Abstract
Natural killer (NK) cells are capable of identifying and killing tumor cells as well as virus infected cells without pre-sensitization. NK cells express activating and inhibitory receptors, and can distinguish between normal and tumor cells. The present study was designed to demonstrate the importance of the expression level of NKG2D ligands on the Burkitt’s lymphoma cell line, Raji, in enhancing NK cell cytolytic activity. Various flavonoids were used as stimulants to enhance the expression of NKG2D ligands. NK cell lysis activity against Raji was not changed by pre-treatment of Raji with luteolin, kaempferol, taxifolin and hesperetin. However, treatment of Raji with naringenin showed increased sensitivity to NK cell lysis than untreated control cells. The activity of naringenin was due to enhanced NKG2D ligand expression. These results provide evidence that narigenin’s antitumor activity may be due to targeting of NKG2D ligand expression and suggests a possible immunotherapeutic role for cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flavonoids are a large family of compounds synthesized by plants. They are included in plant secondary metabolites, and are broadly distributed in vegetables, herbs and flowers (Middleton 1998). Flavonoids have been classified according to their chemical structure. Apigenin, luteolin, kaempferol, quercetin, naringenin, taxifolin and hesperetin are included in flavones, and further divided into the subgroups of flavone (apigenin, luteolin), flavonol (kaempferol, quercetin), flavanone (hesperetin, naringenin) and flavanonol (taxifolin). Experimental evidence indicated that flavonoids have immune modulatory activities including anti-allergic, anti-inflammatory, anti-microbial and anti-cancer properties (Havsteen 1983; Maggioni et al. 2014; Parhiz et al. 2015). In this study, we investigated the immunomodulatory activity of a variety of flavonoids, and found that narigenin has predominant activity. This flavonoid is the predominant flavanone in grapefruit and is considered to have a bioactive effect on human health as an antioxidant, free radical scavenger, anti-inflammatory compound, carbohydrate metabolism promoter, and immune system modulator (Felgines et al. 2000).
Natural killer (NK) cells are a subpopulation of lymphocytes that do not express CD3 (general T lymphocyte marker), or CD19 (general B lymphocyte marker). These cells are capable of identifying and killing tumor cells as well as virus infected cells without pre-sensitization (Vivier et al. 2008). NK cell express activating and inhibitory receptors to distinguish between normal and tumor cells. Representative activation receptors include NKp30, NKp44, NKp46, DNAM-1 and NKG2D (Moretta et al. 2001; Houchins et al. 1991). Among these, NKG2D is expressed on NK and T cells (Houchins et al. 1991) and considered as a primary activation receptor and trigger of cytolysis activity. KIR and CD94/NKG2A are representative inhibitory receptors, which are highly expressed on resting NK cells. On the other hand, cytolysis activity of NK cells is managed by inhibitory receptors, such as MHC class I molecules, which are usually expressed on most healthy cells, but are down regulated by virus infection or during cancer development (Moretta et al. 2001).
The present study was designed to demonstrate the importance of the expression level of NKG2D ligands on the Burkitt’s lymphoma cell line, Raji, in enhancing NK cell cytolysis activity. Raji is the stable human B-lymphocyte cell line from a Burkitt’s lymphoma male patient (Karpova et al. 2005; Pulvertaft 1964; Epstein et al. 1966). It expresses certain complement receptors as well as Fc receptors for immunoglobulin G (Theofilopoulos et al. 1976). This report describes the effect of naringenin in the induction of NKG2D ligands on the Raji cell line.
Materials and methods
Material and reagents
Apigenin (A), luteolin (B), kaempferol (C), quercetin (D), naringenin (E), taxifolin (F) and hesperetin (G) (Fig. 1) were purchased from Sigma-Aldrich (St Louis, MO, USA). Unless indicated, all other chemicals were purchased from Sigma-Aldrich.
Cell lines
K562 (human leukemia cell line), Raji (human Burkitt’s lymphoma cell line) and NK-92MI were obtained from American Type Culture Collection (ATCC, Rockville, MD). K562 and Raji cell lines were maintained in culture flasks with 10 % fetal bovine serum/RPMI 1640 (Hyclone Laboratories, Logan, UT) supplemented with penicillin (100 U/mL)/streptomycin (100 μg/mL) (Gibco BRL, Grand Island, NY). NK92-MI was maintained in culture flasks with 12.5 % horse serum, 12.5 % fetal bovine serum/Alpha Minimum Essential medium (Hyclone Laboratories, Logan, UT) supplemented with 0.2 mM inositol; 0.1 mM 2-mercaptoethanol; 0.02 mM folic acid and penicillin (100 U/mL)/streptomycin (100 μg/mL).
