangi J (2006) New genes in inflammatory bowel disease: lessons for complex diseases? Lancet 367:1271–1284. https://doi.org/10.1016/S0140-6736(06)68345-1
Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F (2011) IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med 208:1127–1133. https://doi.org/10.1084/jem.20101712
Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ (2003) Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869–8872. https://doi.org/10.1074/jbc.C200651200
Gnatenko DV, Dunn JJ, McCorkle SR, Weissmann D, Perrotta PL, Bahou WF (2003) Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood 101:2285–2293. https://doi.org/10.1182/blood-2002-09-2797
Grewal IS, Flavell RA (1998) CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol 16:111–135. https://doi.org/10.1146/annurev.immunol.16.1.111
Gunaydin NC, Chou J, Karaca NE, Aksu G, Massaad MJ, Azarsiz E, Ertan Y, Geha RS, Kutukculer N (2014) A novel disease-causing CD40L mutation reduces expression of CD40 ligand, but preserves CD40 binding capacity. Clin Immunol 153:288–291. https://doi.org/10.1016/j.clim.2014.05.001
Hanauer SB (2017) Combination therapy for inflammatory bowel disease. Gastroenterol Hepatol NY 13:296–298
Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA (1998) CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391:591–594. https://doi.org/10.1038/35393
Henn V, Steinbach S, Buchner K, Presek P, Kroczek RA (2001) The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood 98:1047–1054. https://doi.org/10.1182/blood.v98.4.1047
Hibi T, Ogata H (2006) Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol 41:10–16. https://doi.org/10.1007/s00535-005-1744-3
Hirota SA, Ng J, Lueng A, Khajah M, Parhar K, Li Y, Lam V, Potentier MS, Ng K, Bawa M, McCafferty DM, Rioux KP, Ghosh S, Xavier RJ, Colgan SP, Tschopp J, Muruve D, MacDonald JA, Beck PL (2011) NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 17:1359–1372. https://doi.org/10.1002/ibd.21478
Hu SK, Mitcho YL, Rath NC (1988) Effect of estradiol on interleukin 1 synthesis by macrophages. Int J Immunopharmacol 10:247–252. https://doi.org/10.1016/0192-0561(88)90055-0
Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ (2003) CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res 92:1041–1048. https://doi.org/10.1161/01.RES.0000070111.98158.6C
Jandinski JJ (1988) Osteoclast activating factor is now interleukin-1 beta: historical perspective and biological implications. J Oral Pathol 17:145–152. https://doi.org/10.1111/j.1600-0714.1988.tb01515.x
Jenne CN, Urrutia R, Kubes P (2013) Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol 35:254–261. https://doi.org/10.1111/ijlh.12084
Jorgensen SP, Hvas CL, Agnholt J, Christensen LA, Heickendorff L, Dahlerup JF (2013) Active Crohn’s disease is associated with low vitamin D levels. J Crohns Colitis 7:e407–e413. https://doi.org/10.1016/j.crohns.2013.01.012
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, International IBDGC, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491:119–124. https://doi.org/10.1038/nature11582
Kaistha A, Levine J (2014) Inflammatory bowel disease: the classic gastrointestinal autoimmune disease. Curr Probl Pediatr Adolesc Health Care 44:328–334. https://doi.org/10.1016/j.cppeds.2014.10.003
