Abstract
A simple, sensitive, and rapid assay based on hydrophilic interaction liquid chromatography (HILIC) with tandem mass spectrometry was developed and validated for the simultaneous determination of metformin and 13 other oral antihyperglycaemic drugs in human urine using metoprolol as an internal standard. A simple sample clean-up procedure using the “dilute and shoot” approach enabled fast and reliable analysis. Chromatographic separation was performed on a HILIC column using an elution gradient of mobile phase A, composed of 1 mM ammonium formate (pH 5), and mobile phase B, composed of acetonitrile, at a flow rate of 0.35 mL/min. Quantitation was performed on a triple quadrupole mass spectrometer operated in multiple reaction monitoring mode by using electrospray ionization in positive ion mode. The total chromatographic run time was 20 min. Calibration curves for each analyte were linear over concentration ranges of 2–300, 5–400, or 20–500 ng/mL, with a coefficient of determination above 0.99. The method was validated for selectivity, sensitivity, recovery, linearity, accuracy and precision, system suitability, robustness, and stability. Inter-batch and intra-batch coefficients of variation across four validation runs were ≤ 13.62%. The present method was successfully applied for the analysis of metformin and nateglinide in urine samples after their oral administration to healthy human subjects under fasted conditions.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many oral antidiabetic drugs with different mechanisms of action have been developed to lower blood sugar and delay the occurrence of serious complications in patients with type 2 diabetes (Lebovitz 2004). For severe cases, monotherapy with an oral antidiabetic agent is not adequate to achieve satisfactory blood glucose control (Bell 2004). Thus, combination regimens, which include drugs with different and complementary mechanisms of action, are recommended (Bell 2004). Metformin (Fig. 1), an insulin-sensitizing agent, has become one of the most commonly prescribed medications for type 2 diabetes (Krentz and Bailey 2005). However, most oral antidiabetics can cause life-threatening hypoglycemia, and can therefore be misused with suicidal or homicidal intent (Wark 2009; Nakayama et al. 2005). Furthermore, in clinical cases of hypoglycemia of unknown etiology, the detection of oral antidiabetics allows differentiation of hypoglycemia due to oral antidiabetics from hypoglycemia due to other reasons (insulin-induced, insulinoma). Metformin is eliminated primarily by the kidneys, and no metabolites have been identified (Eyal et al. 2010). Thus, reliable, sensitive, and rapid bioanalytical methods are required to simultaneously detect and quantify metformin and other antihyperglycaemic drugs in human urine.
In the last few years, the use of LC–MS/MS has shown several advantages, such as enhanced sensitivity and the ability to analyze complex matrices with high selectivity, over other techniques. Various analytical strategies have been used to measure metformin in plasma, including normal phase chromatography (silica and cyano) and reversed phase chromatography with mass spectrometry (Wang et al. 2004; Marques et al. 2007; Koseki et al. 2005; Chen et al. 2004; Mistri et al. 2007; Li et al. 2013; Zhang et al. 2007; Georgita et al. 2007; Zhong et al. 2005; Polagani et al. 2013; Jagadeesh et al. 2013). Most of these techniques require large sample volumes, use complex sample preparation procedures, and require long chromatographic run times because metformin is difficult to extract and measure in biological fluids because of its high polarity (log P octanol/water = − 2.64). Hydrophilic interaction liquid chromatography (HILIC), first introduced in 1990 (Alpert 1990), has proven to be an effective way to separate polar analytes compared to traditional reversed-phase chromatography (Yang et al. 2009; Alpert 1990). In recent years, interest in HILIC–MS/MS methods has increased, because HILIC uses high amounts of organic solvent in the mobile phase, which favors ionization of compounds and increases detection sensitivity (Yang et al. 2009). Several recently published methods have described the use of HILIC–MS/MS for quantitative analysis of metformin in human plasma (Liu and Coleman 2009; Pontarolo et al. 2014), in dietary supplements and herbal medicines (Wu et al. 2012), or in the plasma and urine of a pregnant patient (Zhang et al. 2015). To date, none of the described HILIC–MS/MS methods have simultaneously monitored metformin, phenformin and other oral hypoglycemia drugs in human urine.
