Abstract
Tryptanthrin, an indoloquinazoline alkaloid, was first obtained by sublimation of natural indigo and later isolated from the culture of fungus Candida lipolytica and a variety of other natural sources. Tryptanthrin showed a variety of intriguing biological properties such as antibacterial, antifungal, antiprotozoal, and antiparasitic activities, inhibitory activities against COX-2, 5-LOX, NO synthase and PGE(2) expression, as well as cytotoxic and antitumor activities. Present review covers recent studies on the natural sources, biological activities and mechanisms of their actions, synthesis, structure–activity relationship, and metabolism of tryptanthrin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
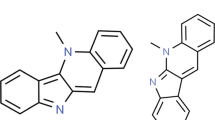
The history of tryptanthrin (1a, 6,12-dihydro-6,12-dioxoindolo[2,1-b]quinazoline) began as early as in 1822 (Siedel 1822). In 1879 Sommargua described the sublimation of natural indigo under reduced pressure to give yellow needles with a molecular formula of C15H8N2O2 (m.p. 258–259 °C) (von Sommaruga 1879). Several research groups additionally reported the same golden-yellow crystals from commercial as well synthetic indigos (O’Neill 1892; Bloxam 1915) and indigotin as well (Perkin, 1906). German patent reported the same yellow compound as an oxidation product of indigo by KMnO4 (German Patent 1913). Friedländer and Roschdestwensky not only prepared the compound by air oxidation of indigo at high temperature and designated it as anhydro-α-isatinanthranilide with a structure but also prepared it chemically from anthranilic acid and its equivalent (Friedländer and Roschdestwensky 1915). 
Tryptanthrin was also isolated from the culture of the yeast Candida lipolytica, grown in an artificial media containing high concentration of tryptophan, hence it was designated as tryptanthrin in 1971 (Schindler and Zähner 1971). It should be noted that Sen et al. (1974) isolated yellow needles (m.p. 265–266 °C, m/e 248) from petroleum extract of dried and powdered fruit of Couroupita guaianensis Abul in 1974 and determined its structure as 2 with a trivial name couroupitine A (Sen et al. 1974). However the structure of couroupitine A was corrected later by Bergman et al. (1977) as the structure 1a originally proposed.
The structure of tryptanthrin is so unique that the preliminary chemical and spectroscopic (UV, IR, and NMR) (Jahng 2012)Footnote 1 data are not sufficiently definite enough to characterize its structure. The exact structure, thus, remained uncertain until X-ray crystallography confirmed the present structure (Brufani et al. 1971; Fedeli and Mazza 1974)Footnote 2. An X-ray crystal structure of tryptanthrin showed that its main structural feature is a nearly planar arrangement within experimental error. However, there are small but significant departures from the planarity, especially for the ‘O’ and probably ‘C(12)’ of carbonyl on the pyrimidine ring (see Table 1).
All the proton resonances have been assigned and are summarized in Table 2 (Jarrah and Thaller 1980). H10 lies in the bay region and thus is the most down-field (δ 8.67) shifted one due to the deshielding effect of oxygen at C(12) carbonyl. Such deshielding effect also leads H1 and H7 to resonate at δ 8.48 and 7.96, respectively, while the lone pair of N5 similarly affects the chemical shift of H4.
Although a plenty of studies for tryptanthrin resulted in a couple of reviews, these were only focused on the synthesis of the tryptanthrin core and reactions of it (Witt and Bergman 2003; Wang et al. 2007; Tucker and Grundt 2012). Present review covers the occurrences, physicochemical and biological properties, synthesis and reactions as well as structure and activity relationships.
Occurrence and derivatives
Tryptanthrin was additionally isolated from fungi such as Schizophyllum commune (Hosoe et al. 1999, 2000) and Leucopaxillus cerealis (Jarrah and Thaller 1980), and higher plants such as Couroupita guaianensis Abul. (the cannon ball tree) (Sen et al. 1974; Bergman et al. 1977, 1985), Strobilanthes cusia (assam indigo) (Honda and Tabata 1979), Polygonum tinctorium Lour. (Japanese and Chinese Indigo) (Honda et al. 1980), Isatis indigotica (wood) (Li 1987; Li et al. 1983, 2000)Footnote 3, known as ‘Qing Dai (leaves)’ and ‘Ban Lan Gen (roots)’ in Chinese, ‘Sentai’ in Japanese, and ‘Cheongdae’ in Korean traditional medicine, Isatis tinctoria (Honda et al. 1980; Seifert and Unger 1994; Danz et al. 2001, 2002a) known as Chinese woad, Wrightia tinctoria (George et al. 1996; Muruganandam and Bhattacharya 2000), two Calanche species including C. discolor and C. liukiuensis (Yoshikawa et al. 1998; Murakami et al. 2001), Phaius mishmensis (Jao et al. 2008), Cissus sucyoides (Xu et al. 2009), and Baphicacanthus cusia (Liu et al. 2009). It is somewhat intriguing that tryptanthrin has also been isolated from the urine of Asian elephant (Elephas maximus (Rasmussen et al. 1993).
The derivatives of tryptanthrin, isolated from the natural sources, are summarized in Fig. 1. Methylisatoid (Baeyer and Oekonomides 1882) and candidine (Laatsch and Leudwig-Köhn 1986) were chemically prepared long before their isolation from natural sources. Methylisatoid is a compound of controversy with a long history (vide infra). On the other hand, candidine (also known as qingdainone) was isolated by Fielder from the culture of Candida lipolytica as an unidentified violet compound with the molecular formula C23H13N3O2 (Fielder 1974), of which the structure was later determined and named candidine by Bergman et al. (1985a; 1985b) Candidine was also additionally isolated from the higher plants such as Isatica indigotica (Zou and Huang 1985) and Baphicacanthus cusia (Wu et al. 1997). Ophiuroidine (4,8,9-trihydroxy tryptanthrin) was recently isolated from the marine invertebrate Carabian britterl star Ophiocoma riisei as the first hydroxylated tryptanthrin (Utkina and Denisenko 2007). An additional hydroxylated tryptanthrin, phaitanthrin C, and tryptanthrin-related compounds phaitanthrin A, B, D and E were also isolated from Phaius mishmensis along with methylisatoid and candidine (Jao et al. 2008).