Kaminska B, Roszko-Kirpsza I, Landowski P, Szlagatys-Sidorkiewicz A, Guzinska-Ustymowicz K, Maciorkowska E (2015) Evaluation of CD40 and CD80 receptors in the colonic mucosal membrane of children with inflammatory bowel disease. Ann Agric Enviorn Med 22:695–699. https://doi.org/10.5604/12321966.1185778
Karnell JL, Rieder SA, Ettinger R, Kolbeck R (2019) Targeting the CD40-CD40L pathway in autoimmune diseases: humoral immunity and beyond. Adv Drug Deliv Rev 141:92–103. https://doi.org/10.1016/j.addr.2018.12.005
Kasran A, Boon L, Wortel CH, Hogezand RA, Schreiber S, Goldin E, Boer M, Geboes K, Rutgeerts P, Ceuppens JL (2005) Safety and tolerability of antagonist anti-human CD40 Mab ch5D12 in patients with moderate to severe Crohn’s disease. Aliment Pharmacol Ther 22:111–122. https://doi.org/10.1111/j.1365-2036.2005.02526.x
Katsanos KH, Papamichael K, Feuerstein JD, Christodoulou DK, Cheifetz AS (2018) Biological therapies in inflammatory bowel disease: beyond anti-TNF therapies. Clin Immunol. https://doi.org/10.1016/j.clim.2018.03.004
Kim TJ, Koo JS, Kim SJ, Hong SN, Kim YS, Yang SK, Kim YH (2017) Role of IL-1ra and Granzyme B as biomarkers in active Crohn’s disease patients. Biomarkers. https://doi.org/10.1080/1354750X.2017.1387933
Kojok K, Akoum SE, Mohsen M, Mourad W, Merhi Y (2018) CD40L priming of platelets via NF-kappaB activation is CD40- and TAK1-dependent. J Am Heart Assoc 7:e03677. https://doi.org/10.1161/JAHA.118.009636
Kojok K, Mohsen M, El Kadiry AEH, Mourad W, Merhi Y (2020) Aspirin reduces the potentiating effect of CD40L on platelet aggregation via inhibition of myosin light chain. J Am Heart Assoc 9:e013396. https://doi.org/10.1161/JAHA.119.013396
Koutroubakis IE, Theodoropoulou A, Xidakis C, Sfiridaki A, Notas G, Kolios G, Kouroumalis EA (2004) Association between enhanced soluble CD40 ligand and prothrombotic state in inflammatory bowel disease. Eur J Gastroenterol Hepatol 16:1147–1152. https://doi.org/10.1097/00042737-200411000-00011
Kurti Z, Ilias A, Gonczi L, Vegh Z, Fadgyas-Freyler P, Korponay G, Golovics PA, Lovasz BD, Lakatos PL (2018) Therapeutic preferences and outcomes in newly diagnosed patients with Crohn’s diseases in the biological era in Hungary: a nationwide study based on the National Health Insurance Fund database. BMC Gastroenterol 18:23. https://doi.org/10.1186/s12876-018-0746-6
Kusters P, Seijkens T, Burger C, Legein B, Winkels H, Gijbels M, Barthels C, Bennett R, Beckers L, Atzler D, Biessen E, Brocker T, Weber C, Gerdes N, Lutgens E (2017) Constitutive CD40 signaling in dendritic cells limits atherosclerosis by provoking inflammatory bowel disease and ensuing cholesterol malabsorption. Am J Pathol 187:2912–2919. https://doi.org/10.1016/j.ajpath.2017.08.016
Lafage-Pochitaloff M, Herman P, Birg F, Galizzi JP, Simonetti J, Mannoni P, Banchereau J (1994) Localization of the human CD40 gene to chromosome 20, bands q12–q13.2. Leukemia 8:1172–1175
Lal S, Steinhart AH (2006) Antibiotic therapy for Crohn’s disease: a review. Can J Gastroenterol 20:651–655. https://doi.org/10.1155/2006/250490
Lampinen M, Vessby J, Fredricsson A, Wanders A, Rorsman F, Carlson M (2019) High serum sCD40 and a distinct colonic T cell profile in ulcerative colitis associated with primary sclerosing cholangitis. J Crohns Colitis 13:341–350. https://doi.org/10.1093/ecco-jcc/jjy170