When developing a method for forensic analysis, a sample cleanup screen should be carefully selected. This is particularly important for LC separations because hundreds of different types of interference can occur when using biological matrixes (Kong et al. 2017). For the analysis of urine samples, a simple sample clean-up procedure, so called “dilute and shoot” has become a trend in the past 10 years in both analytical toxicology and doping-control analysis. The benefits such as easy sample preparation, omission of time-consuming extractions, thus lowering the total uncertainty budgets are the driving forces behind this trend (Deventer et al. 2014).
Recently, assay methods for other oral antihyperglycaemic drugs in biological samples using LC–MS/MS have also been reported. LC–MS/MS methods have been used to quantitate drugs and their metabolites in biological samples in vitro and in vivo. Hess et al. succeeded in simultaneously quantifying 11 oral hypoglycemia drugs (glimepiride, gliclazide, glipizide, glibenclamide, glibornuride, gliquidone, glisoxepide, repaglinide, nateglinide, rosiglitazone, and pioglitazone) in human plasma by using LC–MS/MS with a C8 column and an electrospray ionization (ESI) source operated in the positive ion detection mode (Hess et al. 2011). Polar antihyperglycaemic drugs such as metformin and phenformin were excluded. An UPLC–QToF-MS method was utilized for the quantification of antidiabetic agents (chlorpropamide, glibenclamide, gliclazide, glimepiride, metformin, nateglinide, pioglitazone, rosiglitazone, and vildagliptin) in human plasma (Fachi et al. 2016) but phenformin was not included in the study.
In the present study, a sensitive, simple, and rapid HILIC method with tandem mass spectrometric detection (HILIC–MS/MS) was developed and validated for quantitative analysis of metformin and 13 other oral antihyperglycaemic drugs (phenformin, tolbutamide, chlorpropamide, glibenclamide, gliclazide, glimepiride, glipizide, mitiglinide, repaglinide, nateglinide, pioglitazone, rosiglitazone, and sitagliptin) in human urine. A simple sample clean-up procedure using the “dilute and shoot” approach enabled fast and reliable preparation for analysis. The chemical structures of all drugs analyzed are shown in Fig. 1.
Materials and methods
Materials
All chemicals and reagents used in this study were of analytical grade. Sitagliptin (≥ 98.0%), pioglitazone (≥ 98.0%), glipizide (≥ 97.0%), nateglinide (≥ 97.0%), glimepiride (≥ 98.0%), metformin (≥ 98.0%) and metoprolol (≥ 99.0%) were gifts from Shinpoong Pharmaceutical Co. Ltd. (Ansan, Korea). Rosiglitazone (≥ 98.0%), gliclazide (≥ 98.0%), repaglinide (≥ 98.0%), glibenclamide (≥ 99.0%), chlorpropamide (≥ 97.0%), tolbutamide (99.9%), and phenformin (99.0%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mitiglinide (≥ 98.0%) was a gift from JW Pharmaceutical (Seoul, Korea). Sodium hydroxide (≥ 98.0%) was purchased from Duksan Pure Chemical Co. Ltd. (Ansan, Korea). Formic acid (≥ 99.5%) was purchased from Daejung (Siheung, Korea). Ammonium formate (≥ 95.0%) was purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Tablets containing 90 mg of nateglinide and 500 mg of metformin were gifts from Ildong Pharmaceutical Co. Ltd. (Seoul, Korea). HPLC-grade acetonitrile, ethanol, and methanol were from Daejung (Siheung, Korea). Purified water was prepared in the laboratory using an Aqua Max water purification system from Young Lin Instrument Co., Ltd. (Anyang, Korea).