Biological activities and reaction mechanisms
Although a variety of biological properties of tryptanthrin-containing herbs themselves and their extracts have been studied, this review will focus on tryptanthrin and its derivatives. Tryptanthrin showed strong inhibitory activities against pathogenic microorganisms such as Bacillus subtilis (MIC’s of 3.1–6.3 μg/mL) (Schindler and Zähner 1971; Honda et al. 1979; Okunade and Elvin-Lewis 2009), Escherlichia coli (Bandekar et al. 2010), Mycobacterium tuberculosis (MIC = 10 μg/mL) (Baker and Mitscher 1995; Mitscher and Baker 1998a, b), and Helicobacter pylori (2.5 μg/mL) (Hashimoto et al. 1999; Kataoka et al. 2001), and antifungal activity against Trichophyton, Microsporum, and Epidermophyron species at the level of MIC = 5 μg/mL (Honda and Tabata 1979; Honda et al. 1980; Li et al. 1983). The antifungal activity of tryptanthrin is comparable to that of clinically using griseofulvin against T. mentagrophytes. Its antiprotozoal activities against Leishmania donovani (IC50 = 600 ng/mL) (Bhattacharjee et al. 2002) and Plasmodium falciparum (<100 ng/mL) (Pitzer et al. 2003; Bhattacharjee et al. 2004), and anti-parasitic activity against Trypanosoma brucei (Scovill et al. 2002) were also strong enough to warrant further studies for its development as an anti-leishmanial agent (Bhattacharjee et al. 2002). The inhibitory activities of tryptanthrin against COX-2 (IC50 = 64 nM) (Danz et al. 2001, 2002b; Hamburger and Danz 2000), 5-LOX (IC50 = 0.15 μM) (Hamburger 2002; Danz et al. 2002a; Oberthür et al. 2005), NO synthase and prostaglandin E(2) expression at the cellular level (Ishihara et al. 2000) opened a vista for a possible lead for anti-inflammatory agents. In addition, the inhibitory activities against hepatocyte growth factor in human fibroblasts (Motoki et al. 2005) and against the multidrug resistance gene MDR1 in breast cancer cells (Yu et al. 2007, 2009) as well as the cytotoxicity against selected human cancer cell lines (IC50 = 10 μM for HT-1376) (Zou and Huang 1985; Hosoe et al. 1999; Sharma et al. 2002; Jao et al. 2008; Camargo et al. 2009; Yu et al. 2007; Liang et al. 2012) and the antitumor activity (Kimoto et al. 2001; Koya-Miyata et al. 2001; Chan et al. 2009) of tryptanthrin were also studied.
However, studies on the mechanism of biological activities are limited to include hemin and hemozoin binding as a factor for antimalarial activity (Dorn et al. 1998; Hicks et al. 2005), DNA intercalation (~10 drug/kbp at 100 μg/mL) for antibacterial activity against E. coli (Bandekar et al. 2010), and down-regulation of MRD1 gene expression in doxorubicin resistance to human breast cancer (Yu et al. 2007, 2009), and modulatory action on murine myeloid leukemia cells (Chan et al., 2009).
Although systematic synthesis and structure–activity relationship studies for anti-tubercular (Mitscher and Baker 1998a), COX inhibitory (Hamburger and Danz 2000), and cytotoxic activity (Zou and Huang 1985; Sharma et al. 2002; Camargo et al. 2009; Liang et al. 2012), have led several promising candidates for further development, none of them has yet been successfully launched to the clinics (vide infra).
In addition to such intriguing biological properties, tryptanthrin can also transport electrons, thus having potential as a photoelectronic photoreceptor (Sugai and Saito 1997; Sugai et al. 1997). The origin of such a property stems from the electron-accepting ability of tryptanthrin. Tryptanthrin shows two reversible waves with cathodic and anodic peaks, separated by approximately 60 mV, indicating two one-electron transfers. The more facile (less negative) reduction potential at −0.75 V covers an electron transfer to the carbonyl oxygen on the more strained five-membered ring, while the more negative potential at –1.40 V covers an electron transfer to that of the less strained six-membered ring. The electron-accepting ability of the carbonyl atoms seems to be crucial for the antileishmanial activity of tryptanthrin (Bhattacharjee et al. 2002).
Total synthesis and other chemical studies
Synthesis
There might be three general approaches for the synthesis of tryptanthrin: oxidative coupling of isatin, indigo, and methylisatoid; the construction of quinzolinone ring at the final key step, and the construction of indol ring at the final step. However, many of the reactions would not allow such simple categorization; the present review was, thus, written in the chronological order on the basis of new synthetic approaches.
The early preparation studies on tryptanthrin included the chemistry of indigo (3, indigotine) and methylisatoid. Meister et al. (1913) isolated the same yellow compound as an oxidation product of indigo by KMnO4 (German Patent belongs to Farbwerke vonmals Meister 1913). The same result was also obtained from isatin (4).
Friedländer and Roschdestwensky prepared anhydro-α-isatinanthranilide three different ways from α-isatinanilide and anthranilic acid, isatin chloride and anthranilic acid, and O-nitrosobenzoic acid and indoxyl (Friedländer and Roschdestwensky 1915) (Scheme 1). These synthetic approaches opened a new vista for the preparation of tryptanthrin chemically.
Heller and Benade reported tryptanthrin by CrO3 oxidation of dihydrotryptanthrin which was prepared by heating methylisatoid with HOAc-HBr (Heller 1919; Heller and Benade 1922). However, the exact structure of methylisatoid and related alkylisatoids had been a long unfruitful controversy between Heller and Hantzsch. Heller favored an O-methylisatin-isatin complex structure for methylisatoid (Heller and Benade 1907, 1922), while Hantzsch preferred one of two monomeric structures (Hantzsch 1921, 1925). Such controversy was ended by Cox et al. who excluded Heller’s structure by X-ray crystallography structure determination (Cox et al. 1936). However, Hantzsch’s structures also turned out to be ill-characterized when Cornforth proved the present structure by three experiments including decomposition and synthesis (Cornforth 1976). 
Air oxidation of indigo blue (also called indigotin) in the presence of Cu and pyridine also afforded tryptanthrin (Machemer 1930), which can also be achieved from indigo and isatin in the presence of Cu(OAc)2 (Heller and Barthel 1936). Such a method has been re-examined using ozone as the oxidant to provide isatin, isatoic anhydride, and tryptanthrin. Isatin formed as a primary intermediate would then undergo further oxidation to isatic anhydride (5) (Matsui et al. 1982), which would be condensed to isatin to form tryptanthrin. Later, oxidation of isatin by oxidizing agent such as CrO3 to isatoic anhydride was proven to be effective (Geckeler and Metz 1979). 
Zeide and Chelintsev employed a nucleophilic substitution reaction of 2-chloroquinoline with anthranilic acid followed by cyclization to form 12H-quino[2,1-b]quinazolin-12-one (6) (the reverse process cannot be excluded) and subsequent oxidation of 6 by KMnO4-mediated oxidative ring contraction to afford tryptanthrin (Zeide and Chelintsev 1937).
Condensation of methyl 2-(2′-aminobenzamido)benzoate and formamide afforded 3,4-dihydro-3-(2-methoxycarbonylphenyl)-4-oxoquinazoline (7), which was then cyclized by heating at 140 °C to give tryptanthrin in 60 % yield. Similarly reaction of methyl anthranilate with ethyl orthoformate afforded 7 in 40 % yield and tryptanthrin, where the yield of trypanthrin was not given (Butler et al. 1960). 
This method has been modified recently. Three-component reaction of anthranilic acid, methyl anthranilate, and ethyl orthoformate in the presence of liquid crystal, [Hbim]BF4, resulted in 7 in 67 % yield. The C2-H of quinazolin-4(3H)-one is so acidic enough to be lithiated by n-BuLi at room temperature, which enables to cyclize to tryptanthrin in 81 % yield (Potewar et al. 2008).
The intermediate, 2-lithiated 3-(2′-methoxycarbonylphenyl)quinazolin-4(3H)-one, could be directly generated from (2-isocyanophenyl)lithium, which was the reacted with methyl 2-isocyanatobenzoate to afford tryptanthrin in 85 % yield (Lygin and de Meijere 2009).
The preparation method of Bird in 1963 involved Schiff base formation of O-methylisatin with 2-aminobenzaldehyde, which then underwent acid-catalyzed cyclization followed by CrO3 oxidation to give tryptanthrin (Bird 1963). 
