Abstract
The study aimed to systematically review the effects of exercise training (EX) on brachial artery flow-mediated dilation (FMD) and inflammatory biomarkers in patients with peripheral artery disease (PAD). Five electronic databases were searched: (i) patients with PAD aged ≥ 18; (ii) structured EX ≥ 2 weeks; (iii) measured brachial artery FMD; and (iv) measured blood inflammatory biomarkers. Eighteen studies met the inclusion criteria. EX increased FMD but had no effect on C-reactive protein, interleukin-6, and tumor necrosis factor-α. Subgroups with moderate intensity had a greater increase in FMD than subgroups with vigorous intensity. There was no difference in effect on FMD and three inflammatory biomarkers between subgroups training for ≤ 12 weeks and > 12 weeks of EX, < 50 min and ≥ 50 min of session duration, and < 150 min and ≥ 150 min of weekly volume, respectively. These results suggest that EX-induced improvement in vascular function can be independent of the improvement of systemic inflammation.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peripheral artery disease (PAD) is a manifestation of systemic atherosclerosis resulting in blood flow restriction by narrowed or blocked peripheral arteries. The vascular endothelium, an inner layer of blood vessels, plays a pivotal role in the development and progression of atherosclerosis [1]. Endothelial dysfunction is defined by an imbalance between vasodilation and vasoconstriction, characterized by reduced bioavailability of vasodilators, particularly nitric oxide (NO), and/or increased endothelium-derived contracting factors [2]. Endothelial (dys)function can be quantified by the measurement of flow-mediated dilation (FMD) of the brachial artery as a non-invasive gold standard [3, 4]. A meta-analysis concluded that every 1% decrease in brachial artery FMD is associated with a 13% increase in the events of cardiovascular diseases (CVD) [4]. Moreover, brachial artery FMD is independently associated with the presence and extent of atherosclerosis [5]. Thus, the improvement of endothelial function measured by brachial artery FMD is a major therapeutic goal for PAD.
Chronic inflammation plays a critical role in the pathogenesis of atherosclerotic diseases [6]. Various inflammatory mediators such as pro-inflammatory cytokines promote endothelial nitric oxide synthase (eNOS) uncoupling [7] and endothelial activation, morphological and functional modifications triggered by nuclear factor-κB (NF-κB) signaling [8], and as a result, these inflammatory mechanisms can directly or indirectly induce vascular dysfunction. Pro-inflammatory cytokines, including certain interleukins (ILs) and tumor necrosis factor (TNF)-α, stimulate the upregulation of adhesion molecules with subsequent monocyte adhesion [8, 9]. Monocytes proceed to differentiate into macrophages that further promote coagulation, platelet adhesion, and aggregation, which consequently induces atherosclerosis [9]. In addition, the pro-inflammatory marker C-reactive protein (CRP) may play a role in the development and progression of PAD [10].
Physical activity increases hemodynamic shear stress, the most powerful physiological activator of eNOS, which promotes the production of a key vasodilator, NO [11]. We previously determined that exercise training (EX) alleviated atherosclerosis and obese-induced vascular dysfunction through increased NO bioavailability and eNOS expression in mice [12, 13]. In patients with PAD, EX increased brachial artery FMD with decreased [14] or unchanged levels of inflammatory biomarkers [15], whereas another study determined that both FMD and inflammatory biomarkers remained unchanged after EX [16]. Similarly, the effects of EX on inflammatory biomarkers seem to be contradictory in patients with PAD. For example, EX decreased levels of CRP or IL-6 [17, 18] in some studies, whereas in other studies CRP, IL-6, and TNF-α were unchanged following EX [15]. Thus, there are contradictory results about the effects of EX on vascular endothelial function and inflammation in patients with PAD.
To our knowledge, no meta-analysis has been conducted to examine the effects of EX on the key prognostic variables for the risk of atherosclerosis and CVD events, vascular endothelial function, and inflammatory biomarkers in patients with PAD. Exercise guidelines for the patients need to be developed based on a quantitative review. Moreover, there are contradictory results about EX-induced changes in endothelial function and inflammation in PAD. Therefore, the purpose of this study was to evaluate the effects of EX on endothelial function assessed by brachial artery FMD and blood inflammatory biomarkers in patients with PAD by conducting a systematic review and meta-analysis. This review carefully examined the specific variables of training regimens (i.e., intensity, duration, and frequency) and demographic characteristics of patients (i.e., age, gender, and body mass index (BMI)) to provide high-level evidence for developing optimal exercise guidelines.