Lappas M (2013) NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol Reprod 89:14. https://doi.org/10.1095/biolreprod.113.110056
Lee JR, Koretzky GA (1998) Production of reactive oxygen intermediates following CD40 ligation correlates with c-Jun N-terminal kinase activation and IL-6 secretion in murine B lymphocytes. Eur J Immunol 28:4188–4197. https://doi.org/10.1002/(SICI)1521-4141(199812)28:12%3c4188::AID-IMMU4188%3e3.0.CO;2-B
Lee JY, Hall JA, Kroehling L, Wu L, Najar T, Nguyen HH, Lin WY, Yeung ST, Silva HM, Li D, Hine A, Loke P, Hudesman D, Martin JC, Kenigsberg E, Merad M, Khanna KM, Littman DR (2020) Serum amyloid A proteins induce pathogenic Th17 cells and promote inflammatory disease. Cell 180(79–91):e16. https://doi.org/10.1016/j.cell.2019.11.026
Leroyer AS, Rautou PE, Silvestre JS, Castier Y, Leseche G, Devue C, Duriez M, Brandes RP, Lutgens E, Tedgui A, Boulanger CM (2008) CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J Am Coll Cardiol 52:1302–1311. https://doi.org/10.1016/j.jacc.2008.07.032
Levin AD, Wildenberg ME, van den Brink GR (2016) Mechanism of action of anti-TNF therapy in inflammatory bowel disease. J Crohns Colitis 10:989–997. https://doi.org/10.1093/ecco-jcc/jjw053
Li Z, Vermeire S, Bullens D, Ferrante M, Van Steen K, Noman M, Bossuyt X, Rutgeerts P, Ceuppens JL, Van Assche G (2015) Anti-tumor necrosis factor therapy restores peripheral blood B-cell subsets and CD40 expression in inflammatory bowel diseases. Inflamm Bowel Dis 21:2787–2796. https://doi.org/10.1097/MIB.0000000000000554
Lievens D, Eijgelaar WJ, Biessen EA, Daemen MJ, Lutgens E (2009) The multi-functionality of CD40L and its receptor CD40 in atherosclerosis. Thromb Haemost 102:206–214. https://doi.org/10.1160/TH09-01-0029
Liu Z, Colpaert S, D’Haens GR, Kasran A, de Boer M, Rutgeerts P, Geboes K, Ceuppens JL (1999) Hyperexpression of CD40 ligand (CD154) in inflammatory bowel disease and its contribution to pathogenic cytokine production. J Immunol 163:4049–4057
Liu G, Mateer SW, Hsu A, Goggins BJ, Tay H, Mathe A, Fan K, Neal R, Bruce J, Burns G, Minahan K, Maltby S, Fricker M, Foster PS, Wark PAB, Hansbro PM, Keely S (2019) Platelet activating factor receptor regulates colitis-induced pulmonary inflammation through the NLRP3 inflammasome. Mucosal Immunol 12:862–873. https://doi.org/10.1038/s41385-019-0163-3
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6:16. https://doi.org/10.1038/s41421-020-0156-0
Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JY, Malekzadeh R, Westra HJ, Yamazaki K, Yang SK, International Multiple Sclerosis Genetics C, International IBDGC, Barrett JC, Alizadeh BZ, Parkes M, Bk T, Daly MJ, Kubo M, Anderson CA, Weersma RK (2015) Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet 47:979–986. https://doi.org/10.1038/ng.3359
Locksley RM, Killeen N, Lenardo MJ (2001) The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487–501. https://doi.org/10.1016/s0092-8674(01)00237-9
Louis E (2018) Stopping biologics in IBD-what is the evidence? Inflamm Bowel Dis 24:725–731. https://doi.org/10.1093/ibd/izx098