The reaction of N-sodioisatin with 2-nitrobenzoyl chloride gave 1-(2′-nitrobenzoyl)isatin (8), in which the nitro group could be readily reduced by a conventional reduction method, such as refluxing with SnCl4 in conc. HCl to afford tryptanthrin in 67 % yield (Kikumoto and Kobayashi 1966; Son et al. 2003). 
In addition, a reaction of 8 in the presence of Ru3(CO)12 and CO (g) under pressure (40 kg/cm3) provided tryptanthrin in fairly good yield (Akazome et al. 1993). This procedure involves well-known deoxygenative reduction of a nitro group by carbon monoxide to an active nitrene intermediate (Nugent and Mayer 1988)Footnote 4. The resulting nucleophilic nitrene then attacks the C2 carbonyl group of isatin to form the desired tryptanthrin. 
Although a detailed preparative methods for N-(2′-aminobenzoyl)isatins has not been described in the literature, a couple of the cyclization of reaction to tryptanthrins via N-(2′-aminobenzoyl)isatins have been reported. The method of Eguchi et al. employed an aza-Wittig reaction of N-(2′-azidobenzoyl)isatin and tri(n-butyl)phosphine to prepare tryptanthrin (Eguchi et al. 1992), in which N-(2′-aminobenzoyl)isatin could be an intermediate. 
Bond and Hooper reported a reaction of copper 2-nitrophenylacetylide (9) with 2-iodobenzoic acid in pyridine to form tryptanthrin in 11 % yield, along with indogenide (10) (Bond and Hooper 1969). Authors also claimed that refluxing 9 with 2-bromopyridine in pyridine produced tryptanthrin as the only identifiable product. However, the reaction mechanism for the conversion is not easy to deduce as the authors have agreed. 
The synthetic procedure of Mitscher et al. employed deprotonation of isatins by NaH to form N-sodioisatins, which were then allowed to condense with isatoic anhydrides to lead the corresponding tryptanthrins (Mitscher et al. 1981). This procedure has advantages, as many of the starting isatins and isatoic anhydride are not only commercially available but also can be readily prepared by well established procedures (Sandmeyer 1919; Gassman et al. 1977), thus applied to synthesize the derivatives of tryptanthrin (Baiocchi et al. 1993; Grandolini et al. 1997; Valiante 2004; Lee et al. 2007; Gilman et al. 2008). 
The reaction conditions of this procedure have been modified numerously to improve or simplify such transformation. The experimentally simplest one involves heating isatin (4) and isatoic anhydride (5) in the presence of an equimolar amount of triethylamine or pyridine (Bergman et al. 1985). Alternative reaction conditions employed involve an inorganic base such as NaOH (Liu et al. 2008) and basic salts such as K2CO3 in the presence of microwave (Azizian et al. 2007). In general, excess 5 has been found effective presumably due to the scavenging any water formed during the reaction. Water scavenging agent such as dicyclohexylcarbodiimide (DCC), diiospropylcarbodiimide (DIC), or 1,1′-carbonyldiimidazole has also been employed (Mason et al. 2009). Application of such a procedure to isatin-d 4 and isatoic anhydride-d 4 yielded tryptanthrin-d 8 (Overthür et al. 2002).
A couple of additional dimerization procedures involves the irradiation of isatin with 10.6 μm wavelength laser light to give tryptanthrin (yield not given) (Karpf and Junek 1978). Batenero and Barba reported electrodimerization of isatin to tryptanthrin (Batanero and Barba 2006). Preparative electrolysis of isatin using Hg cathode at the first reduction potential (−0.9 V vs. CSE) provided tryptanthrin in 92 % yield. The low-energy consumption process may involve a single electron transfer to the oxygen in air, from which a possible reaction mechanism has been deduced as shown in Scheme 2.
Dimerization can also be induced by halogenating agents such as PCl5 and POCl3 (Moskovkina 1997; Moskovkina et al. 2012; Jao et al. 2008), in which authors claimed ‘Baeyer’s isatin chloride’ (2-chloro-3H-indol-3-one, 11) as a possible intermediate.
Baeyer claimed that the reaction of isatin with PCl5 afforded isatin chloride as early as in 1878 (Baeyer 1878, 1879) and a variety of reactions of isatin chloride with nucleophiles such as amines, phenols, and ‘active methylene’ compounds were pursued (Hill and Henze 1924; Begley and Grimshaw 1975; Katritzky et al. 1989). Although, the reaction of 11 with anthranilic acid afforded tryptanthrin (Baker and Duke 1972), isatin chloride remained as a ‘phantom’ molecule until Cornforth et al. confirmed the structure in 1996 (Cornforth et al. 1996). Cornforth et al. 1996 repeated Baker and Duke’s procedure [dilute (4 %) solution of isatin and PCl5 in benzene and long period of reaction time (6 h)] to get red crystals and confirmed the structure (12) by x-ray crystallography. Reaction mechanism of the conversion was proposed as shown in Scheme 3.
Thionyl chloride has long been a condensing agent for the construction of quinazolin-4(3H)-one skeleton from anthranilic acid and lactams (Kametani et al. 1977), and has also been applied to the synthesis of tryptanthrin with a modification (Lee et al. 2003). Attempts to develop more efficient and practical synthetic methods for tryptanthrin have been continued and eventually have established a one-pot reaction of isatin and anthranilic acid in the presence of thionyl chloride (Jahng et al. 2008).
Nucleophilic substitution of a carbanion generated from 3-N-(2-chlorophenyl)-2-methyl-4(3H)-quinazolinone and n-BuLi afforded indolo[2,1-b]quinaolin-12(6H)-one (13), to which a keto group at C6 was introduced by either direct air oxidation (Staskun and Wolfe 1992) or Thummel’s two-step procedure (Son et al. 2003).
Bowman et al. employed a radical cyclization for the preparation of tryptanthrin (Bowman et al. 2007). The starting material, prepared from 2-(4-oxoquinazolin-3(4H)-yl)benzoic acid and 1,2-diphenyldiselane in the presence of tri(n-butyl)phosphine, was converted to acyl selenide which was then subjected radical reaction condition to yield tryptanthrin in 3–13 % yield.
It should be noted that an efficient and green method for the synthesis of tryptanthrin and its derivatives from isatin and isatoic anhydride was recently reported (Kumar et al. 2011), in which the reaction was performed in aqueous medium at room temperature by employing β-cyclodextrin as a catalyst thus opening a new vista in alkaloid synthesis.
Biosynthesis of tryptanthrin
Biosynthetic pathway of tryptanthrin by Candida lipolytica has been proposed by Schindler and Zähner as shown in Scheme 4 (Schindler and Zähner 1971). Oxidative deamination of tryptophan would lead 3-(1H-indol-3-yl)pyruvic acid, which was then condensed with anthranilic acid to form tryptanthrin (Scheme 4).
Additional studies reveal that Candida lipolytica synthesizes tryptanthrin from equimolar amounts of tryptophan and anthranilic acid. Such a procedure allowed to pursue biosynthesis of a series of tryptanthrins by employing either substituted tryptophans and anthranilic acid or tryptophan and substituted anthranilic acids (Fiedler et al. 1976).
Enzymatic hydrolysis of a novel indole S,O-bisdesmoside, calanthoside, with β-galactosidase in 0.2 M acetate buffer (pH 4.4) provided tryptanthrin in 63 % yield, along with a small amount of indirubin and isatin (Yoshikawa et al. 1998; Murakami et al. 2001). This result may imply that calanthoside is a common genuine glycoside of tryptanthrin and other related alkaloids such as indirubin and isatin in plants. Here again, the reaction mechanism for this conversion was not easy to deduce. 