Cell viability
The cells were treated with various concentrations of the samples, and cell viability was measured using the Wst-8 based colorimetric assay (Dojindo, Japan), which relies on the ability of living cells to reduce a tetrazolium salt into a soluble, colored formazan product. The cell suspension of the 5 × 104 cells/well was cultured in triplicate in a flat-bottomed 96-well plate for 30 h. The Wst-8 reagent was added to both the cells and the blank samples, which were then incubated at 37 °C and 5 % CO2, respectively, for 3 h. Next, the level of dye that had formed was measured using a spectrophotometer (Bio-rad 680, CA, U.S.A.) at the wavelength of 450 nm. The blank value without cells was subtracted from each experiment value, as the background.
NK cell cytotoxicity assay
The lytic capacity of NK cells was examined in lactate dehydrogenase (LDH) assay (Takara, Japan). Target cells (6 × 104) were planted into 96-cell plates respectively and the effector cells (1.8 × 105) were added into the plates and incubated for 4 h at 37 °C in a humidified atmosphere containing 5 % CO2. In blocking assay, an NKG2D molecule, the counterpart of NKG2D ligands, was blocked by an anti-human NKG2D monoclonal antibody. NK cells were incubated with the indicated concentration of anti-NKG2D monoclonal antibody (clone 1D11; BD Biosciences) or mouse IgG1 as isotype control for 1 h, washed, and then used as effector cells. Cytotoxicity assay plate was centrifuged at 250×g for 10 min. And then, 100 μL of supernatant was transferred to a new 96-well plate and 100 μL substrate mix was added. After 30 min incubation in the room temperature, the absorbance was measured at 490 nm. Percentage of cytotoxicity was calculated as follows: Cytotoxicity (%) = [(A−low control)/(high control−low control)] × 100. A: [effector-target cell mix]−[effector cell control], low control: Measure the spontaneous LDH release, that is the LDH activity released from the target cells, High control: Measure the maximum releasable LDH activity in the target cells that is the maximum LDH release induced by the addition of Triton X-100.
Phenotypic analysis
Cancer cell lines were washed twice in phosphate buffer saline (PBS) and resuspended in FACS staining buffer (PBS with 0.5 % bovine serum albumin). Cells were blocked with mouse serum for 30 min at 4 °C and then stained with PE-conjugated anti-human ULBP-1, anti-human ULBP-2, anti-human MIC-A/B and isotype-matched control antibody. After incubation for 30 min at 4 °C, cells were washed three times in PBS and fixed with PBS containing 1 % paraformaldehyde (Sigma-Aldrich, St. Loice, MO). At least 104 events were analyzed by flow cytometry (FACScan, BD Biosciences) with the cellquest software. The PE-conjugated anti-human ULBP-1, anti-human ULBP-2, anti-human MIC-A/B and isotype-matched control antibodies were purchased from BD PharMingen (San Diego, CA, U.S.A.). The dead cells were gated out by their low forward-angle light scatter intensity.
Statistics
Statistical analyses of data were performed by the Student’s t test to determine statistical significance. Values are given as mean ± S.D. (standard deviation).
Results
NK cell lysis activity is dependent on the cancer cell line
To determine whether NK cell lysis activity is affected by changes in the target cancer cell line, we first confirmed the cellular cytotoxicity of NK cells against two different hematological tumor cell lines, human leukemia cell line (K562) and human Burkitt’s lymphoma cell line (Raji). As shown in Fig. 2, NK cell cytotoxicity against K562 increased according to target cell numbers. NK cell lysis of the K562 was approximately 65.3 % (E/T 3:1); however, very little NK cell lysis activity was observed against Raji, regardless of the target cell concentration.