Ludwiczek O, Kaser A, Tilg H (2003) Plasma levels of soluble CD40 ligand are elevated in inflammatory bowel diseases. Int J Colorectal Dis 18:142–147. https://doi.org/10.1007/s00384-002-0425-4
Luther J, Gala M, Patel SJ, Dave M, Borren N, Xavier RJ, Ananthakrishnan AN (2018) Loss of response to anti-tumor necrosis factor alpha therapy in Crohn’s disease is not associated with emergence of novel inflammatory pathways. Dig Sci 63:738–745. https://doi.org/10.1007/s10620-018-4932-8
Magnusson MK, Strid H, Sapnara M, Lasson A, Bajor A, Ung KA, Ohman L (2016) Anti-TNF therapy response in patients with ulcerative colitis is associated with colonic antimicrobial peptide expression and microbiota composition. J Crohns Colitis 10:943–952. https://doi.org/10.1093/ecco-jcc/jjw051
Magro F, Soares JB, Fernandes D (2014) Venous thrombosis and prothrombotic factors in inflammatory bowel disease. World J Gastroenterol 20:4857–4872. https://doi.org/10.3748/wjg.v20.i17.4857
Mao L, Kitani A, Strober W, Fuss IJ (2018) The role of NLRP3 and IL-1beta in the pathogenesis of inflammatory bowel disease. Front Immunol 9:2566. https://doi.org/10.3389/fimmu.2018.02566
Monteleone M, Stow JL, Schroder K (2015) Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine 74:213–218. https://doi.org/10.1016/j.cyto.2015.03.022
Morowitz DA, Allen LW, Kirsner JB (1968) Thrombocytosis in chronic inflammatory bowel disease. Ann Intern Med 68:1013–1021. https://doi.org/10.7326/0003-4819-68-5-1013
Moss AC (2015) Optimizing the use of biological therapy in patients with inflammatory bowel disease. Gastroenterol Rep Oxf 3:63–68. https://doi.org/10.1093/gastro/gou087
Nagalla S, Shaw C, Kong X, Kondkar AA, Edelstein LC, Ma L, Chen J, McKnight GS, Lopez JA, Yang L, Jin Y, Bray MS, Leal SM, Dong JF, Bray PF (2011) Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood 117:5189–5197. https://doi.org/10.1182/blood-2010-09-299719
Naismith JH, Sprang SR (1998) Modularity in the TNF-receptor family. Trends Biochem Sci 23:74–79. https://doi.org/10.1016/s0968-0004(97)01164-x
Nakarai A, Kato J, Hiraoka S, Takashima S, Inokuchi T, Takahara M, Sugihara Y, Harada K, Okada H (2018) An elevated platelet count increases the risk of relapse in ulcerative colitis patients with mucosal healing. Gut Liver 12:420–425. https://doi.org/10.5009/gnl17236
Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E (2008) Platelets and wound healing. Front Biosci 13:3532–3548
Ogrinc Wagner A, Friedrich V, Barthels C, Marconi P, Blutke A, Brombacher F, Brocker T (2019) Strain specific maturation of dendritic cells and production of IL-1beta controls CD40-driven colitis. PLoS ONE 14:e0210998. https://doi.org/10.1371/journal.pone.0210998
Owczarek D, Rodacki T, Domagala-Rodacka R, Cibor D, Mach T (2016) Diet and nutritional factors in inflammatory bowel diseases. World J Gastroenterol 22:895–905. https://doi.org/10.3748/wjg.v22.i3.895
Pankratz S, Bittner S, Kehrel BE, Langer HF, Kleinschnitz C, Meuth SG, Gobel K (2016) The inflammatory role of platelets: translational insights from experimental studies of autoimmune disorders. Int J Mol Sci. https://doi.org/10.3390/ijms17101723