Metabolism
Tryptanthrin is an agonist of the rat aryl hydrocarbon receptor (AhR) implying that tryptanthrin can induce cytochrome P450 (CYP) 1A1 as confirmed by immunoblotting and CTP 1A1 activity assay (Schrenk et al. 1997). The LC masses of the two identifiable metabolites from the culture of cytosolic cytochrome P450-mediated metabolism of tryptanthrin were 264 and 280. The first metabolite (M1) was identified as a metabolite monohydroxylated on the aromatic ring of the indole moiety from the MS2 spectra of protonated tryptanthrin and M1. The structure of M1 was confirmed as 8-hydroxytryptanthrin with a chemically synthesized authentic tryptanthrin (Lee et al. 2007), while the metabolite M2 (mw 280) remained to be characterized (Scheme 4).
The experimental evidence, the reaction mechanism of the cytochrome P450-mediated oxidation process (King 2009), and the electronic aspects of the benzene ring of an indole moiety are enough to deduce a possible metabolic pathway as shown in Scheme 5. Among the possible tryptanthrin epoxides formed by cytochrome P450-mediated oxidation, epoxide 12 is expected to be the most favorable one to undergo either spontaneous rearrangement followed by NIH shift or ring opening by epoxide hydrolase, followed by aromatization to yield 8-hydroxytryptanthirn and 2,8-dihydroxytrypanthrin, respectively, as first metabolites of tryptanthrin. However, it is somewhat surprising that 7-hydroxytryptanthrin (phaitanthrin) (Jao et al. 2008) and 4,8,9-trihydroxytryptanthrin (ophiuroidine) (Utkina and Denisenko 2007) are two hydroxylated tryptanthrins isolated so far, which may imply that an alternative metabolic process operates in nature.
Structural modification and structure–activity relationship
Cytotoxicity
Effect of substituents
A couple of systematic structural modifications of tryptanthrin have led some promising results for anticancer agents. A substituent at C8 and oxime ethers of C6 carbonyl improved the activity significantly (Table 3).

Promising in vitro activity of the compound 1f and 1g allowed testing their in vivo activities. Based on the promising results (optimum T/C 23 %, 17 days) of hollow fibre assay (HFA), 1f was tested in nude mice bearing HT-29 colon cancer xenograpfts to be active enough to pursue additional studies.
Effect of benzo-annulation
Introduction of additional benzene ring, especially by benzo-annulation would lead the system more delocalized electron state which resulted in changes in chemical and biological properties (Costes et al. 2000; Hong et al. 2010). Benzo-annulated tryptanthrins (16) were recently prepared by employing one-pot synthesis from corresponding either (benzo)isatins and (benzo)anthranilic acids in the presence of SOCl2 or (benzo)isatins and (benzo)isatoic anhydrides, in which the prerequisite benzoisatins and benzoisatoic anhydrides could be prepared by employing previously reported methods (Liang et al. 2012). 
Although the benzo-annulation on quinazolin-4(3H)-one ring did not affect significantly on the inhibitory activities against topo I and II, the benzoannulation on indolin-3-one ring affected the inhibitory activity very much especially by linear annulation. However, bezno-annulated tryptanthrins did not show any significant increase in cytotoxicity against selected human cancer cell lines, which were not directly related either to the inhibitory activities against topo I and II or to the reduction potentials.
Antiinflammatory activity
Hamburger and Danz prepared 110 derivatives of tryptanthrin and examined their inhibitory activities on COX-1, COX-2 and NF-κB and the results are summarized in Table 4 (Hamburger and Danz 2000; Danz et al. 2001). Introduction of a substituent at C3 (R3) and C8 (R8) as in 1s and 1u generally increased inhibitory activities on COX-2 and selectivity on COX-2 while substituents at C4 (R4) (1o and 1q) and C9 (R9) (1p) decreased the activity significantly.

Antimalarial activity
One of the early biological properties of tryptanthrin was strong inhibitory activity against microbes for tuberculosis such as Mycobacterium smegmatis, M. tuberculosis, and M. avium (MAC). Mitscher and his coworkers (Baker and Mitscher 1995; Mitscher and Baker 1998a, b) prepared a series of deriviatives of tryptanthrins as well as its aza-analogues to lead a general SAR for antimalarial activity (Tables 5, 6).


Inhibitory activity on topoisomerases
The inhibitory activity of 1a and its benzoannulated derivatives (16) on topoisomerases I and II (topo I and II) were evaluated to show that tryptanthrin and its benzo-annulated derivatives showed selective inhibitory activity on topo I with an increase of activity on topo II by benzo-annulation on quinazolin-4(3H)-one moiety. Although the benzo-annulation on quinazolin-4(3H)-one ring did not affect significantly on the inhibitory activities against topo I and II, the benzoannulation on indolin-3-one ring affected the inhibitory activity very much especially by linear annulations (Liang et al. 2012) (Table 7).
Conclusions and outlook
Despite a long period of time elapsed since its discovery, tryptanthrin continues to be one of the intriguing alkaloids for which more chemistry and biological studies are required. Studies on tryptanthrin are still ongoing to include not only to searches for the new natural sources of itself as well as derivatives of tryptanthrin, developments of more efficient and practical synthetic methods, and structural modifications for stronger biological activities, but also possible candidates for optical usages as photoreceptor.
Notes
Spectral data for tryptanthrin were collected as follows: IR(KBr) υ 1725, 1688, 1610, 1519, 1550, 1350, 1310, 760 cm−1; UV (EtOH) λmax (ε) 225 (4.46), 251 (4.68), 280–420 (4.04) nm; MS m/z (rel. intensity) 248 (M, 100), 220 (45), 192 (30), 102 (15). 13C NMR (CDCl3, 62.5 MHz) δ 182.6 (C6), 158.1 (C12), 146.6 (C4a), 146.3 (C10a), 144.3 (C5a), 138.3 (C9), 135.1 (C3), 130.7 (C4), 130.2 (C2), 127.5 (C1), 127.2 (C8), 125.4 (C7), 121.9 (C6a), 118.0 (C10).
In which crystal data of tryptanthrin were given: C15H8N2O2, MW = 248.2; monoclinic, a = 7.46 (6), b = 7.66 (6), c = 20.78 (16) Å, β = 109.0 (5)o, V = 1122.7 Å 3; F (000) = 512; Space group P21/c (C 52h , No. 14) from systematic absences; Z = 4, D = 1.47 g cm−3.
Chem Abstr., 134, 128466(2001), Wikipedia encyclopedia has claimed that Isatis indigotica is a wrong expression of Isatis tinctoria: http://www.wikipedia.org.
Where the reactions of nucleophilic nitrene complexes to the carbonyl group of aldehydes and ketones to provide the corresponding amines are well described.
References
Akazome, M., T. Kondo, and Y. Watanabe. 1993. Transition-metal complex-catalyzed reductive N-heterocyclization: Synthesis of 4(3H)-quinazolinone derivatives from N-(2-nitrobenzoyl)amides. Journal of Organic Chemistry 58: 310–312.
Azizian, J., M.R. Mohammadizadeh, S. Zomorodbakhsh, A.A. Mohammadi, and A.R. Karimi. 2007. Microwave-assisted one-pot synthesis of some dicyanomethylene derivatives of indenoquinoxaline and tryptanthrin under solvent free conditions. ARKIVOC xv: 24–30.
