Abstract
The sap from the succulent Desmidorchis flava (N.E.Br) Meve and Liede yielded a new pregnane glycoside, named nizwaside whose structure was established using 1D and 2D NMR techniques as well as mass spectrometry (ESIMS). Nizwaside was tested for anticancer, DPPH antioxidant, urease enzyme inhibition, α-glucosidase enzyme inhibition and acetylcholinesterase inhibition activities. Interestingly, nizwaside showed significant anti-proliferative effects on MDA MB231 breast cancer cells with an IC50 of 23.5 µg/ml. Moreover, nizwaside was more effective than Doxorubicin, a well-known clinical anticancer drug, in suppressing MDA MB231 cell proliferation even at concentrations lower than that of Doxorubicin (75 µg/ml nizwaside vs. 100 µg/ml Doxorubicin). On the other hand, nizwaside showed relatively weak antioxidant activity with 15 % inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pregnane glycosides are C21 steroidal natural products attached to sugars and have generally been reported to be present in the family Asclepiadaceae. Furthermore, these compounds demonstrate a fair degree of diversity in the aglycone part with different numbers and types of sugar units being attached at the aglycone C-3 position (Araya et al. 2012). Interestingly, these pregnane glycosides have been reported to have antitumor, anti-inflammatory, analgesic, antimicrobial, platelet pro-aggregating and digitalis receptor binding activities (Abdel-Sattar et al. 2007; Babu et al. 2008). Furthermore, these compounds have been shown to exhibit anti-acetylcholinesterase and anti-amnesic properties (Lee et al. 2005).
In this study, we investigated the sap of Desmidorchis flava (N.E.Br) Meve & Liede (Caralluma flava N.E.Br) and isolated a new pregnane glycoside named nizwaside (1) whose anticancer activity towards breast cancer cells (MDA-MB-231 cells) is being reported for the first time.
Materials and methods
General
Optical rotations were measured on a KRUSS P P3000 polarimeter (A. Kruss Optronic, Germany). IR spectra were recorded on a Bruker, ATR-Tensor 37 spectrophotometer. ESI–MS were recorded on a Waters Quattro Premier XE Mass Spectrometer (Waters, Milford, MA). The 1H and 13C NMR spectra were recorded on Bruker NMR spectrometers operating at 600 MHz (150 MHz for 13C). The chemical shift values are reported in ppm (δ) units and the coupling constants (J) are given in Hz. For TLC, pre-coated aluminum sheets (silica gel 60 F-254, E. Merck) were used. Visualization of the TLC plates was achieved under UV light at 254 and 366 nm and by spraying with ceric sulfate reagent.
Sample collection and identification
Fresh D. flava was collected from various mountains of Oman including Jebel Akhdar, Jabel Shams and Jebel Hatt. The plant specimen was identified and authenticated by plant taxonomist Dr. Syed Abudallh Gilani, Department of Biological Sciences and Chemistry, University of Nizwa. A voucher specimen (No: BSHR-01/2014) was deposited in the herbarium of the Department of Biological Sciences and Chemistry.
Collection of sap, crystallization and chromatographic purification of the isolated product
The sap of D. flava was collected by the prosess of physically gently squeezing the sliced juicy stem segments by hand. The collected sap was then dissolved in the minimum of dichloromethane after which the solution was slowly triturated with methanol to form a milky type precipitate which was allowed to coagulate over a 48 h period. The resulting two phased system was very gently filtered using whatman filter paper which separated the milky solids and afforded a clear filtrate which was allowed to evaporate at ambient temperature to afford yellow shiny crystals (1.5 g). These were adsorbed and loaded onto a silica gel column and chromatographed using gradients of n-hexane/CH2Cl2 (85:15, 50:50, 0:100) followed by CH2Cl2/MeOH (85:15) to give 10 subfractions (F1–10). The nizwaside (1), obtained from this column was purified by re-chromatography with CH2Cl2/MeOH (96:4) as eluent, to yield 12 mg of pure material.
Nizwaside (1)
White solid; mp: 172 °C; [α] 25D = −4.0 (c 0.04, MeOH); IR (KBr): 3400, 1710, 1620, 1450, 1060 cm−1; UV (CH2Cl2) λmax (log ε) 219 (3.04), 229 (2.90) nm. 1H and 13C NMR (600 MHz and 150 MHz, CD3OD): Table 1; ESIMS: m/z (rel. int.): m/z 1031.1 [M + Na]+ (88); HRESIMS: 1031.5332 (calcd for C56H80NaO16, 1031.5338).