Instrumentation and LC–MS/MS operation conditions
The LC–MS/MS system consisted of an Agilent 1200 series (Agilent Technologies, Santa Clara, USA) system coupled with an API 3200 Q Trap triple-quadrupole mass spectrometer (Absciex, Foster city, CA, USA) equipped with a Turbo V Ion Spray source. Separation was performed on a YMC-Triart Diol-HILIC column (50 × 3.0 mm i.d., 3 µm) from YMC Co. Ltd. (Kyoto, Japan) combined with a C18 guard column (4.0 × 3.0 mm i.d.) from Phenomenex (Torrance, CA, USA) using an elution gradient of 1 mM ammonium formate pH 5 (mobile phase A) and acetonitrile (mobile phase B) at a flow rate of 0.35 mL/min, 0% mobile phase B for 0.2 min, 0–70% mobile phase B for 9.8 min, 100–0% mobile phase B for 1 min, and 0% mobile phase B for 9 min. The temperature of the column was 35 °C. The analytical run time was 20.0 min. The positive ESI settings for analysis of the analytes and internal standard (IS) were as follows: ion source gas 1 (nebulizer gas), 40 (arbitrary units); ion source gas 2 (turbo heater gas), 70 (arbitrary units); curtain gas, 30 (arbitrary units); turbo-gas temperature, 500 °C; ion spray voltage 4000 V. Multiple reaction monitoring (MRM) mode was used for quantification (Table 1). Analyst 1.6 software was used for LC–MS/MS system control and data processing.
Preparation of calibration standards and quality control samples
Stock solutions of metformin, chlorpropamide, phenformin, sitagliptin, tolbutamide, gliclazide, glipizide, mitiglinide, nateglinide, rosiglitazone, pioglitazone, glibenclamide, glimepiride, repaglinide, and IS were prepared by dissolving the respective accurately weighed compounds in methanol to obtain final concentrations of 1000 μg/mL. Working standard mixture solutions of 100, 500, and 5000 ng/mL were prepared by serial dilutions of the stock solutions (5 µg/mL) in ammonium formate 1 mM (pH 5). IS stock solutions (5 µg/mL) were prepared in methanol, and a working IS mixture solution (5000 ng/mL) was prepared by dilution in ammonium formate 1 mM (pH 5). All solutions were stored at 4 °C, and were thawed at room temperature before use. Calibration standards (CSs) and quality control (QC) samples were prepared by spiking drug-free human urine with the respective working solutions. CSs were prepared at concentrations of 2, 5, 10, 20, 50, 100, 200, and 300 ng/mL for sitagliptin, rosiglitazone, pioglitazone, repaglinide, phenformin, and mitiglinide; 5, 10, 20, 50, 100, 200, 300, and 400 ng/mL for glipizide, gliclazide, nateglinide, glibenclamide, glimepiride, and metformin; and 20, 50, 100, 200, 300, 400, and 500 ng/mL for tolbutamide and chlorpropamide. Double blank (blank urine processed without IS) and single blank (blank processed with IS) samples were prepared using the same matrix. QCs were prepared at 2 ng/mL (the limit of quantitation, LOQ), 6 ng/mL (low-QC, LQC), 120 ng/mL (middle-QC, MQC), and 225 ng/mL (high-QC, HQC) for sitagliptin, rosiglitazone, pioglitazone, repaglinide, phenformin and mitiglinide; 5 ng/mL (LOQ), 15 ng/mL (LQC), 160 ng/mL (MQC), and 300 ng/mL (HQC) for glipizide, gliclazide, nateglinide, glibenclamide, glimepiride, and metformin; and 20 ng/mL (LOQ), 60 ng/mL (LQC), 200 ng/mL (MQC), and 375 ng/mL (HQC) for tolbutamide and chlorpropamide. Spiked urine samples were aliquoted into microcentrifuge tubes, and stored at − 20 °C until further use.
Sample preparation
One thousand microliters of human blank urine, CSs, and QC samples were vortex-mixed with 40 µL of IS solution and 200 µL of ammonium formate 1 mM (pH 3) buffer for 1 min at high speed. After centrifugation at 15,000 rpm at 15 °C for 5 min, 10 µL of supernatant was injected into the LC–MS/MS.