Baeyer, A. 1878. Synthese des Indigoblaus. Chemische Berichte 11: 1296–1297.
Baeyer, A. 1879. Über die Einwirkung des Fünffachchlorphosphors auf Isatin und auf verwandte Substanzen. Chemische Berichte 12: 456–461.
Baeyer, A., and S. Oekonomides. 1882. Über das Isatin. Chemische Berichte 15: 2093–2102.
Baiocchi, L., M. Giannangeli, V. Rossi, V. Ambrogi, G. Grandolini, and L. Perioli. 1993. Synthesis and antimicrobial activity of some new indolo[2,1-b]quinazolin-6(12H)ones. Farmaco 48: 487–501.
Baker, J.T., and C.C. Duke. 1972. Reactions of α-chloroisatin (2-Chloroindoleninone) with methanethiol and 1,2-ethanedithiol. Tetrahedron Letters 13: 307–308.
Baker, W.R., and L.A., Mitscher. 1995. Preparation of indolo[2,1-b]quinazoline-6,12-dione tuberculostatics. U.S. Patent 5,441,955.
Bandekar, P.P., K.A. Roopnarine, V.J. Parekh, T.R. Mitchell, M.J. Novak, and R.R. Sinden. 2010. Antimicrobial activity of tryptanthrins in Escherichia coli. Journal of Medicinal Chemistry 53: 3558–3565.
Batanero, B., and F. Barba. 2006. Electrosynthesis of tryptanthrin. Tetrahedron Letters 47: 8201–8203.
Begley, W.J., and J. Grimshaw. 1975. Condensation between 2-chloro-3H-indol-3-one and phenols. Journal of Chemical Society, Perkin Transactions 1: 1840–1845.
Bergman, J., B. Egestad, and J.O. Lindström 1977. The structure of some indolic constituents in Couroupita guaianesis Abul. Tetrahedron Letters 18: 2625–2626.
Bergman, J., J.-O. Lindstöm, and U. Tilstam. 1985. The structure and properties of some indolic constituents in Couroupita guaianesis Abul. Tetrahedron 41: 2879–2881.
Bergman, J., and U. Tilstam. 1985. Structure determination of candidine, A violet indolic constituents from culture solutions of Couroupita guaianesis Abul. Tetrahedron 41: 2883–2884.
Bhattacharjee, A.K., D.J. Skanchy, B. Jennings, T.H. Hudson, J.J. Brendle, and K. Werbovetz. 2002. Analysis of stereoelectronic properties, mechanism of action and pharmacophore of synthetic indolo[2,1-b]quinazolin-6,12-dione derivatives in relation to antileishmanial activity using quantum chemical, cyclic voltammetry and 3-D-QSAR CATALYST procedures. Bioorganic and Medicinal Chemistry 10: 1979–1989.
Bhattacharjee, A.K., M.G. Hartell, D.A. Nichols, R.P. Hicks, B. Stanton, J.E. van Hamont, and W.K. Milhous. 2004. Structure-activity relationship study of antimalarial indolo[2,1-b]quinazoline-6,12-diones (tryptanthrins). Three dimensional pharmacophore modeling and identification of new antimalarial candidates. European Journal of Medicinal Chemistry 39: 59–67.
Bird, C.W. 1963. The structure of methylisatoid. Tetrahedron 19: 901–904.
Bloxam, W.P. 1915. Our present knowledge of the chemistry of indigo. Journal of Chemical Society, Perkin Transactions 974–987.
Bond, C.C., and M. Hooper. 1969. Isatogens. part VI. Synthesis of isatogens via tolan (diphenylacetylene) intermediate. Journal of Chemical Society C 2453–2460.
Bowman, W.R., M.R.J. Elsegood, T. Stein, and G.W. Weaver. 2007. Radical reactions with 3H-quinazolin-4-ones: synthesis of deoxyvasicinone, mackinazolinone, luotonin A, rutaecarpine and tryptanthrin. Organic and Biomolecular Chemistry 5: 103–113.
Brufani, M., W. Fedeli, F. Mazza, A. Gerhardt, and W. Keller-Schierlein. 1971. The structure of tryptanthrin. Experimentia 27: 1249–1250.
Butler, K., M.W. Partridge, and J.A. Waite (1960) Cyclic amides. XIV. Derivatives of 7H-5,6a,12-triazabenzo[a]anthracene. Journal of Chemical Society 4970–4976.
Camargo, L.T.F.M., M.M. Sena, and A.J.A. Camargo. 2009. A quantum chemical and chemometrical study of indolo[2,1-b]quinazoline and their analougues with cytotoxic activity against breast cancer cells. SAR and QSAR in Environmental Research 20: 537–549.
Chan, H.-L., H.-Y. Yip, N.-K. Mak, and K.-N. Leung. 2009. Modulatory effects and action mechansims of tryptanthrin on murine myeloid leukemia cells. Cellular and Molecular Immunology 6: 335–342.
Cornforth, J., P.B. Hitchcock, and P. Rozos. 1996. Isatin chlorides: a phantom reactions of 2-(2,2-dichloro-2, 3-dihydro-3-oxoindol-1-yl)-3H-indol-3-one. Journal of Chemical Society, Perkin Transactions 1: 2787–2792.
Cornforth, J.W. 1976. Structure of isamic acid and methylistoid. Journal of Chemical Society, Perkin Transactions 1: 2004–2009.
Costes, N., H. Le Deit, S. Michel, F. Tillequin, M. Koch, B. Pfeiffer, P. Renard, S. Leonce, N. Guilbaud, L. Kraus-Berthier, A. Pierre, and Gh Atassi. 2000. Synthesis and cytotoxic and antitumor activity of benzo[b]pyrano[3,2-h]acridin-7-one analogues of acronycine. Journal of Medicinal Chemistry 43: 2395–2402.
Cox, E.G., T.H. Goodwin, and A.I. Wagstaff. 1936. The structure of isatin. Proceedings of the Royal Society 157: 399–411.
Danz, H., S. Stoyanova, P. Wippich, A. Brattstrom, and M. Hamburger. 2001. Identification and isolation of the cyclooxygenase-2 inhibitory principles in Isatis tinctoria. Planta Medica 67: 411–416.
Danz, H., D. Baumann, and M. Hamburger. 2002a. Quantitative determination of the dual COX-2/5-LOX inhibitor tryptanthrin in Isatis tinctoria by ESI-LC-MS. Planta Medica 68: 152–157.
Danz, H., S. Stoyanova, O.A.R. Thomet, H.-U. Simon, G. Dannhardt, H. Ulbrich, and M. Hamburger. 2002b. Inhibitory activity of tryptanthrin on prostaglandin and leukotriene synthesis. Planta Medica 68: 875–880.
Dorn, A., S.R. Vippagunta, H. Matile, A. Bubendorf, J.L. Vennerstrom, and R.G. Ridley. 1998. A comparison and analysis of several ways to promote haematin (Haem) polymerisation and an assessment of its initiation in vitro. Biochemical Pharmacology 55: 737–747.
Eguchi, S., H. Takeuchi, and Y. Matsushita. 1992. Short-step synthesis of rutaecarpine and tryptanthrin via intramolecular Aza-Wittig reaction. Heterocycles 33: 153–156.
Fedeli, W., and F. Mazza. 1974. Crystal structure of tryptanthrin (indolo[2,1-b]quinazoline-6,12-dione). Journal of Chemical Society, Perkin Transactions 2: 1621–1623.