Methods
This current systematic review followed the strategy of the PRISMA statement [19].
Data Sources
Five electronic databases (CINAHL, Cochrane, EMBASE, MEDLINE, and Web of Science) were searched for eligible studies published in English from the earliest date available to April 2023. The following keywords were used for searches: “exercise or training or rehabilitation” AND “endothelial function or flow-mediated dilation or inflammation or inflammatory or biomarker or cytokine” AND “peripheral artery disease or peripheral arterial disease.” Reference lists of included studies and relevant reviews were manually searched to ensure all relevant studies were captured. Two reviewers (JL and AZ) searched all the articles and applied the inclusion and exclusion criteria to the titles and abstracts searched. Disagreements about inclusion and exclusion were resolved by reaching a consensus through discussion, which involved a senior researcher (YP). When the information was not clear, the full-text papers of the studies were obtained for review. Corresponding authors of potentially eligible studies were contacted if studies reported missing or unclear data.
Study Selection
The inclusion criteria for eligible studies were as follows: (i) patients with PAD of all stages aged ≥ 18; (ii) structured exercise intervention ≥ 2 weeks, such as aerobic training (AT), resistance training (RT), or combined AT and RT including supervised intervention, home-based intervention, and mixed intervention in both randomized and non-randomized controlled studies; (iii) measured endothelial function by brachial artery FMD (% of change); and (iv) measured blood inflammatory biomarkers. Measurements had to be administered both at baseline and post-intervention. Non-exercise trials, such as medication treatment, or multi-interventional trials involving additional non-exercise interventions on EX, such as exercise intervention combined with dietary supplements, were excluded from multi-intervention studies to focus on the effects of EX alone. Studies investigating the acute effect of one single exercise session were also excluded. Duplicate studies or sub-studies of included trials were also excluded from the analysis.
Quality Assessment
Two reviewers assessed the quality of the included studies using the PRISMA recommendations [19]. The quality assessment consisted of six items: (i) appropriate generation of random allocation sequence; (ii) concealment of the allocation sequence; (iii) blinding of the assessment and collection outcomes; (iv) proportion of participants lost to follow-up; (v) complete outcome data; and (vi) the intention-to-treat principle [19]. The overall quality of the evidence was also assessed by the Grading of Recommendations Assessment, Development, and Evaluations (GRADE) [20, 21]. GRADE assesses outcomes across five areas: risk of bias, inconsistency, indirectness, imprecision, and publication bias. According to GRADE, interventions were graded as follows: ‘high quality’—we are very confident that the true effect lies close to that of the estimate of the effect; “moderate quality”—we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; “low quality”—our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect; “very low quality”—we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of the effect [22].
Data Extraction
Data were extracted from all selected studies to record detailed information in terms of subject characteristics, study methods, interventions, outcomes, and adverse events. Means and standard deviation (SD) were used, but where standard errors or 95% confidence interval (CI) were provided, they were converted to SD.
In terms of population characteristics, age, gender, BMI, relative body fat, the number of participants, post-PAD period, and medication were recorded to compare the similarity of participants between trials. The primary outcome was FMD and the secondary outcomes were inflammatory biomarkers. The brachial artery was only selected instead of a femoral or popliteal artery for the measurement of FMD because brachial artery endothelial function is inversely associated with CVD risk and atherosclerosis as a non-invasive gold standard to assess endothelial function [3,4,5]. Regarding the intervention, total duration, frequency (days per week), intensity, session duration, weekly training volume, type and order of exercise, names of exercise machines or tools, supervisors, and places of intervention were recorded to compare the similarity of training methods between trials. The median values were used for calculation if the studies reported a range of data (e.g., 16 from 15 to 17 repetitions). Detailed interventions about control groups (CON) and any additional interventions were recorded. Measurement technique and region were also extracted.