Method validation
The method was validated for system suitability (SST), selectivity, sensitivity (the detection limit LOD, the quantification limit LOQ), linearity, carryover, qualitative matrix effect, precision and accuracy, robustness, stability, and dilution integrity following International Council for Harmonisation (ICH) guidelines for impurity in new drugs (Q3A 2008), ICH guidelines for validation of an analytical procedure (Q2 2005), and guidelines for bio-analytical method validation.
The selectivity of this method was investigated by preparing and analyzing six individual human blank urine samples. Selectivity was assessed by comparing signals for analytes and IS in these blank samples with signals obtained from an analysis of human urine sample spiked with a concentration of analytes at LOQ and IS at the working concentration (200 ng/mL).
The linearity of the method was determined by analysis of standard plots associated with 11-point standard calibration curves. A total of six linearity curves containing ten non-zero concentrations were analyzed. Best-fit calibration curves of peak area ratio versus concentration were drawn. The calibration curve was constructed by plotting peak area ratios (y) of analytes relative to the IS versus analyte concentrations (x). Concentrations of analytes were calculated from calibration curve equations (y = mx + c). The regression equation for each calibration curve was also used to back-calculate the measured concentration at each QC level, and slope, intercept and correlation coefficient were calculated as regression parameters.
Intra-day and inter-day assay precision and accuracy were evaluated by determining analyte concentrations in 11 replicates of QC samples at four different concentrations [lower limit of quantification (LLOQ), LQC, MQC, and HQC] daily for three separate days. These four concentrations cover the LOQ, low, medium, and high range of the standard curve. Each run consisted of CSs, blank urine samples with and without IS in duplicate, and QC samples in 11 replicates. The accuracy of the method was determined as relative error (RE) in percentage ((difference between the mean calculated and added concentration)/added concentration) × 100, whereas precision was evaluated by intra- and inter-day coefficients of variation (CV, %). Precision (CV, %) and accuracy (RE, %) values of ≤ 15% for all QCs tested with the exception of that related to the LOQ (≤ 20%) were considered acceptable.
Carryover effects were also examined. Carryover on the autosampler was observed with some high-concentration samples prior to application of an automated two-step wash procedure. After application of the wash procedure, carryover for all analytes was evaluated by injecting a blank sample immediately after a high pool sample. Carryover in this case is defined as the area counts measured in the blank as a percentage of those present in the preceding sample for each analyte. The percent carryover was found to be minimal for all analytes, and concentrations in the blank sample were below the limits of detection for all analytes. Carryover in the blank sample following the high concentration standard should not be more than 20% of the LLOQ and not more than 5% for the IS.
Matrix effect values (%) in the matrixes were evaluated by comparing peak area values of screening transitions from matrix-matched standard (MMS) samples at three different concentrations (LQC, MQC and HQC) with those from standard solutions in water at the same concentrations as MMS samples (n = 6). Recovery values (%) in each matrix were assessed by comparing the peak area of a screening transition from a QC sample with that of an MMS sample at the same concentration (n = 6). A total of three QC samples (LQC, MQC, and HQC samples) per matrix were tested to determine the recovery rate of the methods. Relative standard deviation (RSD, %) values calculated for the concentrations should not be more than ± 15%.
An important part of method validation is SST, details of which are usually given in Pharmacopoeias. SST analysis was performed under optimized chromatographic conditions. In mass spectrometric methods, only repeatability of retention times and peak areas were checked.
The robustness of the developed method was investigated by evaluating the influence of small deliberate variations in procedure variables like flow rate (± 0.05 mL/min) and pH of the mobile phase (± 0.5 units).
Stability tests were performed using LOQ and QC samples or standard solutions at the same concentrations, as follows: (1) freeze and thaw stability of the standard solutions for three cycles at − 20 °C, (2) short-term stability of standard solutions at room temperature for 6 h, (3) long-term stability of standard solutions at − 20 °C for 3 weeks, and (4) stability of QC samples in an autosampler at 4 °C for 24 h.
Application of the method
This method was applied for the detection and quantitation of metformin and nateglinide in a urine sample obtained from a 31-year-old healthy male volunteer after oral administration of a tablet containing a combination of metformin (500 mg) and nateglinide (90 mg). Urine samples were collected after intake of the drug, and were stored at − 20 °C until further analysis.