Fiedler, H.-P. 1974. Diss. Tübingen.
Fiedler, E., H.-P. Fiedler, A. Gerhard, W. Keller-Schierlein, W.A. Koenig, and H. Zähner. 1976. Stoffwechselprodukte von Mikroorganismen. 156. Syntheses und Biosynthesese of substituierter Tryptanthrine. Archives of Mikrobiologie 107: 249–256.
Friedländer, P., and N. Roschdestwensky. 1915. Über ein Oxydationspdukt des Indigblaus. Chemische Berichte 48: 1841–1847.
Gassman, P.G., B.W. Cue Jr, and T.-Y. Luh. 1977. A general method for the synthesis of isatins. Journal of Organic Chemistry 42: 1344–1348.
Geckeler, K., and J. Metz. 1979. A simple synrthesis of 1,2,3-benzotriazin-4(3H)-one. Archiv der Pharmazie 312: 842–844.
German Patent belongs to Farbwerke vonmals Meister, Lucius and Bruning 1913. Oxidation product. DE 276,808.
George, V., A.S. Koshy, O.V. Singh, M.N.S. Nayar, and P. Pushpangadan. 1996. Tryptanthrin from Wrightia tinctoria. Fitoterapia 67: 553–554.
Gilman, R.E., M.J. Novak, J.C. Baum, and J.A. Olson. 2008. Scanning tunneling microscopy of 8-fluoroindolo[2,1-b]quinazoline-6,12-dione (8-fluorotryptanthrin) at the graphite-solution interface: fully resolved molecular orbitals. Journal of Physical Chemistry C 112: 14545–14548.
Grandolini, G., V. Ambrogi, L. Perioli, M. Giannangeli, L. Jovicevic, and V. Rossi. 1997. Synthesis and antimicrobial activity of some new derivatives of 6,12-dihydroindolo[2,1-b]quinazolin-6,12-dione. Farmaco 52: 679–683.
Hamburger, M. and H. Danz 2000. Indoloquinazolinones for inhibiting NF-κB and antiinflammatory applications. PCT Appl. WO 0061159.
Hamburger, M. 2002. Isatis tinctoria: From the rediscovery of an ancient medicinal plant towards a novel anti-inflammatory phytopharmaceutical. Phytochemistry Reviews 1: 333–344.
Hantzsch, A. 1921. Über die wirklichen und angeblichen Isomerien in der Isatin-Reihe. Chemische Berichte 54: 1221–1257.
Hantzsch, A. 1925. Über Isatoid und sogen. Isatol. Chemische Berichte 58: 685–692.
Hashimoto, T., H. Aga, H. Chaen, S. Fukuda, and M. Kurimoto. 1999. Isolation and identification of anti-Helicobacter pylori compounds from Polygonum tinctorium Lour. The Journal of Natural Medicines (Tokyo) 53: 27–31.
Heller, G., and W. Benade. 1907. Über die Farbenerscheinung der alkalischen Isatinlösung. Chemische Berichte 40: 1291–1300.
Heller, G. 1919. Neue Isomerien in der Isatinreihe. III. Chemische Berichte 52: 437–446.
Heller, G., and W. Benade. 1922. Über die Natur der Isatoide. Chemische Berichte 55: 1006–1014.
Heller, G., and R. Barthel. 1936. Zur Kenntnis des Indigos: cis-indigo. Chemische Berichte 69B: 563–565.
Hicks, R.P., D.A. Nichols, C.A. DiTusa, D.J. Sullivan, M.G. Hartell, B.W. Koser, and A.K. Bhattacharjee. 2005. Evaluation of 4-azaindolo[2,1-b]quinazoline-6,12-diones’ interaction with hemin and hemozoin: A spectroscopic, X-ray crystallographic and molecular modeling study Internet Electron. Journal of Molecular Design 4: 751–764.
Hill, A.J., and H.R. Henze. 1924. Condensation reactions of cyclic ketones. I. The action of isatin and isatin alph chloride upon certain hydantoins. Journal of the American Chemical Society 46: 2806–2810.
Honda, G., and M. Tabata. 1979. Isolation of antifungal principle tryptanthrin, from Strobilanthes cusia O. Kuntze Planta Medica 36: 85–86.
Honda, G., M. Tabeta, and M. Tsuda. 1979. The microbial specificity of tryptanthrin. Planta Medica 37: 172–174.
Honda, G., V. Tosirisuk, and M. Tabata. 1980. Isolation of an antidermatophytic, tryptanthrin, from indigo plants. Polygonum tinctorium and Isatis tinctoria. Planta Medica 38: 275–276.
Hong, Y.H., W.J. Lee, S.H. Lee, J.K. Son, H.-L. Kim, M. Nam, Y. Kwon, and Y. Jahng. 2010. Synthesis and biological properties of benzo-annulated rutaecarpines. Biological and Pharmaceutical Bulletin 33: 1704–1709.
Hosoe, T., K. Nozawa, N. Kawahara, K. Fukushima, K. Nishimura, M. Miyaji, and K. Kawai. 1999. Isolation of a new potent cytotoxic pigment along with indigotin from the pathogenic Basicdiomycetous fungus Schzophyllum commune. Mycopathologia 146: 9–12.
Hosoe, T., K. Nozawa, N. Kawahara, K. Fukushima, K. Nishimura, M. Miyaji, and K. Kawai. 2000. Isolation of a new potent cytotoxic pigment along with indigotin from the pathogenic basidiomycetous fungus Schizophyllum commune. Mycopathologia 146: 9–12.
Ishihara, T., K. Kohno, S. Ushio, K. Iwaki, M. Ikeda, and M. Kurimoto. 2000. Tryptanthrin inhibits nitric oxide and prosataglandin E2 synthesized by murine macrophases. European Journal of Pharmacology 407: 197–204.
Jahng, Y. 2012. Unpublished results.
Jao, C.-W., W.-C. Lin, Y.-T. Wu, and P.-L. Wu. 2008. Isolation, structure elucidation, and synthesis of cytotoxic tryptanthrin analogues from Phaius mishmensis. Journal of Natural Products 71: 1275–1279.
Jahng, K.C., S.I. Kim, D.H. Kim, C.S. Seo, J.-K. Son, S.H. Lee, E.-S. Lee, and Y. Jahng. 2008. One-pot synthesis of simple alkaloids: 2,3-Polymethylene-4(3H)-quinazolinones, luotonin A, tryptanthrin, and rutaecarpine. Chemical and Pharmaceutical Bulletin 56: 607–609.
Jarrah, M.Y. and V. Thaller 1980. 300 MHz 1H n. m. r. spectra of indolo[2,1-b]quinazoline-6,12-dione, tryptanthrin, and its 2- and 8-chloro-derivatives. Chemical Research (S) 186.
Kametani, T., C.V. Loc, T. Higa, M. Koizmi, and K.J. Fukumoto. 1977. Iminoketene cycloaddition. 2. Total syntheses of arborine, glycosminine, and rutecarpine by condensation of iminoketene with amides. Journal of the American Chemical 99: 2306–2309.
Karpf, H., and H. Junek. 1978. Thermolyse von Isatin Mittels eines CO2-Lasers. Tetrahedron Letters 33: 3007–3008.