Data Analysis
Heterogeneity between studies was assessed using the Cochran Q statistic [23] and the I2 test [24]. I2 ranges from 0 to 100%: a value < 25% indicates a low risk of heterogeneity; 25–75% indicates a moderate risk of heterogeneity, and > 75% indicates a high risk of heterogeneity. In each study, the effect size (ES) for the intervention was calculated by the standardized mean difference between pre-and post-intervention using Cohen’s d. Separate meta-analyses of trials with FMD and inflammatory biomarkers were performed to generate the mean ES and 95% CI. ESs were classified according to Cohen’s definition (1988), where 0.2 is considered small, 0.5 moderate, and 0.8 large [25]. We used a fixed-effects model when homogeneity was verified or a random-effects model when heterogeneity was shown by the Q statistic [24]. When the Q value exceeds the degrees of freedom of the estimate, significant heterogeneity is considered to exist. Publication bias was assessed using Egger’s regression test [26]. To evaluate whether an individual cohort had undue influence on the overall meta-analysis result, sensitivity analyses were performed on all outcomes by omitting one of the trials at a time and determining whether statistical conclusions remained the same. The percent changes in FMD and inflammatory biomarkers between pre-and post-intervention were calculated using the weighted averages. Meta-analyses were performed with SPSS version 20 and STATA version 14.2. Data coding and other calculations were conducted with Microsoft Excel 365.
Subgroup analyses were performed to identify potential factors influencing the effect of EX on outcomes and accounting for the heterogeneity between studies: (i) ≤ 12 weeks versus > 12 weeks of EX; (ii) < 50 min versus ≥ 50 min of session duration; (iii) < 150 min versus ≥ 150 min of weekly volume; and (iv) moderate-versus vigorous-intensity. Random effects meta-analysis regression was conducted to compare the effect estimates (effect size) in different subgroups by considering the meta-analysis results from each subgroup separately. To interpret the results of subgroup analyses, P value (P < 0.05) between study variations was considered for the statistical difference between subgroups.
Results
Study Selection and Characteristics
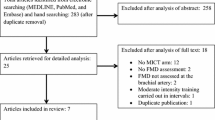
The selection process resulted in 3640 potential studies and is documented in the PRISMA flow diagram (Fig. 1). From the titles and abstracts, 3497 studies were excluded based on the inclusion and exclusion criteria, and then 143 full-text studies were reviewed for eligibility by applying the criteria. Of these, 125 studies were excluded after assessment, and finally, a total of 18 studies [14,15,16,17,18, 27,28,29,30,31,32,33,34,35,36,37,38,39] met the criteria. In the selected studies, 9 studies [14,15,16, 28, 29, 32, 34, 35, 38] had two EX cohorts, and one study had one EX cohort (treadmill) and another EX cohort (treadmill) in a water pool of 24–30 °C [14]. Another heat therapy cohort without EX [27] was excluded from this analysis. Consequently, 29 exercise cohorts in 18 studies were included. Of 18 studies, 13 studies measured brachial artery FMD and 6 studies measured inflammatory biomarkers, such as CRP, IL-6, and/or TNF-α following EX.
Quality Assessment and Potential Bias
In the quality assessment, 83% reported appropriate generation of a random allocation sequence (15 of 18); 44% presented concealment of the allocation sequence (8 of 18); 56% described blinding of the assessment and collection outcomes (10 of 18); 100% explained proportion of participants lost to follow-up (18 of 18); 100% exhibited complete outcome data (18 of 18); and 33% reported that the intention-to-treat principle was used for statistical analyses (6 of 18). Egger’s test showed significant publication bias for FMD (P = 0.04) and no significant bias for CRP, IL-6, and TNF-α (P = 0.13, P = 0.78, and P = 0.17, respectively) (Fig. 2).
Adverse Events
The presence or absence of adverse events was recorded in three [18, 27, 39] of the eighteen studies. Those three studies reported that there were no adverse events.
Participants
Table 1 shows the characteristics of all the studies included. Studies were published from February 2001 [31] to December 2021 [36]. A total of 1301 participants completed their interventions (EX: 980, CON: 321, female%: 33.2%), with sample size ranging from 11 [27] to 185 [33]. The average age of the participants was 66.0 ± 7.9 years (EX: 68.3 ± 8.2, CON: 66.7 ± 8.4 years). Average BMI was 27.5 ± 5.0 kg/m2 (EX: 27.7 ± 5.0, CON: 26.9 ± 5.0 kg/m2). Most patients with PAD in included studies had some comorbidities including diabetes, hypertension, hyperlipidemia, myocardial infarction, coronary artery disease, or cancer, but it was not reported in four studies [14, 28, 31, 33]. Their medications were maintained throughout interventions but were withheld for at least 24 h before evaluation. The duration of PAD was ≥ 1 month [18] or ≥ 3 months [28, 29], and other studies did not report it.