Results
Mass spectrometry
It was important to optimize chromatographic conditions and mass spectrometry parameters to develop and validate a selective and rapid assay method for simultaneous quantitation of metformin and 13 oral antihyperglycaemic drugs in human urine. MS parameters were optimized by infusing a standard analyte solution of 500 ng/mL into the mass spectrometer operating in the MRM mode by using electrospray as the ionization source. Signal intensities obtained in the positive mode were much higher than those obtained in the negative ion mode, as analytes and IS have the ability to accept protons. The protonated form of each analyte and IS, [M+H]+ ion was the parent ion in the Q1 spectrum and was used as the precursor ion to obtain Q3 product ion spectra. Fragmentation was initiated using sufficient nitrogen for collision-activated dissociation, and by applying 20 V collision energy to break parent ions. It was observed that higher nebulizer gas pressure positively affected spectral response. Ion spray voltage and temperature did not have a considerable effect on the behavior of compounds, and were maintained at 4000 V and 500 °C, respectively. Q1 and Q3 were set at unit resolution, and dwell time was maintained at 150 ms. No crosstalk between the MRMs of the analytes and of the IS was found. Fine tuning of gas 1 (nebulizer gas), gas 2 (heater gas), and CAD gas was done to get a consistent and stable response with a high signal-to-noise ratio (S/N). Table 1 shows parent and product ions for analytes and IS, respectively. ESI was selected as the ionization source, as it gave high spectral responses for both the analytes, and the regression curves obtained were linear. Moreover, the ESI source provided reliable data on method validation and quantitation of samples from human volunteers. We did not test an atmospheric pressure chemical ionization (APCI) source. Some studies indicate that APCI is much less susceptible to analyte-ion suppression compared with ESI and EI techniques (Mallet et al. 2004; Jemal et al. 2003). However, APCI is often used only when ESI fails to provide the required sensitivity, as APCI requires strict operating conditions such as a high flow rate, high temperature, and high voltage, and is thus incompatible with thermally labile compounds (Jemal et al. 2003).
Liquid chromatography
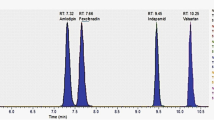
As metformin and the other 13 oral antihyperglycaemic drugs have different physicochemical properties, it was difficult to set chromatographic conditions that produced sharp peak shapes and adequate response. This included mobile phase selection, flow rate, column type, and injection volume. Methanol and acetonitrile were investigated at different ratios with buffers, such as ammonium acetate and ammonium formate, as well as acid additives, such as formic acid and acetic acid at varying strengths. LC–MS/MS mobile phase combinations included organic solutions (methanol or acetonitrile) with formic acid (0.1 or 0.5%) and/or ammonium formate (1, 10, 50, 100, or 200 mM) at pH 3, 4, or 5 as additives. It was observed that using an elution gradient of 1 mM ammonium formate (pH 5.0) (mobile phase A) and acetonitrile (mobile phase B) provided the best sensitivity, efficiency, and peak shape. The elution gradient was as follows: 0% mobile phase B for 0.2 min, 0–70% mobile phase B for 9.8 min, 100–0% mobile phase B for 1 min, and 0% mobile phase B for 9 min. An acidic buffer helped improve peak shape and spectral response. The use of a short HILIC column (50 × 3.0 mm i.d., 3 µm) connected to a C18 guard column (4.0 × 3.0 mm i.d.) was useful in separating and eluting all compounds in a short time. The total chromatographic run time was 20.0 min for each run. In Fig. 2, chromatograms of the quantitative ion transitions in MRM mode for a spiked urine sample with 200 ng/mL of each analyte are shown.
Validation
Selectivity and lower limit of quantification
No interfering peaks from endogenous compounds were observed at the retention times of analytes or IS. The total chromatographic run time was 20 min. The LLOQ was the concentration at which the precision and variance of accuracy were ≤ 20%, and the S/N was ≥ 10. RSD (%) was used to assess precision. RE (%) was used to estimate accuracy, which was calculated as ((measured conc. − nominal conc.)/nominal conc.) × 100%.