NK cell mediated cytotolysis against K562 and Raji. Cancer cell lines and NK cells were added into the plates and incubated for 4 h at 37 °C. NK cell cytotoxicity towards cancer cell lines was measured using LDH release assay. The data of three independent experiments, each of which was performed in triplicate, are expressed as means ± SD. **Significant differences, p < 0.01
Cell viability following application of flavonoids on Raji
To assess the cancer cell sensitivity to NK cell cytotoxicity following application of flavonoid, the cell viability of Raji was measured in a broad concentration range of 1.5625–100 μM, using a WST-8 assay. The negative control (cell only) group was treated with just DMSO as a solvent for flavonoids. As shown in Fig. 3, flavonoids showed cytotoxic effects on the Raji at different concentrations. Taxifolin showed cytotoxic effects at a concentration higher than 50 μM. Apigenin, kaempferol, naringenin and hesperetin showed cytotoxic effects at a concentration higher than 25 μM. Luteolin and quercetin showed cytotoxic effects at a concentration higher than 12.5 μM. These results demonstrated that luteolin and quercetin are the most toxic flavonoids and taxifolin is the safest flavonoid. To rule out the direct toxic effects of flavonoid on Raji, we used flavonoids below the toxic concentration.
Effects of flavonoids on NK cell lysis activity
To determine whether NK cell lysis activity on cancer cells is affected by treatment of flavonoids, we confirmed the cytolytic activity of NK cells against flavonoid-treated Raji. Raji were treated with flavonoids for 72 h, and then used to assess the cytolytic activity of NK cells. The lytic capacity of NK cells was examined with LDH assay. As shown in Fig. 4a, very little NK cell cytotoxicity was observed against Raji. NK cell lysis activity against Raji was unchanged by pre-treatment of luteolin, kaempferol, taxifolin and hesperetin on Raji cells. However, treatment of Raji with naringenin resulted in higher sensitivity to NK cell lysis than untreated control cells (mean ± SD 68.2 ± 4.8 % vs. 26.4 ± 2.8 % at E/T 3:1, P < 0.05). NK cells killing activity was elevated almost 2.5 fold with naringenin treatment. The treatment of Raji with apigenin and quercetin showed less sensitivity to NK cell lysis. Naringenin activity on NK cell lysis was then assessed at various concentrations. As shown in Fig. 4b, NK cell lysis activity against Raji was increased according to naringenin concentrations.
Enhanced NK cell–mediated lysis of Raji following treatment with flavonoids. Raji were incubated with various kinds of flavonoids (a) or various concentrations of naringenin (b) for 72 h and then used in the cytolytic activity of NK cells. The data of three independent experiments, each of which was performed in triplicate, are expressed as means ± SD. *p < 0.05; **p < 0.01, compared to cell only control
Expression of NKG2D ligands on cancer cell lines
We next investigated whether naringenin treatment of Raji resulted in increased NKG2D ligand expression, which would enable the enhanced NK cytotoxic effect through improved recognition of cancer cells. Raji were treated with flavonoids for 72 h and then the expression of NKG2D ligands (MIC-A/B, ULBP-1, and ULBP-2) was observed by flow cytometry. As shown in Fig. 5, ULBP-1 (A), ULBP-2 (B) and MIC-A/B (C) expressions obviously increased following incubation with naringenin. The expression of NKG2D ligands also increased according to the concentration of naringenin. Quercetin also increased expressions of ULBP-1 (5.89), ULBP-2 (40.08) and MIC-A/B (7.97), but less than naringenin did. However, the expression of NKG2D ligands was not increased by pretreatment with luteolin, kaempferol, taxifolin, apigenin and hesperetin (data not shown).
Expression of NKG2D ligands on Raji cells after treatment with naringenin. Raji were treated with various concentrations of naringenin for 72 h and then the expressions of ULBP-1 (a), ULBP-2 (b) and MIC-A/B (c) were observed by flow cytometry. Figures in parenthesis refer to percent of M1 gate. The data shown are representative of three experiments
Blocking assay with NKG2D monoclonal antibody
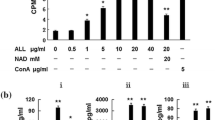
To verify that high NK cell cytolysis requires high expression of NKG2D ligands on the surface of a cancer cell, an NKG2D molecule, the counter part of NKG2D ligands, was blocked by anti-human NKG2D monoclonal antibody. NK cells were incubated with the indicated concentration of anti-NKG2D monoclonal antibodies or isotype antibodies for 1 h, washed, and then used as effector cells. As expected, the blockade of NKG2D dramatically attenuated NK cytolysis of naringenin-treated Raji in a dose dependent manner (Figs. 5, 6). In particular, the cytolysis of NK cells against the naringenin-treated Raji decreased by almost 41 % at a high concentration (50 μg/mL) of NKG2D antibodies. However, blocking of NKG2D did not completely attenuate the cytolytic effect of NK cells.