Paschou SA, Kothonas F, Lafkas A, Myroforidis A, Loi V, Terzi T, Karagianni O, Poulou A, Goumas K, Vryonidou A (2018) Favorable effect of anti-TNF therapy on insulin sensitivity in nonobese, nondiabetic patients with inflammatory bowel disease. Int J Endocrinol 2018:6712901. https://doi.org/10.1155/2018/6712901
Peppercorn MA (1993) Is there a role for antibiotics as primary therapy in Crohn’s ileitis? J Clin Gastroenterol 17:235–237
Perazzio SF, Soeiro-Pereira PV, Dos Santos VC, de Brito MV, Salu B, Oliva MLV, Stevens AM, de Souza AWS, Ochs HD, Torgerson TR, Condino-Neto A, Andrade LEC (2017) Soluble CD40L is associated with increased oxidative burst and neutrophil extracellular trap release in Behcet’s disease. Arthritis Res Ther 19:235. https://doi.org/10.1186/s13075-017-1443-5
Petrovic SS, Vasiljevska MM, Obradovic SD, Tarabar DK, Doder RB, Majstorovic IJ, Petrovic MD, Magic ZM, Cikota BM, Perisic NJ, Brcerevic IA, Manojlovic NS, Rancic NK (2020) Antiplatelet agents’-ticagrelol and eptifibatide-safety in experimental colitis in mice. Turk J Gastroenterol 31:451–458. https://doi.org/10.5152/tjg.2020.19454
Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE (2014) NOD proteins: regulators of inflammation in health and disease. Nat Rev Immunol 14:9–23. https://doi.org/10.1038/nri3565
Ple H, Maltais M, Corduan A, Rousseau G, Madore F, Provost P (2012) Alteration of the platelet transcriptome in chronic kidney disease. Thromb Haemost 108:605–615. https://doi.org/10.1160/TH12-03-0153
Polese L, Angriman I, Cecchetto A, Norberto L, Scarpa M, Ruffolo C, Barollo M, Sommariva A, D’Amico DF (2002) The role of CD40 in ulcerative colitis: histochemical analysis and clinical correlation. Eur J Gastroenterol Hepatol 14:237–241. https://doi.org/10.1097/00042737-200203000-00006
Rachakonda SP, Dai H, Penack O, Blau O, Blau IW, Radujkovic A, Muller-Tidow C, Kumar R, Dreger P, Luft T (2018) Single nucleotide polymorphisms in CD40L predict endothelial complications and mortality after allogeneic stem-cell transplantation. J Clin Oncol 36:789–800. https://doi.org/10.1200/JCO.2017.76.4662
Rendu F, Brohard-Bohn B (2001) The platelet release reaction: granules’ constituents, secretion and functions. Platelets 12:261–273. https://doi.org/10.1080/09537100120068170
Riordan AM, Ruxton CH, Hunter JO (1998) A review of associations between Crohn’s disease and consumption of sugars. Eur J Clin Nutr 52:229–238. https://doi.org/10.1038/sj.ejcn.1600556
Ripoche J (2011) Blood platelets and inflammation: their relationship with liver and digestive diseases. Clin Res Hepatol Gastroenterol 35:353–357. https://doi.org/10.1016/j.clinre.2011.02.012
Rizvi M, Pathak D, Freedman JE, Chakrabarti S (2008) CD40-CD40 ligand interactions in oxidative stress, inflammation and vascular disease. Trends Mol Med 14:530–538. https://doi.org/10.1016/j.molmed.2008.09.006
Rosen A, Ursing B, Alm T, Barany F, Bergelin I, Ganrot-Norlin K, Hoevels J, Huitfeldt B, Jarnerot G, Krause U, Krook A, Lindstrom B, Nordle O (1982) A comparative study of metronidazole and sulfasalazine for active Crohn’s disease: the cooperative Crohn’s disease study in Sweden. I Des Methodologic Considerations Gastroenterol 83:541–549
Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS (2011) Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood 118:e101–e111. https://doi.org/10.1182/blood-2011-03-339705