Qualitative matrix effect and recovery
The matrix factors of analytes and IS were evaluated by comparing the analyte/IS ratio in human urine and water samples at low, medium, and high concentrations. The matrix factor for the 14 analytes in urine samples ranged from 80 to 99%: metformin (82–93%), chlorpropamide (82–96%), phenformin (80–84%), sitagliptin (81–95%), tolbutamide (80–86%), gliclazide (80–82%), glipizide (86–99%), mitiglinide (81–89%), nateglinide (80–81%), rosiglitazone (81–90%), pioglitazone (80–87%), glibenclamide (80–85%), glimepiride (80–82%), and repaglinide (82–93%), with a RSD of ≤ 15%. Recovery results are presented in Table 2. The matrix factor for urine samples was also acceptable and in agreement with US Food and Drug Administration guidelines.
Carryover
The carryover effect was tested by instantly analyzing blank samples following samples at the upper limit of quantification (ULOQ). No residual peaks were observed at the retention times of metformin and chlorpropamide. Carryover of other compounds was low enough to meet acceptable criteria.
Linearity and LLOQ
The urine calibration curve was constructed using CSs of 2–300 ng/mL for sitagliptin, rosiglitazone, pioglitazone, repaglinide, phenformin, and mitiglinide; 5–400 ng/mL for glipizide, gliclazide, nateglinide, glibenclamide, glimepiride, and metformin; 20–500 ng/mL for tolbutamide and chlorpropamide. The calibration curve was prepared by determining the best fit of peak-area ratios (peak area analyte/peak area IS) versus concentration, and was fitted to the y = mx + c linear equation form using the weighting factor (1/X2). The average correlation coefficient (n = 6) was found to be ≥ 0.99. The lowest concentration with an RSD ≤ 20% was considered to be LLOQ. Linearity results are presented in Table 3.
Precision and accuracy
Accuracy and intra- and inter-assay precision were determined by analyzing 11 replicates of QC samples at four concentrations on three different days. The results are shown in Table 4.
System suitability
SST was assessed by analyzing six consecutive injections of the standard mixture of analytes at 200 ng/mL each. Parameters such as repeatability of reference standard solution injections were established (retention times and peak areas were checked, and the repeatability was expressed as RSD in %). SST results are presented in Table 3.
Robustness
Minor deliberate changes in different experimental parameters such as flow rate (± 0.05 mL/min) and pH of the mobile phase (± 0.5 units) did not significantly affect recoveries, peak areas, or retention times for all analytes, indicating that the proposed method is robust.
Stability
Analytes are considered stable in biological matrices when 85–115% of the initial concentration can be detected. The stabilities of all analytes in human urine under different storage conditions are presented in Table 5. No significant degradation was detected under the conditions described in this study, as their concentrations deviated by no more than 14.34% relative to the reference nominal concentrations. No degradation products were detected under the selected MS conditions. All analytes in human urine can therefore be stored at room temperature for 4 h, or at − 20 °C for 21 days, and can tolerate three freeze–thaw cycles. Analysis of QC samples showed no significant degradation after 6 h at room temperature. These results indicate that analytes are stable under routine laboratory conditions, and no specific procedure (e.g., acidification, or addition of organic solvents) is required to stabilize compounds for daily clinical drug monitoring.
Application
The present method was applied to detect and quantify metformin and nateglinide in a urine sample obtained from a 31-year-old healthy male volunteer after oral administration of a tablet containing a combination of metformin (500 mg) and nateglinide (90 mg). After 2 days of administration, the concentration of nateglinide was 17.3 ng/mL and the concentration of metformin was 692.7 ng/mL in urine (Fig. 3).
Samples for dilution tests were prepared by spiking blank human urine with respective working solutions. The following concentrations were used for dilution tests: 700 ng/mL (the highest concentration of QC sample). An ultrahigh dilution QC (700 ng/mL) was prepared and diluted to the concentration close to the concentration used for the assay. The diluted concentration (350 ng/mL) was analyzed as part of method validation. The accuracy of dilution was 103.4%, and the precision of dilution integrity was 4.83%. These results indicate that samples containing metformin at concentrations higher than the ULOQ may be diluted and re-assayed.