Kataoka, M., K. Hirata, T. Kunikata, S. Ushio, K. Iwaki, K. Ohashi, M. Ikeda, and M. Kurimoto. 2001. Antibacterial action of tryptanthrin and kaempferol, isolated from the indigo plant (Polygunum tinctotium Lour.) against Helicobacter pylori-induced mongolian gerbils. Journal of Gastroenterology 36: 5–9.
Katritzky, A.R., W.Q. Fan, A.E. Koziol, and G.J. Palenik. 1989. 2-Chloro-3H-indol-3-one and its reactions with nucleophiles. Journal of Heterocyclic Chemistry 26: 821–828.
Kikumoto, R., and T. Kobayashi. 1966. The reactions of oxindoles and isatin with nitrobenzyl chlorides : Formation of 2′-hydroxy-spiro[2H-indole-2,3′-3′H-indole]. Tetrahedron 22: 3337–3343.
Kimoto, T., K. Hino, S. Koya-Miyata, Y. Yamamoto, M. Takeuchi, Y. Nishizaki, M.J. Micallef, S. Ushio, K. Iwaki, M. Ikeda, and M. Kurimoto. 2001. Cell differentiation and apoptosis of monocyclic and promyelocytic leukemia cells (U-937 and HL-60) by tryptanthrin. Pathology International 51: 315–325.
King, R. A. 2009. Biotransformations in drug metabolism. In Drug metabolism handbook, concepts and applications. ed Nassar, A. F., Hollenber, P. F., and J. Scatina, 17–40. New York: Wiley.
Koya-Miyata, S., T. Kimoto, M.J. Micallef, K. Hino, M. Taniguchi, S. Ushio, K. Iwaki, M. Ikeda, and M. Kurimoto. 2001. Prevention of azoxymethane-induced intestinal tumors by a cute ethyl acetate-extract and tryptanthrin extracted from Polygonum tinctorium lour. Anticancer Research 21: 3295–3300.
Kumar, A., V. Deepak, and P. Kumar. 2011. β-Cyclodextrin catalyzed synthesis of tryptanthrin in water. Green Chemistry 13: 51–54.
Laatsch, H. and H. Ludwig-Köhn 1986. Isolierung des indigoiden Pigmentes Candidin aus Urin und Hämofiltrat von Urämikern. Liebigs Annalen der Chemie 1847–1857.
Lee, E.S., J.G. Park, and Y. Jahng. 2003. A facile synthesis of simple alkaloids – Synthesis of 2,3-polymethylene-4(3H)-quinazolinones and related alkaloids. Tetrahedron Letters 44: 1883–1886.
Lee, S.K., G.H. Kim, D.H. Kim, D.H. Kim, Y. Jahng, and T.C. Jeong. 2007. Biological and Pharmaceutical Bulletin 30: 1991–1995.
Li, Q., J. Jin, M. Chong, and Z. Song. 1983. Studies on the antifungal constituent of Qing Dai (Isatis indigotica). Zhongcaoyao 14: 440–441.
Li, Q. 1987. The chemical constituents of Qing-Dai. Zhiwu Xuebao 29: 67–72.
Li, B., W. Chen, S. Zheng, G. Yang, and C. Qiao. 2000. Two new alkaloids isolated from tetraploid Banlangen. Acta Pharmaceutica Sinica B 35: 508–510.
Liang, J.L., J.-E. Eom, Y. Kwon, and Y. Jahng. 2012. Synthesis of benzo-annulated tryptanthrins and their biological properties. Bioorganic and Medicinal Chemistry 20: 4962–4967.
Liu, J., C. Wang, Z. Liu 2008. Method for preparation of tryptanthrin derivatives and its application for preparation of indoleamine 2,3-dioxygenase inhibitor. Chin. Pat CN101177428.
Liu, Y., F. Ouyang, H. Yu, L. Li, N. Wang, and X. Yao. 2009. Chemical constituents in the leaves of Baphicaanthus cusia. Chinese Journal of Medicinal Chemistry 19: 273–275.
Lygin, A.V., and A. de Meijere. 2009. ortho-Lithiophenyl isocyanide: A versatile precursor for 3H-quinazolin-4-ones and 3H-quinazolin-4-thiones. Organic Letters 11: 389–392.
Machemer, H. 1930. Die Autoxydation der Metalkomplexe des Indigos. Chemische Berichte 63B: 1341–1347.
Mason, J.J., T. Janosik, J. Bergman. 2009. A new approach to methoxyisatins leading to the total synthesis of ophiuroidine and other hydroxytryptanthrins. Synthesis 3642–3648.
Matsui, M., M. Morita, K. Shibata, and Y. Takase. 1982. Ozonolysis of indigo. Nippon Kagaku Kaishi 7: 1268–1269.
Mitscher, L.A., W.-C. Wong, T. DeMeulenaere, J. Sulko, and S. Crake. 1981. Antimicrobial agents from plants. New synthesis and bioactivity of tryptanthrin (indolo[2,1-b]quinazoline-6,12-dione) and its derivatives. Heterocycles 15: 1017–1021.
Mitscher, L.A., and W.R. Baker. 1998a. Tuberculosis: A search for novel therapy starting with natural products. Medicinal Research Reviews 18: 363–374.
Mitscher, L.A., and W.P. Baker. 1998b. A search for novel chemotherapy against tuberculosis amongst natural products. Pure and Applied Chemistry 70: 365–371.
Moskovkina, T.V. 1997. New synthesis of 6,12-dihydro-6,12-dioxoindolo[2,1-b]quinazoline (tryptanthrine, couropitine A). Russian Journal of Organic Chemistry 33: 125–126.
Moskovkina, T.V., A.I. Kalinovskii, and V.V. Makhan’kov. 2012. Synthesis of tryptanthrin (couroupitine) derivatives by reaction of substituted isatins with phosphoryl chloride. Russian Journal of Organic Chemistry 48: 123–126.
Motoki, T., Y. Takami, Y. Yagi, A. Tai, I. Yamamoto, and E. Gohda. 2005. Inhibition of hepatocyte growth factor induction in human dermal fibroblasts by tryptanthrin. Biological and Pharmaceutical Bulletin 28: 260–266.
Murakami, T., A. Kishi, T. Sakurama, H. Matsuda, and M. Yoshikawa. 2001. Chemical constituents of two oriental orchids, Calanthe discolor and C. liukiuensis: Precursor indole glycoside of tryptanthrin and indirubin. Heterocycles 54: 957–966.
Muruganandam, A.V., and S.K. Bhattacharya. 2000. Indole and flavonoid constituents of Wrightia tinctoria, W. tomentosa and W. coccinea. Indian Journal of Chemistry - Section B 39B: 125–131.
Nugent, W.A., and J.M. Mayer. 1988. Metal-Ligand Multiple Bonds. New York: Wiley-Science.
Oberthür, C., R. Jaeggi, and M. Hamburger. 2005. HPLC based activity profiling for 5-lipoxygenase inhibitory activity in Isatis tinctoria leaf extracts. Fitoterapia 76: 324–332.
Okunade, A.L., and M.P.F. Elvin-Lewis. 2009. Novel Therapeutic Agents from Plants, 405–452. Enfield: Science Publishers.
O’Neill, C. 1892. Chemistry News 65: 124.
Overthür, C., B. Hoffmann, and M. Hamburger. 2002. Synthesis of d-tryptanthrin. Pharmazie 57: 586–587.
Perkin, A.G. 1906. An oxidation product of indigotin. Proceedings of the Chemical Society London 22: 198–199.