Blocking assay with anti-NKG2D monoclonal antibody. NK cells were incubated with the indicated concentration of anti-NKG2D antibody or isotype antibody for 1 h, washed, and then mixed with Raji. NK cell cytotoxicity towards Raji was measured using LDH assay. The data of three independent experiments, each of which was performed in triplicate, are expressed as means ± SD. **p < 0.01, compared to isotype antibody control
Discussion
The increased expression of NKG2D ligands on the surface of cancer cells is of great interest and numerous reports have suggested new methods for increasing the expression of NKG2D ligands to contribute to cancer therapy. Armeanu et al. (2005) reported that valproic acid, a histone deacetylase inhibitor, increases the transcription of MIC-A and MIC-B in hepatocellular carcinoma cell lines (HepG2 and Hep3B), leading to increased cell surface, soluble and total MIC protein expression, but no significant changes in the expression of ULBP-1, ULBP-2, and ULPB-3. Bae et al. demonstrated that quercetin-treated cancer cell lines showed enhanced susceptibility to NK cells through induction of NKG2D ligands. These effects were found to be caused by blocking of nuclear factor-kappa B and phosphatidylinositol 3-kinase pathways (Davis et al. 2009; Bae et al. 2010).
Among the flavonoids, quercetin has shown potential for anticancer activity. Quercetin is a flavonol, a subclass of flavonoids, and a potent antioxidant; providing cardiovascular protection by reducing oxidation of LDL cholesterol (Williams et al. 2004; Barnes et al. 2011). In this study, we used quercetin as a positive control to up-regulate the NKG2D ligands. Ironically, kaempferol, which is also a flavonol, did not show enhancement of NKG2D ligands. Based on these results, we found that there is a difference in the stimulatory activity of NKG2D ligand expression between the flavonoids, and so these were screened to find the most powerful flavonoid for NKG2D ligand expression. As shown in Fig. 4a, naringenin showed stronger activity than quercetin in increasing the NKG2D ligand expression. Naringenin is already reported to have anti-proliferative effects in various cancer cell lines (Kawaii et al. 1999; Frydoonfar et al. 2002; Virgili et al. 2004) and among several proposed mechanisms for naringenin’s induced anticancer effects (Verhoeyen et al. 2002; Harmon and Patel 2004; Middleton et al. 2000; Harmon and Patel 2003), its ability to inhibit cell proliferation via estrogen receptor binding is considered significant. The other mechanisms of the anticancer activity include a direct effect on cancer cells. However, in this study, we demonstrated indirect anticancer activity of naringenin through NK cell lysis.
We have demonstrated that the sensitivity of cancer cell to NK cells can be enhanced by increasing the expression of NKG2D ligands. Animal experiments and human clinical studies have shown that the expression of NKG2D ligands on cancer cells is directly associated with tumor treatment (Conejo-Garcia et al. 2004; Guerra et al. 2008; Friese et al. 2004). NKG2D ligands are not expressed constitutively in all cells and their expression is gradually upregulated by cancer progression. NK cells can recognize cancer cells among the surrounding normal tissue however Raji expresses low level of NKG2D ligands compared with other lymphoma cell lines, such as K562 and Jurkat (Bae et al. 2012).
Interestingly, K562 and Raji, both derived from lymphocytes, have different sensitivity to NK cells. The sensitivity of Raji is significantly lower than that of K562, which is thought to be due to the higher expression of MHC class I by Raji. Because MHC class I is a representative inhibitory receptor for NK cell activity, its expression is decreased with viral infection or cancer cell progression (Vivier et al. 2008). NK cells preferentially kill cells that express low levels of MHC class I molecules. This mode is known as “missing-self recognition”. Therefore, the high expression cells with MHC class I in spite of virus infection such as Raji, may be exposed to NK cells with the help of special compounds, such as naringenin.