Anticancer activity
Cell line and reagents
The breast cancer cell line MDA-MB-231 was maintained in DMEM (Invitrogen, Carlsbad, CA, USA). The medium was supplemented with 10 % fetal bovine serum (FBS) and 1 % antimycotic antibiotic (Invitrogen, Carlsbad, CA, USA). Cells were cultured in a 5 % CO2—humidified atmosphere at 37 °C. Stock solutions of compound 1 and Doxorubicin were made in DMSO at a final concentration of 2 mg/ml and were always made fresh just prior to experiments.
Cell growth inhibition studies by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cells were seeded at a density of 1 × 105 cells per well in 96-well microtiter culture plates. After overnight incubation, the normal growth medium was removed and replaced with either fresh medium (untreated control) or different concentrations of compound 1 in growth medium diluted from a 2 mg/ml stock solution. After 24 h of incubation, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml in PBS) was added to each well and incubated for a further 4 h at 37 °C. Upon termination, the supernatant was aspirated and the MTT formazan, formed by metabolically viable cells, was dissolved in a solubilization solution containing DMSO (100 µl) by mixing for 5 min on a gyratory shaker. The absorbance was measured at 540 nm on an Ultra Multifunctional Microplate Reader (Bio-Rad, USA). Each treatment had eight replicate wells and each experiment was repeated at least three times.
Results
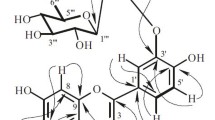
Phytochemical investigation of the Omani medicinal plant D. flava provided a pregnane glycoside, viz., nizwaside (1) (Fig. 1).
The molecular formula of compound 1 was established as C56H80O16 based on a quasi-molecular ion peak at m/z 1031.5332 (calcd for C56H80NaO16, 1031.5338) and 13C NMR analysis. The IR spectrum of compound 1 displayed hydroxyl, ester and aromatic absorption bands at 3400, 1710, and 1610 cm−1, respectively. The NMR spectrum of the aglycone portion (Table 1) displayed signals for three methyl groups [δH 0.82 (s, 3H, Me-19); δC 12.4 (C-19); δH 1.09 (s, 3H, Me-18); δC 10.0 (C-18); δH 1.35 (d, J = 6.6 Hz, 3H, Me-21); δC 18.9 (C-21)] and three oxygenated methine signals [δH 3.62 (m, 1H, H-3); δC 78.5 (C-3); δH 4.95 (dd, J = 4.8, 12.0 Hz, 1H, H-12); δC 79.4 (C-12); δH 5.24 (q, J = 6.6 Hz, 1H, H-20); δC 75.7 (C-20)].
The complete structure confirmation of the aglycone part of compound 1 was established by COSY and HMBC experiments (Fig. 2). The HMBC correlations between Me-19 and C-10, C-1, C-5, and C-9, Me-18 with C-13, C-12, C-14, and C-17; CH2-11 with C-9, C-13, C-12, and C-8; H-20 and C-13, C-16, C-17, and C-21; H-12 and C-9, C-11, C-13, C-14, C-17, and Me-18 confirmed the 3,12,20-trioxygenated pregnane skeleton (Araya et al., 2012). Furthermore, the oxygenated quaternary carbon signal at δ 87.2 is typical for pregnanes with an OH group at C-14 (Araya et al., 2012). The presence of two benzoyl units was confirmed by the 1H NMR data [12-Bz: δ 8.03 (dd, J = 1.2, 8.4 Hz, H-2′/H-6′), 7.67 (t, J = 8.4 Hz, H-4′), 7.50 (t, J = 8.4 Hz, H-3′/5′); 20-Bz: δ 7.73 (dd, J = 1.2, 8.4 Hz, H-2′/H-6′), 7.52 (t, J = 8.4 Hz, H-4′), 7.22 (t, J = 8.4 Hz, H-3′/5′)] (Abdel-Sattar et al. 2007; Araya et al. 2012). The presence of two benzoyl units was further confirmed by two ester C=O signals at 168.0 and 167.4 in the 13C NMR spectrum (Abdel-Sattar et al. 2007; Araya et al. 2012). The attachment of the two benzoyl units at C-12 and C-20 were confirmed based on the 3 J HMBC correlations of H-12 (δ 4.95) and H-20 (δ 5.24) to the benzoyl ester carbonyls (δ 168.0 and 167.4 respectively).