Discussion
An HILIC–MS/MS method for simultaneous quantitative analysis of metformin and 13 oral antihyperglycaemic drugs in human urine was developed and validated. The use of HILIC with a high level of organic solvents in the mobile phase solved the problem of poor retention of metformin on an analytical column, and increased the sensitivity of the assay by improving ionization efficiency for ESI–mass spectrometry (Liu and Coleman 2009). To the best of our knowledge, this is the first report of simultaneous quantitative analysis of the levels of metformin and 13 oral antihyperglycaemic drugs in human urine using HILIC–MS/MS. This method provides the sensitivity, accuracy, and precision required for quantitative measurements of metformin and 13 oral antihyperglycaemic drugs in human urine. This method is useful for the rapid and accurate determination of metformin and other oral antihyperglycaemic drugs using a small amount of urine in forensic and clinical toxicology.
References
Alpert AJ (1990) Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J Chromatogr A 499:177–196
Bell DS (2004) Type 2 diabetes mellitus: what is the optimal treatment regimen? Am J Med 116:23–29
Chen X, Gu Q, Qiu F, Zhong D (2004) Rapid determination of metformin in human plasma by liquid chromatography–tandem mass spectrometry method. J Chromatogr B 802:377–381
Deventer K, Pozo OJ, Verstraete AG, Van Eenoo P (2014) Dilute-band-shoot-liquid chromatography–mass spectrometry for urine analysis in doping control and analytical toxicology. Trends Anal Chem 55:1–13
Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GD, Clark SM, Risler L, Wang J, Kelly EJ, Shen DD, Hebert MF (2010) Pharmacokinetics of Metformin during Pregnancy. Drug Metab Dispos 38(5):833–840
Fachi MM, Cerqueira LB, Leonart LP, de Francisco TMG, Pontarolo R (2016) Simultaneous quantification of antidiabetic agents in human plasma by a UPLC–QToF-MS method. PLoS ONE 11(12):e0167107
Georgita C, Albu F, David V, Medvedovici A (2007) Simultaneous assay of metformin and glibenclamide in human plasma based on extraction-less sample preparation procedure and LC/(APCI)MS. J Chromatogr B 854:211–218
Hess C, Musshoff F, Madea B (2011) Simultaneous quantification and validated quantification of 11 oral hypoglycaemic drugs in plasma by electrospray ionisation liquid chromatography–mass spectrometry. Anal Bioanal Chem 400:33–41
Jagadeesh B, Bharathi DV, Pankaj C, Narayana VS, Venkateswarulu V (2013) Development and validation of highly selective and robust method for simultaneous estimation of pioglitazone, hydroxypioglitazone and metformin in human plasma by LC–MS/MS: application to a pharmacokinetic study. J Chromatogr B 930:136–145
Jemal M, Schuster A, Whigan DB (2003) Liquid chromatography/tandem mass spectrometry methods for quantitation of mevalonic acid in human plasma and urine: method validation, demonstration of using a surrogate analyte, and demonstration of unacceptable matrix effect in spite of use of a stable isotope analog internal standard. Rapid Commun Mass Spectrom 17:1723–1734
Kong TY, Kim JH, Kim JY, In MK, Choi KH, Kim HS, Lee HS (2017) Rapid analysis of drugs of abuse and their metabolites in human urine using dilute and shoot liquid chromatography–tandem mass spectrometry. Arch Pharmacal Res 40:180–196
Koseki N, Kawashita H, Niina M, Nagae Y, Masuda N (2005) Development and validation for high selective quantitative determination of metformin in human plasma by cation exchanging with normal-phase LC/MS/MS. J Pharm Biomed Anal 36:1063–1072
Krentz AJ, Bailey CJ (2005) Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 65:385–411
Lebovitz HE (2004) Oral antidiabetic agents: 2004. Med Clin N Am 88:847–863