In the previous study, we compared the cytotoxic activity of human naïve NK cells to six different cancer cell lines (K562, Jurkat, MCF-7, Ramos, Hep3B, and Raji) (Bae et al. 2012). NK cells showed a high cytolytic effect against the high expression cell lines with NKG2D ligands, such as K562 and Jurkat, but showed low cytolytic effect against Ramos, Hep3B and Raji, which express low levels of NKG2D ligands. The sensitivity of Hep3B and Raji to NK cell cytolysis was increased by the enhancement of NKG2D ligands expression. Zang et al. (Zhang et al. 2008) reported similar results that K562 expressed the highest level of MIC-A, while Raji expressed a much lower level of MIC-A compared with K562. This result suggested a correlation between the expression of MIC-A and the sensitivity of cancer cells to NK cytolysis. Summarizing the results; it appears that NKG2D ligands have a decisive effect on the susceptibility to NK cell killing, even converting the inhibitory activity of MHC class I. Therefore, we designed this study as a follow-up experiment and used Raji cells as target cells of NK cell cytotocxicity assay.
To demonstrate the importance of NKG2D ligands on NK lysis activity, we performed blocking assay using NKG2D monoclonal antibody. When NK cells were blocked with anti-human NKG2D receptor antibodies, NK lysis of naringenin-activated Raji was obviously weakened. However, blocking NKG2D did not completely attenuate the cytolytic effect of NK cells. This may be due to the interaction between NK cells and cancer cells with other receptors and ligands, such as DNAM-1 and CD112 (or CD155).
Taken together, we demonstrated that naringenin increased the expression of NKG2D ligands and the susceptibility to NK cell killing on Burkitt’s lymphoma cells. Our results indicate that increased sensitivity of naringenin-treated myeloma cells to NK cell lysis is caused by higher NKG2D ligand expression. These results provide evidence that targeting NKG2D ligand expression may be an additional mechanism supporting the antitumor activity of naringenin and suggest its possible immunotherapeutic value for cancer treatment.
References
Armeanu, S., M. Bitzer, U.M. Lauer, S. Venturelli, A. Pathil, M. Krusch, S. Kaiser, J. Jobst, I. Smirnow, A. Wagner, A. Steinle, and H.R. Salih. 2005. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Research 65: 6321–6329.
Bae, D.S., Y.K. Hwang, and J.K. Lee. 2012. Importance of NKG2D-NKG2D ligands interaction for cytolytic activity of natural killer cell. Cellular Immunology 276: 122–127.
Bae, J.H., J.Y. Kim, M.J. Kim, S.H. Chang, Y.S. Park, C.H. Son, S.J. Park, J.S. Chung, E.Y. Lee, S.H. Kim, and C.D. Kang. 2010. Quercetin enhances susceptibility to NK cell-mediated lysis of tumor cells through induction of NKG2D ligands and suppression of HSP70. Journal of Immunotherapy 33: 391–401.
Barnes, S., J. Prasain, T. D’alessandro, A. Arabshahi, N. Botting, M.A. Lila, G. Jackson, E.M. Janle, and C.M. Weaver. 2011. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food and Function 2: 235–244.
Conejo-Garcia, J.R., F. Benencia, M.C. Courreges, P.A. Gimotty, E. Khang, R.J. Buckanovich, K.A. Frauwirth, L. Zhang, D. Katsaros, C.B. Thompson, B. Levine, and G. Coukos. 2004. Ovarian carcinoma expresses the NKG2D ligand Letal and promotes the survival and expansion of CD28- antitumor T cells. Cancer Research 64: 2175–2182.
Davis, J.M., E.A. Murphy, and M.D. Carmichael. 2009. Effects of the dietary flavonoid quercetin upon performance and health. Current Sports Medicine Reports 8: 206–213.
Epstein, M.A., B.G. Achong, Y.M. Barr, B. Zajac, G. Henle, and W. Henle. 1966. Morphological and virological investigations on cultured Burkitt tumor lymphoblasts (strain Raji). Journal of the National Cancer Institute 37: 547–559.
Felgines, C., O. Texier, C. Morand, C. Manach, A. Scalbert, F. Regerat, and C. Remesy. 2000. Bioavailability of the flavanone naringenin and its glycosides in rats. American Journal of Physiology Gastrointestinal and Liver Physiology 279: G1148–G1154.
Friese, M.A., J. Wischhusen, W. Wick, M. Weiler, G. Eisele, A. Steinle, and M. Weller. 2004. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Research 64: 7596–7603.