The NMR spectra also displayed the presence of three sugar anomeric units [δH 4.71 (d, J = 8.4 Hz, H-1″); δC 102.6 (C-1″); δH 4.57 (dd, J = 2.0, 9.0 Hz, H-1″′); δC 102.2 (C-1″′); δH 4.84 (dd, J = 2.0, 9.0 Hz, H-1′′′′); δC 97.1 (H-1′′′′′)], three Me groups [δH 1.19 (d, J = 6.0 Hz); δC 18.9; δH 1.22 (d, J = 6.0 Hz); δC 18.2; δH 1.20 (d, J = 6.0 Hz); δC 18.5] and three sugar methoxy groups [δH 3.59 (s); δC 62.5; δH 3.40 (s); δC 57.4; δH 3.41 (s); δC 58.4], suggesting it to be a triglycoside. The β-linkages of the three sugars units were confirmed by the large coupling constants (J = 8.4–9.0) of the anomeric protons (Abdel-Sattar et al. 2007; Araya et al. 2012). The 1D (1H and 13C) and 2D (COSY, HMBC, and NOESY) NMR data suggested that the sugar part of compound 1 consists of two 2,6-dideoxy sugars and one 6-deoxy sugar (Araya et al. 2012; Abdel-Sattar et al. 2007). From the NMR data the three sugar units were identified as two cymarose (=2,6-dideoxy-3-O-methyl-ribohexose) and one 6-deoxy-3-O-methyl-D-allopyranose (Abdel-Sattar et al. 2007; Araya et al. 2012). Most importantly the connectivity of the sugar units and the connectivity of the sugar unit to the pregnane skeleton in compound 1 were found via key HMBC correlations (Fig. 2): 6-deoxy-3-O-methyl-D-allopyranose anomeric proton H-1″ and C-3; H-3 and H-1″; D-cymaropyranose anomeric proton H-1″′ and C-4″; H-4″ and C-1″′; D-cymaropyranose anomeric proton H-1′′′′ and C-4″′; and H-4″′ and C-1′′′′.
The relative stereochemistry at C-3 was confirmed by comparison of NMR data [1: δH 3.65 (m, H-3); δC 78.5 (C-3); Lit: δH 3.64-3.68 (m, H-3); δC 78.1-78.4 (C-3)] with those of related pregnane glycosides (Leo et al. 2005) as well as from NOESY correlation of H-3 with H-5. Moreover NOE correlations of H-12 with H-11α and H-17α, with no correlations with Me-18, suggested the α-configuration of H-12 and H-17. The stereochemistry of C-20 was deduced as S in a same manner as reported in the literature for related pregnane glycosides: the NOE correlations between H-20/H-21, H-20/H-18 and H-l6a/H-21 and with no NOE correlation between H-21 and H-l8 (Itokawa et al. 1988; Qiu et al. 1997; E. Abdel-Sattar et al. 2001). From the above evidence, the structure of compound 1 was established as 12,20-di-O-benzoyl-pregnane-3β,12β,14β,20-tetraol 3-O-β-D-cymaropyranosyl-(1 → 4)-β-D-cymaropyranosyl-(1 → 4)-6-deoxy-3-O-methyl-β-D-allopyranoside.
Anticancer, DPPH antioxidant, urease, α-glucosidase, and acetylcholinesterase activities of nizwaside (1) are compiled in Table 2 and Fig. 3.
Effect of compound 1 on cancer cell viability. Values are presented as Mean ± SD of two duplicates. Cells (1 × 105/well) were seeded and allowed to adhere firmly in the cell culture medium with 10 % FBS. The cells were treated with compound 1 for 24 h and medium was removed. The cells were then incubated with MTT reagent (5 mg/ml) and crystal violet dissolved in DMSO for 4 h, and absorbance was read at 540 nm (reference wavelength—690 nm). Absorbance of control (without treatment) was considered as 100 % cell survival
Discussion
It has been reported that plant-based drugs are playing a most crucial role in the public health sector and the WHO estimated that 80 % people of the world employed traditional medicines for their primary health care (Farnsworth et al. 1985). Natural products have played a crucial role in drug discovery and are the main source for producing effective anticancer agents. It has been reported that natural products introduced more than 60 % of anticancer drugs in last three decades (Kingston 2009).