Interventions
Most studies performed supervised EX in research centers by qualified trainers or researchers, but four cohorts had partial [32] or complete home-based EX sessions [16, 28, 36]. All studies conducted AT using a bicycle or a treadmill, but seven studies [14, 15, 27, 32, 34, 36, 38] additionally performed resistance training (RT). One EX cohort performed treadmill exercise in a water pool of 24–30 °C [14]. The mean training period was 17 weeks (minimum–maximum: 6 [30]–40 [16] weeks). The training frequency was most often 3 days per week; 2 [15, 27], 4 [16], or 5 [36] days per week were also used. The mean session duration was 50 min (minimum–maximum: 30 [18, 31]–90 [27] min). The mean weekly training volume was 142 min (minimum–maximum: 90 [18, 31]–225 [36] min). AT trials expressed intensity as a percent of maximum heart rate (HRmax), walking ability, the Borg scale, or the metabolic equivalent of task (MET). RT trials established their intensity by a percentage of one-repetition maximum (1-RM) or the Borg scale. Intensities ranged from moderate (64–76% of HRmax, 50–69% of 1-RM, Borg scores 12–14, or MET scores 3.0–5.9) to vigorous (77–95% of HRmax, 70–90% of 1-RM, Borg score 15–17, or MET scores ≥ 6.0). AT trials monitored HR or walking speed of participants during exercise for accurate intensity guidance. RT trials monitored HR or progressively increased their intensity throughout training by increasing loads.
Measurements
All studies measured FMD using a high-resolution ultrasound machine to assess brachial artery endothelial function. There were slight differences in the inflation pressure for measuring FMD between studies: 30 [30] or 50 [16, 17, 32,33,34,35, 37] mmHg above systolic blood pressure, 200 mmHg [31], 250 mmHg [36], or unknown [27,28,29]. The duration of cuff inflation was 3 min [30], 4 min [33, 34, 36, 37], 5 min [16, 17, 28, 29, 31, 32, 35], or not specified [27]. Inflammatory biomarkers were measured by ELISA [14, 15, 18, 38] or not specified [17, 39].
Effects of Exercise Training
Flow-mediated Dilation
EX in twenty trials of thirteen studies significantly increased brachial artery FMD (mean ES = 0.54, 95% CI = 0.25 to 0.83, P < 0.001) (Fig. 3). The absolute increase in FMD between the pre-and post-intervention was 19.7%. Univariate meta-regression showed heterogeneity between studies (Q = 108.8, df = 19, P < 0.001, I2 = 82.5%). In subgroup analyses, subgroups with moderate intensity had a significantly greater increase of FMD than subgroups with vigorous intensity (P < 0.01). There was no significant difference in effect between subgroups training for ≤ 12 weeks and > 12 weeks of EX (P = 0.41), < 50 min and ≥ 50 min of session duration (P = 0.80), and < 150 min and ≥ 150 min of weekly volume (P = 0.74), respectively. Age and baseline BMI were excluded from subgroup analyses as most participants had similar levels of age (65–70 years) and BMI (25–30 kg/m2). Weekly frequency was also excluded from subgroup analyses as most trials performed EX 3 days per week.
Inflammatory Biomarkers
EX in eight trials of five studies had no significant effect on CRP (mean ES = − 0.07, 95% CI = − 0.23 to 0.10, P > 0.05) (Fig. 4). EX in six trials of four studies had no significant effect on IL-6 (mean ES = 0.01, 95% CI = − 0.22 to 0.24, P > 0.05) (Fig. 5). EX in five trials of three studies had no significant effect on TNF-α levels (mean ES = − 0.15, 95% CI = − 0.34 to 0.05, P > 0.05) (Fig. 6). Univariate meta-regression did not show heterogeneity between studies in CRP (Q = 3.46, df = 7, P > 0.05, I2 = 0.0%), IL-6 (Q = 1.94, df = 5, P > 0.05, I2 = 0.0%), and TNF-α (Q = 1.92, df = 4, P > 0.05, I2 = 0.0%). In subgroup analyses for CRP, there was no significant difference in effect between subgroups training for ≤ 12 weeks and > 12 weeks of EX (P = 0.71), and < 150 min and ≥ 150 min of weekly volume (P = 0.37), respectively. For IL-6, there was no significant difference in effect between subgroups training for ≤ 12 weeks and > 12 weeks of EX (P = 0.55). For TNF-α, there was no significant difference in effect between subgroups’ training for < 150 min and ≥ 150 min of weekly volume (P = 0.38). Other training variables, such as intensity and session duration were excluded from subgroup analyses as most trials performed EX with similar levels of intensity (3.2 km/h), session duration (50–60 min), and frequency (3 days per week).