Ruiz MA, Kaiser RL Jr, de Quadros LG, Piron-Ruiz L, Pena-Arciniegas T, Faria MAG, Siqueira RC, Pirozzi FF, Kaiser FSL, Burt RK (2017) Low toxicity and favorable clinical and quality of life impact after non-myeloablative autologous hematopoietic stem cell transplant in Crohn’s disease. BMC Res Notes 10:495. https://doi.org/10.1186/s13104-017-2824-1
Saluk J, Bijak M, Ponczek MB, Wachowicz B (2014) The formation, metabolism and the evolution of blood platelets. Postepy Hig Med Dosw Online 68:384–391. https://doi.org/10.5604/17322693.1098145
Sandborn WJ (2016) The present and future of inflammatory bowel disease treatment. Gastroenterol Hepatol N 12:438–441
Sandborn WJ, Su C, Panes J (2017) Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 377:496–497. https://doi.org/10.1056/NEJMc1707500
Sands BE, Chen J, Feagan BG, Penney M, Rees WA, Danese S, Higgins PDR, Newbold P, Faggioni R, Patra K, Li J, Klekotka P, Morehouse C, Pulkstenis E, Drappa J, van der Merwe R, Gasser RA Jr (2017) Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 153(77–86):e6. https://doi.org/10.1053/j.gastro.2017.03.049
Schaefer AK, Wastyk HC, Mohanan V, Hou CW, Lauro ML, Melnyk JE, Burch JM, Grimes CL (2017) Crohn’s disease variants of Nod2 are stabilized by the critical contact region of Hsp70. Biochemistry 56:4445–4448. https://doi.org/10.1021/acs.biochem.7b00470
Schonbeck U, Mach F, Libby P (2000) CD154 (CD40 ligand). Int J Biochem Cell Biol 32:687–693. https://doi.org/10.1016/s1357-2725(00)00016-9
Semple JW, Freedman J (2010) Platelets and innate immunity. Cell Mol Life Sci 67:499–511. https://doi.org/10.1007/s00018-009-0205-1
Semple JW, Italiano JE Jr, Freedman J (2011) Platelets and the immune continuum. Nat Rev Immunol 11:264–274. https://doi.org/10.1038/nri2956
Senhaji N, Kojok K, Darif Y, Fadainia C, Zaid Y (2015) The contribution of CD40/CD40L axis in inflammatory bowel disease: an update. Front Immunol 6:529. https://doi.org/10.3389/fimmu.2015.00529
Shanahan F, Quigley EM (2014) Manipulation of the microbiota for treatment of IBS and IBD-challenges and controversies. Gastroenterology 146:1554–1563. https://doi.org/10.1053/j.gastro.2014.01.050
Sharifi A, Vahedi H, Honarvar MR, Amiriani T, Nikniaz Z, Rad EY, Hosseinzadeh-Attar MJ (2020) Vitamin D decreases CD40L gene expression in ulcerative colitis patients: a randomized, double-blinded, placebo-controlled trial. Turk J Gastroenterol 31:99–104. https://doi.org/10.5152/tjg.2020.181028
Shih DQ, Targan SR (2008) Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol 14:390–400
Shuai K, Liu B (2003) Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3:900–911. https://doi.org/10.1038/nri1226
Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K, Jewell DP, Karban A, Loftus EV Jr, Pena AS, Riddell RH, Sachar DB, Schreiber S, Steinhart AH, Targan SR, Vermeire S, Warren BF (2005) Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 19(Suppl A):5A-36A. https://doi.org/10.1155/2005/269076
Simon LM, Edelstein LC, Nagalla S, Woodley AB, Chen ES, Kong X, Ma L, Fortina P, Kunapuli S, Holinstat M, McKenzie SE, Dong JF, Shaw CA, Bray PF (2014) Human platelet microRNA-mRNA networks associated with age and gender revealed by integrated plateletomics. Blood 123:e37-45. https://doi.org/10.1182/blood-2013-12-544692