Nizwaside (1) was tested for its effect on the proliferation of breast cancer cells. As shown in Fig. 3 and Table 2, treatment of MDA MB231 breast cancer cells with compound 1 at different concentrations caused a significant decline in cell viability. This anti-proliferative effect of compound 1 against breast cancer cells, being a concentration-dependent one, resulted in 50 % inhibition of cell proliferation (IC50) of MDA MB231 breast cancer cells in culture at a concentration of 23.5 µg/ml. Compound 1 also turned out to be more effective than Doxorubicin, a well-known clinical anticancer drug, in suppressing MDA MB231 cell proliferation even at concentrations lower than that of Doxorubicin (75 µg/ml compound 1 vs. 100 µg/ml Doxorubicin). This suggests that compound 1 has the potential to effectively suppress the growth of breast cancer cells and may possibly have similar effects on other cancer cell lines. Therefore, this compound may serve as a good candidate for future anticancer drug development and consequently needs to be explored further for its anticancer action and an understanding of the mechanisms thereof.
Nizwaside (1) was also tested for DPPH antioxidant, urease enzyme inhibition, α-glucosidase enzyme inhibition, and acetylcholinesterase inhibition activities (Table 2) and demonstrated moderately weak antioxidant activity with 15 % inhibition.
References
Abdel-Sattar, E., M.A.A. Al-Yahya, N. Nakamura, and M. Hattori. 2001. Penicillosides A-C, C-15 oxypregnane glycosides from Caralluma penicillata. Phytochemistry 57: 1213–1217.
Abdel-Sattar, E., A.A. Ahmed, M.E.F. Hegazy, M.A. Farag, and M.A.A. Al-Yahya. 2007. Acylated pregnane glycosides from Caralluma russeliana. Phytochemistry 68: 1459–1463.
Araya, J.J., F. Binns, K. Kindscher, and B.N. Timmermann. 2012. Verticillosides A–M: Polyoxygenated pregnane glycosides from Asclepias verticillata L. Phytochemistry 78: 179–189.
Babu, K.S., V.R.S. Rao, S.V. Radhakrishnan, J.M. Rao, and S.S. Rambabu. 2008. A new pregnane steroid from the stems of Caralluma umbellata. Journal of Asian Natural Products Research 10: 1013–1016.
Farnsworth, N.R., O. Akerele, A.S. Bingel, D.D. Soejarto, and Z. Guo. 1985. Medicinal plants in therapy. The Bulletin of the World Health Organization 63: 965–972.
Itokawa, H., J. Xu, and K. Takeya. 1988. Pregnane glycosides from an antitumour fraction of Periploca sepium. Phytochemistry 27: 1173–1179.
Kingston, D.I.G. 2009. Tubulin-interactive natural products as anticancer agents. Journal of Natural Products 72: 507–515.
Lee, K.Y., J.S. Yoon, E.S. Kim, S.Y. Kang, and Y.C. Kim. 2005. Anti-acetylcholinesterase and anti-amnesic activities of a pregnane glycoside, cynatroside B, from Cynanchum atratum. Planta Medica 71: 7–11.
Leo, M.D., N.D. Tommasi, R. Sanogo, G. Autore, S. Marzocco, C. Pizza, I. Morelli, and A. Braca. 2005. New pregnane glycosides from Caralluma dalzielii. Steroids 70: 573–585.
Qiu, S.-X., L.-Z. Lin, G.A. Cordell, M. Ramesh, B.R. Kumar, M. Radhakrishna, G.K. Mohan, B.M. Reddy, Y.N. Rao, B. Srinivas, S.N. Thomas, and A.V.N. Appa Rao. 1997. Acylated C-21 steroidal bisdesmosidic glycosides from Caralluma umbellata. Phytochemistry 46: 333–340.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hussain, H., Raees, M.A., Rehman, N.U. et al. Nizwaside: a new anticancer pregnane glycoside from the sap of Desmidorchis flava . Arch. Pharm. Res. 38, 2137–2142 (2015). https://doi.org/10.1007/s12272-015-0653-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0653-0