Sensitivity Analysis
Sensitivity analysis reported that by excluding any of all cohorts from the meta-analysis the estimated effects will still be within the 95% CI of the mean ES in all four outcomes (Supplementary Fig. 1). These results indicate the results of the meta-analysis will not significantly change after the removal of any one cohort.
Discussion
The primary results of this meta-analysis are that EX significantly increased brachial artery FMD by 20% but had no significant effect on CRP, IL-6, and TNF-α levels in patients with PAD aged 66.0 ± 7.9 years. These results suggest that EX-induced improvement in vascular endothelial function can be independent of the improvement of systemic inflammation (Fig. 7). Brachial artery FMD is negatively associated with the risk of CVD events and the extent of atherosclerosis [4, 5]. Accordingly, EX could substantially contribute to reducing the rate of cardiovascular mortality in patients with PAD. In addition to the main findings, subgroup analyses determined that EX with moderate intensity had a significantly greater effect on FMD than EX with vigorous intensity.
The exercise training improves brachial artery endothelial function in patients with peripheral artery disease through increased nitric oxide availability without changes in inflammatory biomarkers, which might be associated with improved eNOS activity. Improved endothelial function potentially reduces future atherogenesis. Moderate intensity is based on ACSM’s cardiac rehabilitation guidelines. HRmax, heart rate max; eNOS, endothelial nitric oxide synthase; CRP, C-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; and PAD, peripheral artery disease
Unchanged Inflammatory Markers Following Exercise Training
Different from our expectation, EX increased FMD but did not affect inflammatory biomarkers. Contrary to our results, a previous meta-analysis [40] found that EX decreased levels of CRP and IL-6 in patients with coronary artery disease. However, another meta-analysis [41] concluded that contradictory inflammatory adaptations to EX can be elicited in patients with CVD according to the type of CVD and training variables. Similarly, included studies in our meta-analysis have differing results of inflammatory biomarkers, which might be attributed to a different training variable. For example, Delaney and Spark [15] determined that IL-1β, IL-2, IL-7, IL-8, IL-10, and IL-12 as well as CRP, IL-6, and TNF-α remained unchanged following 12 weeks of EX. Other two included studies [38, 39] had similar findings that soluble intercellular adhesion molecules, CRP, and IL-6 remained unchanged following 6 months of EX. On the other hand, neutrophils, CRP, IL-6, and/or TNF-α decreased following 12 weeks [17, 18] or 12 months [14] of EX. Our subgroup analyses also indicated no significant difference in effect on the biomarkers between ≤ 12 weeks and > 12 weeks of EX. Accordingly, the training duration does not seem to influence the alteration of systemic inflammation. Another examined training variable, weekly volume (< 150 min vs ≥ 150) did not reveal a significant effect on the inflammatory biomarkers. However, the lowest weekly training volume (90 min) [18] had the greatest favorable ES (− 0.37) compared to ESs (− 0.19–0.47) of a relatively higher weekly training volume (120–180 min) [14, 15, 17, 38, 39]. Considering PAD mostly occurs in the lower extremities and about 30% of these patients have claudication symptoms [42], a prolonged session duration of EX with a treadmill or bicycle might damage the tissue of the legs and contribute to chronic inflammation [43]. Thus, session duration might be one of the variables to induce different inflammatory adaptations to EX. Although our results indicate no significant improvement in systemic inflammation by EX, it has been also documented that EX exerts anti-inflammatory effects by several molecular mechanisms, e.g., reduced Toll-like receptor expression on monocytes and macrophages [44], which prevents increased expression and secretion of pro-inflammatory cytokines [45]. EX also reduces the expressions of three key proteins, NF-κB p65, cysteinaspartate specific proteinase 1, and NOD-like receptor protein 3 that directly trigger the activation of proinflammation [46]. However, EX with moderate intensity has more favorable effects on those factors compared to vigorous EX [46]. Taken together, EX is suggested to induce anti-inflammatory adaptations but exaggerated physical stress induced by vigorous intensity or prolonged session duration could decline its benefits. Future studies are warranted to determine inflammatory responses to different exercise durations and intensities.