Sims JE, Smith DE (2010) The IL-1 family: regulators of immunity. Nat Rev Immunol 10:89–102. https://doi.org/10.1038/nri2691
Singh J, Garber E, Van Vlijmen H, Karpusas M, Hsu YM, Zheng Z, Naismith JH, Thomas D (1998) The role of polar interactions in the molecular recognition of CD40L with its receptor CD40. Protein Sci 7:1124–1135. https://doi.org/10.1002/pro.5560070506
Stokes KY, Calahan L, Hamric CM, Russell JM, Granger DN (2009) CD40/CD40L contributes to hypercholesterolemia-induced microvascular inflammation. Am J Physiol Heart Circ Physiol 296:H689–H697. https://doi.org/10.1152/ajpheart.00962.2008
Su RC, Warner EA, Breidenbach JD, Lad A, Blomquist TM, Kleinhenz AL, Modyanov N, Malhotra D, Kennedy DJ, Haller ST (2020) CD40 receptor knockout protects against microcystin-LR (MC-LR) prolongation and exacerbation of dextran sulfate sodium (DSS)-induced colitis. Biomedicines. https://doi.org/10.3390/biomedicines8060149
Suzuki K, Murano T, Shimizu H, Ito G, Nakata T, Fujii S, Ishibashi F, Kawamoto A, Anzai S, Kuno R, Kuwabara K, Takahashi J, Hama M, Nagata S, Hiraguri Y, Takenaka K, Yui S, Tsuchiya K, Nakamura T, Ohtsuka K, Watanabe M, Okamoto R (2018) Single cell analysis of Crohn’s disease patient-derived small intestinal organoids reveals disease activity-dependent modification of stem cell properties. J Gastroenterol. https://doi.org/10.1007/s00535-018-1437-3
Swanson GR, Sedghi S, Farhadi A, Keshavarzian A (2010) Pattern of alcohol consumption and its effect on gastrointestinal symptoms in inflammatory bowel disease. Alcohol 44:223–228. https://doi.org/10.1016/j.alcohol.2009.10.019
Tariket S, Guerrero JA, Garraud O, Ghevaert C, Cognasse F (2019) Platelet alpha-granules modulate the inflammatory response under systemic lipopolysaccharide injection in mice. Transfusion (Paris) 59:32–38. https://doi.org/10.1111/trf.14970
Tekelioglu Y, Uzun H, Sisman G (2014) Activated platelets in patients suffering from inflammatory bowel disease. Bratisl Lek Listy 115:83–85. https://doi.org/10.4149/bll_2014_018
Torres MI, Rios A (2008) Current view of the immunopathogenesis in inflammatory bowel disease and its implications for therapy. World J Gastroenterol 14:1972–1980. https://doi.org/10.3748/wjg.14.1972
Tuvlin JA, Raza SS, Bracamonte S, Julian C, Hanauer SB, Nicolae DL, King AC, Cho JH (2007) Smoking and inflammatory bowel disease: trends in familial and sporadic cohorts. Inflamm Bowel Dis 13:573–579. https://doi.org/10.1002/ibd.20043
Urbich C, Dernbach E, Aicher A, Zeiher AM, Dimmeler S (2002) CD40 ligand inhibits endothelial cell migration by increasing production of endothelial reactive oxygen species. Circulation 106:981–986. https://doi.org/10.1161/01.CIR.0000027107.54614.1A
Ursing B, Alm T, Barany F, Bergelin I, Ganrot-Norlin K, Hoevels J, Huitfeldt B, Jarnerot G, Krause U, Krook A, Lindstrom B, Nordle O, Rosen A (1982) A comparative study of metronidazole and sulfasalazine for active Crohn’s disease: the cooperative Crohn’s disease study in Sweden. II Result Gastroenterol 83:550–562
van Kooten C, Banchereau J (2000) CD40-CD40 ligand. J Leukoc Biol 67:2–17. https://doi.org/10.1002/jlb.67.1.2
Vatn MH, Sandvik AK (2015) Inflammatory bowel disease. Scand J Gastroenterol 50:748–762. https://doi.org/10.3109/00365521.2015.1033000
Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, Klopocka M, Goldis A, Wisniewska-Jarosinska M, Baranovsky A, Sike R, Stoyanova K, Tasset C, Van der Aa A, Harrison P (2017) Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 389:266–275. https://doi.org/10.1016/S0140-6736(16)32537-5
Voudoukis E, Karmiris K, Koutroubakis IE (2014) Multipotent role of platelets in inflammatory bowel diseases: a clinical approach. World J Gastroenterol 20:3180–3190. https://doi.org/10.3748/wjg.v20.i12.3180
Wallace KL, Zheng LB, Kanazawa Y, Shih DQ (2014) Immunopathology of inflammatory bowel disease. World J Gastroenterol 20:6–21. https://doi.org/10.3748/wjg.v20.i1.6
Weyrich AS, Lindemann S, Zimmerman GA (2003) The evolving role of platelets in inflammation. J Thromb Haemost 1:1897–1905. https://doi.org/10.1046/j.1538-7836.2003.00304.x
Xu XR, Liu CQ, Feng BS, Liu ZJ (2014) Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol 20:3255–3264. https://doi.org/10.3748/wjg.v20.i12.3255
Yacoub D, Hachem A, Theoret JF, Gillis MA, Mourad W, Merhi Y (2010) Enhanced levels of soluble CD40 ligand exacerbate platelet aggregation and thrombus formation through a CD40-dependent tumor necrosis factor receptor-associated factor-2/Rac1/p38 mitogen-activated protein kinase signaling pathway. Arter Thromb Vasc Biol 30:2424–2433. https://doi.org/10.1161/ATVBAHA.110.216143
Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, Khalki L, Limami Y, Zaid N, Sadki K, Ben El Haj R, Mahir W, Belayachi L, Belefquih B, Benouda A, Cheikh A, Langlois MA, Cherrah Y, Flamand L, Guessous F, Boilard E (2020) Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ Res. https://doi.org/10.1161/CIRCRESAHA.120.317703
Zallot C, Quilliot D, Chevaux JB, Peyrin-Biroulet C, Gueant-Rodriguez RM, Freling E, Collet-Fenetrier B, Williet N, Ziegler O, Bigard MA, Gueant JL, Peyrin-Biroulet L (2013) Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm Bowel Dis 19:66–72. https://doi.org/10.1002/ibd.22965
Zhang YZ, Li YY (2014) Inflammatory bowel disease: pathogenesis. World J Gastroenterol 20:91–99. https://doi.org/10.3748/wjg.v20.i1.91
Zhang B, Wu T, Chen M, Zhou Y, Yi D, Guo R (2013) The CD40/CD40L system: a new therapeutic target for disease. Immunol Lett 153:58–61. https://doi.org/10.1016/j.imlet.2013.07.005
Zhao M, Gu J, Wang Z (2020) B cells in Crohn’s patients presented reduced IL-35 expression capacity. Mol Immunol 118:124–131. https://doi.org/10.1016/j.molimm.2019.12.005
Zirlik A, Maier C, Gerdes N, MacFarlane L, Soosairajah J, Bavendiek U, Ahrens I, Ernst S, Bassler N, Missiou A, Patko Z, Aikawa M, Schonbeck U, Bode C, Libby P, Peter K (2007) CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation 115:1571–1580. https://doi.org/10.1161/CIRCULATIONAHA.106.683201
Acknowledgements
We thank Dr Nabil El Bilali for his intellectual input.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anka Idrissi, D., Senhaji, N., Aouiss, A. et al. IL-1 and CD40/CD40L platelet complex: elements of induction of Crohn’s disease and new therapeutic targets. Arch. Pharm. Res. 44, 117–132 (2021). https://doi.org/10.1007/s12272-020-01296-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-020-01296-1