Li N, Deng Y, Qin F, Yu J, Li F (2013) Simultaneous quantification of metformin and glipizide in human plasma by high-performance liquid chromatography–tandem mass spectrometry and its application to a pharmacokinetic study. Biomed Chromatogr 27:191–196
Liu A, Coleman SP (2009) Determination of metformin in human plasma using hydrophilic interaction liquid chromatography–tandem mass spectrometry. J Chromatogr B 877:3695–3700
Mallet CR, Lu Z, Mazzeo JR (2004) A study of ion suppression effects in electrospray ionization from mobile phase additives and solid-phase extracts. Rapid Commun Mass Spectrom 18:49–58
Marques MAS, Soares AdSS, Pinto OW, Barroso PTW, Pinto DP, Ferreira-Filho M, Werneck-Barroso E (2007) Simple and rapid method determination for metformin in human plasma using high performance liquid chromatography tandem mass spectrometry: application to pharmacokinetic studies. J Chromatogr B 852:308–316
Mistri HN, Jangid AG, Shrivastav PS (2007) Liquid chromatography tandem mass spectrometry method for simultaneous determination of antidiabetic drugs metformin and glyburide in human plasma. J Pharm Biomed Anal 45:97–106
Nakayama S, Hirose T, Watada H, Tanaka Y, Kawamori R (2005) Hypoglycemia following a nateglinide overdose in a suicide attempt. Diabetes Care 28:227–228
Polagani SR, Pilli NR, Gajula R, Gandu V (2013) Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC–MS/NS and its application to a human pharmacokinetic study. J Pharm Anal 3:9–19
Pontarolo R, Gimenez AC, Francisco TMGd, Ribeiro RP, Pontes FL, Gasparetto JC (2014) Simultaneous determination of metformin and vildagliptin in human plasma by a HILIC–MS/MS method. J Chromatogr B 965:133–141
Wang Y, Tang Y, Gu J, Fawcett JP, Bai X (2004) Rapid and sensitive liquid chromatography–tandem mass spectrometric method for the quantitation of metformin in human plasma. J Chromatogr B 808:215–219
Wark G (2009) How to look for insulin etc. Guildford Peptide Hormones Scheme, SAS Peptide Hormone Section, Royal Surrey County Hospital
Wu X, Zhu B, Lu L, Huang W, Pang D (2012) Optimization of a solid phase extraction and hydrophilic interaction liquid chromatography–tandem mass spectrometry method for the determination of metformin in dietary supplements and herbal medicines. Food Chem 133:482–488
Yang Y, Boysen RI, Hearn MTW (2009) Hydrophilic interaction chromatography coupled to electrospray mass spectrometry for the separation of peptides and protein digests. J Chromatogr A 1216:5518–5524
Zhang L, Tian Y, Zhang Z, Chen Y (2007) Simultaneous determination of metformin and rosiglitazone in human plasma by liquid chromatography/tandem mass spectrometry with electrospray ionization: application to a pharmacokinetic study. J Chromatogr B 854:91–98
Zhang X, Wang X, Vernikovskaya DI, Fokina VM, Nanovskaya TN, Hankins GD, Ahmed MS (2015) Quantitative determination of metformin, glyburide and its metabolites in plasma and urine of pregnant patients by LC–MS/MS. Biomed Chromatogr 29:560–569
Zhong GP, Bi HC, Zhou S, Chen X, Huang M (2005) Simultaneous determination of metformin and gliclazide in human plasma by liquid chromatography–tandem mass spectrometry: application to a bioequivalence study of two formulations in healthy volunteers. J Mass Spectrom 40:1462–1474
Acknowledgements
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors thank the Institute of New Drug Development Research and the Central Laboratory of Kangwon National University for the analytical instrument.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared no conflicts of interest.
Rights and permissions
About this article
Cite this article
Truong, QK., Mai, XL., Lee, JY. et al. Simultaneous determination of 14 oral antihyperglycaemic drugs in human urine by liquid chromatography–tandem mass spectrometry. Arch. Pharm. Res. 41, 530–543 (2018). https://doi.org/10.1007/s12272-018-1011-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-018-1011-9