Pitzer, K.K., Scovill, J.P., Kyle, D.E., and L. Gerena 2003. Antimalarial and antiproliferative pharmacophore models, novel trytptanthrin compounds having increased solubility, and methods of making and using thereof. U.S. Patent, 6,531,487.
Potewar, T.M., S.A. Ingale, and K.V. Srinivasan. 2008. Synthesis of tryptanthrin and deoxyvasicinone by a regioselective lithiation-intramolecular electrophilic reaction approach. ARKIVOC 14: 100–108.
Rasmussen, L.E.L., T.D. Lee, D. Daves Jr, and M. Schmidt. 1993. Female-to-male sex pheromones of low volatility in the Asian elephant, Elephas maximus. Journal of Chemical Ecology 19: 2115–2128.
Sandmeyer, T. 1919. Isonitrosoacetanilides and their condensation to isatins. Helvetica Chimica Acta 2: 234–242.
Schindler, W., and H. Zähner. 1971. Stoffwechselprodukte von Mikroorganismen—91. Mittelung: Tryptanthrin, ein von Tryptophan abzuleittendes Antibioticum aus Candida lipolitica. Archive der Mikrobiolologie 79: 187–203.
Schrenk, D., D. Riebniger, M. Till, S. Vetter, and H.F. Fiedlerf. 1997. Tryptanthrins: A novel class of agonists of the aryl hydrocarbon receptor. Biochemical Pharmacology 54: 165–171.
Scovill, J., E. Blank, M. Konnick, E. Nenortas, and T. Shapiro. 2002. Antitrypanosomal activities of tryptanthrins. Antimicrobial Agents and Chemotherapy 46: 882–883.
Seifert, K., and W. Unger. 1994. Insecticidal and fungicidal compounds from Isatis tinctoria. Zeit Naturforsch C 49: 44–48.
Sen, A. K., Mahato, S. B., and Dutta, N. L., Couroupitine A 1974. A new alkaloid from Courouita guianensis. Tetrahedron Letters 15: 609–610.
Sharma, V.M., P. Prasanna, K.V. Seshu, B. Renuka, C.V. Rao, G.S. Kumar, C.P. Narasimhulu, P.A. Babu, R.C. Puranik, D. Subramanyam, A. Venkateswarlu, S. Rajagopal, K.B. Kumar, C.S. Rao, N.V. Mamidi, D.S. Deevi, R. Ajaykumar, and R. Rajagopalan. 2002. Novel indolo[2,1-b]quinazoline analogues as cytostatic agents: synthesis, biological evaluation and structure-activity relationship. Bioorganic and Medicinal Chemistry Letters 12: 2303–2307.
Siedel, P. 1822. BASF, Ludwigshafen 1938, in which a preparation of tryptanthrin had been described as early as 1822 by Dumas, Journ. Pharm. VIII, 377 (1822), author could not find the original paper.
Son, J.K., J.G. Park, and Y. Jahng. 2003. A simple synthesis of tryptanthrin. Heterocyclic Communication 9: 621–623.
Staskun, B., and H.F.S. Wolfe. 1992. New approach to the indolo[2, 1-b]quinazoline ring system by cyclization of 3-(o-chlorophenyl)-2-methyl-4(3H)-quinazolinone and its m-isomer. Synthesis of the antibiotic tryptanthrin. African Journal of Chemistry 45: 5–7.
Sugai, A. and S. Saito 1997. Preparation of tryptanthrin derivatives and electrophotographic photoreceptors containing them. Jpn. Kokai Tokyo Koho JP 09040672.
Sugai, A., S. Matsumoto, N. Akiba, Y. Watanabe, H. Kawaguchi 1997. Tryptanthrin derivatives suitable for electron transporting agents and highly sensitive electronic photographic photoconductors using the same. Jpn. Kokai Tokyo Koho JP 09095621.
Tucker, A.M. and P. Grundt 2012. The chemistry of tryptanthrin and its derivatives. ARKIVOC (i): 546–569.
Utkina, N.K., and V.A. Denisenko. 2007. Ophiuroidine, the first indolo[2,1-b]quinazoline alkaloid from the Caribbean brittle star Ophiocoma riisei. Tetrahedron Letters 48: 4445–4447.
Valiante, N. 2004. Use of tryptanthrin compounds for immune potentiation. PCT Int. Appl. WO 2004064759.
von Sommaruga, E. 1879. Über die Moleculargröfse des Indigos. Justus Libigs Annalen der Chemie 195: 302–313.
Wang, C., L. Jianli, and S. Xiaoli. 2007. Progress in the synthesis of natural product tryptanthrin and its derivatives. Chemistry Online 70: 89–95. 化學通報.
Witt, A., and J. Bergman. 2003. Recent developments in the field of quinazoline chemistry. Current Organic Chemistry 7: 659–677.
Wu, X.Y., Y.H. Liu, W.Y. Sheng, J. Sun, and G.W. Qin. 1997. Chemical constituents of Isatis indigotica. Planta Medica 63: 55–57.
Xu, F., H. Matsuda, H. Hata, K. Sugawara, S. Nakamura, and M. Yoshikawa. 2009. Structure of new flavonoids and benzofuran-type stilbene and degranulation inhibitors of rat basophillic leukemia cells from the Brazilian herbal medicine Cissus sucyoides. Chemical and Pharmaceutical Bulletin 57: 1089–1095.
Yoshikawa, M., T. Murakami, A. Kishi, T. Sakurama, H. Matsuda, M. Nomura, H. Matsuda, and M. Kubo. 1998. Novel indole S, O-bisdesmoside, calanthoside, the precursor glycoside of tryptanthrin, indirubin, and isatin, with increasing skin blood flow promotic effects, from two Calanthe species (Orchidaceae). Chemical and Pharmaceutical Bulletin 46: 886–888.
Yu, S.T., T.M. Chen, S.Y. Tseng, and Y.H. Chen. 2007. Tryptanthrin inhibits MDR1 and reverse doxorubicin resistance in breast cancer cells. Biochemical and Biophysical Research Communications 358: 79–84.
Yu, S.-T., T.-M. Chen, J.-W. Chern, S.-Y. Tseng, and Y.-H. Chen. 2009. Downregulation of GSTπ expression by tryptanthrin contributing to sensitization of doxorubicin-resistant MCF-7 cells through c-Jun NH2-terminal kinase-mediated apoptosis. Anti-Cancer Drugs 20: 382–388.
Yu, S.-T., J.-W. Chern, T.-M. Chen, Y.-F. Chiu, H.-T. Chen, and Y.-H. Chen. 2010. Cytotoxicity and reversal of multindrug resistance by tryptanthrin-derived indoloquinazolines. Acta Pharmaceutica Sinica B 31: 259–264.
Zeide, O.A., and G.V. Chelintsev. 1937. The action of chloropyridine on anthranilic acid. Journal of General Chemistry 7: 2318–2323.
Zou, J., and L. Huang. 1985. Minor constituents of Qing Dai, a traditional Chinese medicine. I. Isolation, structure determination and synthesis of tryptanthrin and qingdainone. Acta pharmaceutica Sinica B 20: 45–51.
Acknowledgments
Present work is partially supported by Korean Science Foundation (KRF-2008-521-E00189).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jahng, Y. Progress in the studies on tryptanthrin, an alkaloid of history. Arch. Pharm. Res. 36, 517–535 (2013). https://doi.org/10.1007/s12272-013-0091-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0091-9