Frydoonfar, H.R., D.R. Mcgrath, and A.D. Spigelman. 2002. Inhibition of proliferation of a colon cancer cell line by indole-3-carbinol. Colorectal Diseases 4: 205–207.
Guerra, N., Y.X. Tan, N.T. Joncker, A. Choy, F. Gallardo, N. Xiong, S. Knoblaugh, D. Cado, N.M. Greenberg, and D.H. Raulet. 2008. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 28: 571–580.
Harmon, A.W., and Y.M. Patel. 2003. Naringenin inhibits phosphoinositide 3-kinase activity and glucose uptake in 3T3-L1 adipocytes. Biochemical and Biophysical Research Communications 305: 229–234.
Harmon, A.W., and Y.M. Patel. 2004. Naringenin inhibits glucose uptake in MCF-7 breast cancer cells: a mechanism for impaired cellular proliferation. Breast Cancer Research and Treatment 85: 103–110.
Havsteen, B. 1983. Flavonoids, a class of natural products of high pharmacological potency. Biochemical Pharmacology 32: 1141–1148.
Houchins, J.P., T. Yabe, C. Mcsherry, and F.H. Bach. 1991. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. The Journal of Experimental Medicine 173: 1017–1020.
Karpova, M.B., J. Schoumans, I. Ernberg, J.I. Henter, M. Nordenskjold, and B. Fadeel. 2005. Raji revisited: cytogenetics of the original Burkitt’s lymphoma cell line. Leukemia 19: 159–161.
Kawaii, S., Y. Tomono, E. Katase, K. Ogawa, and M. Yano. 1999. Quantitation of flavonoid constituents in citrus fruits. Journal of Agricultural and Food Chemistry 47: 3565–3571.
Maggioni, D., L. Biffi, G. Nicolini, and W. Garavello. 2014. Flavonoids in oral cancer prevention and therapy. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation.
Middleton Jr, E. 1998. Effect of plant flavonoids on immune and inflammatory cell function. Advances in Experimental Medicine and Biology 439: 175–182.
Middleton Jr, E., C. Kandaswami, and T.C. Theoharides. 2000. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacological Reviews 52: 673–751.
Moretta, A., C. Bottino, M. Vitale, D. Pende, C. Cantoni, M.C. Mingari, R. Biassoni, and L. Moretta. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annual Review of Immunology 19: 197–223.
Parhiz, H., A. Roohbakhsh, F. Soltani, R. Rezaee, and M. Iranshahi. 2015. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytotherapy Research 29: 323–331.
Pulvertaft, J.V. 1964. Cytology of Burkitt’s tumour (African Lymphoma). Lancet 1: 238–240.
Theofilopoulos, A.N., C.B. Wilson, and F.J. Dixon. 1976. The Raji cell radioimmune assay for detecting immune complexes in human sera. The Journal of Clinical Investigation 57: 169–182.
Verhoeyen, M.E., A. Bovy, G. Collins, S. Muir, S. Robinson, C.H. De Vos, and S. Colliver. 2002. Increasing antioxidant levels in tomatoes through modification of the flavonoid biosynthetic pathway. Journal of Experimental Botany 53: 2099–2106.
Virgili, F., F. Acconcia, R. Ambra, A. Rinna, P. Totta, and M. Marino. 2004. Nutritional flavonoids modulate estrogen receptor alpha signaling. IUBMB Life 56: 145–151.
Vivier, E., E. Tomasello, M. Baratin, T. Walzer, and S. Ugolini. 2008. Functions of natural killer cells. Nature Immunology 9: 503–510.
Williams, R.J., J.P. Spencer, and C. Rice-Evans. 2004. Flavonoids: antioxidants or signalling molecules? Free Radical Biology and Medicine 36: 838–849.
Zhang, C., J. Zhang, J. Niu, Z. Zhou, and Z. Tian. 2008. Interleukin-12 improves cytotoxicity of natural killer cells via upregulated expression of NKG2D. Human Immunology 69: 490–500.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.H., Lee, J.K. Naringenin enhances NK cell lysis activity by increasing the expression of NKG2D ligands on Burkitt’s lymphoma cells. Arch. Pharm. Res. 38, 2042–2048 (2015). https://doi.org/10.1007/s12272-015-0624-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0624-5