Increased FMD Following Exercise Training
It is noteworthy that FMD was improved following EX. Exercise increases blood flow, which activates vascular endothelium that produces the potent vasodilator, NO [11,12,13]. Two included studies [28, 29] observed that EX significantly increased plasma levels of nitrite, a key marker of vascular NO bioavailability [47]. The majority (about 70%) of increased NO bioavailability is attributable to the enhanced activity of eNOS in humans and other mammalian species [48]. We recently determined that EX improved vascular function via increased NO levels and expressions of eNOS in arteries of mice with apolipoprotein-E-knockout as models of atherosclerosis [13] and obesity [12]. Accordingly, it seems that NO plays a predominant role in modulating a balance between vasodilators and vasoconstrictors, and increased NO bioavailability may be the major beneficial consequence following EX. Taken together, EX can improve PAD-induced endothelial dysfunction by increased NO availability due to enhanced synthesis and activity of eNOS, which might be independent of systemic inflammation levels. However, pro-inflammatory cytokines can contribute to endothelial dysfunction through eNOS uncoupling [7] and NF-κB signaling [8]. Thus, further studies are needed to investigate the association between vascular endothelial function and cytokines following EX.
A Greater Increase in FMD with Moderate Intensity
Interestingly, subgroups with moderate intensity had a greater increase in FMD than subgroups with vigorous intensity (P < 0.01). Considering hemodynamic shear stress is directly proportional to the velocity of blood flow, it may be reasonable that the improvement in vascular endothelial dysfunction is proportional to exercise intensity by increased cardiac output. However, our previous meta-analysis observed similar results that EX with low–moderate intensity increased brachial artery FMD greater than EX with vigorous intensity in patients with type 2 diabetes [49]. These results suggest several notable points. First, increased endothelial NO bioavailability might not solely rely on an increase in shear stress. There are other factors to determine endothelial function such as vascular endothelial growth factor, intracellular calcium concentration, and hormones [50], which might lead to different responses to EX. Second, a stimulus augmenting NO bioavailability could be fully achieved through EX at moderate intensity. Lastly, vigorous exercise can elevate both endothelial inflammation and oxidative stress [43], which eventually reduce NO bioavailability [7, 8, 51]. Thus, EX with moderate intensity might have a greater increase in FMD in this analysis. However, a previous meta-analysis [52] on the population primarily consisting of CVD and healthy conditions determined that EX with higher intensity induced a greater increase in brachial artery FMD compared to EX with lower intensity. Such contradictory results might be due to different populations/pathologies and analytic methods. Future research is warranted to investigate the role of exercise intensity in the improvement of endothelial function.
Recommendation for Exercise Prescription in Patients with PAD
According to subgroup analyses, a significant difference in effect on FMD was not observed between subgroups training for ≤ 12 weeks and > 12 weeks of EX, < 50 min, and ≥ 50 min of session duration, and < 150 min and ≥ 150 min of weekly volume, respectively. CRP, IL-6 and TNF-α were not influenced by any training variables. These results indicate that EX with a relatively lower weekly training volume for a short duration can be a sufficient exercise regimen to improve FMD in patients with PAD. Age and baseline BMI were excluded from subgroup analyses as most participants had similar levels of age (65–70 years) and BMI (25–30 kg/m2). Aging is a key factor for developing vascular endothelial dysfunction by different mechanisms including exaggerated endothelial oxidative stress and apoptosis which decrease NO bioavailability [51]. Every 10 kg decrease in body weight is related to a 1.1% increase in FMD [53]. Thus, different training strategies could be required for obese or younger patients.
Limitations and Strengths
There are some limitations in this study. Significant publication bias was found in brachial artery FMD (P = 0.04). A few studies (e.g., measured FMD of a popliteal artery) were excluded to enhance homogeneity between studies, and only studies published in English were retrieved, potentially increasing the risk of bias. However, approximately 1300 patients in 20 trials were included in this meta-analysis for FMD. Sensitivity analysis also indicated that any one cohort did not predominantly influence our results in all four outcomes. Of 18 includes studies, 8 studies did not have CON [14, 15, 17, 27, 29,30,31,32], although differences in ESs are insignificant between randomized and nonrandomized trials [54].
This study first investigated the key prognostic variables for the risk of atherosclerosis and CVD events, brachial artery FMD and inflammatory biomarkers following EX in patients with PAD. This analysis observed that brachial artery endothelial function was improved by EX with moderate intensity and a relatively lower weekly training volume for a short-term without the changes in inflammatory biomarkers. Therefore, this study not only reveals the association between EX-induced improvement in vascular dysfunction and inflammation but also contributes to developing a specified EX strategy for patients with PAD.
Conclusions
This meta-analysis determined that EX significantly increased brachial artery FMD but had no effect on CRP, IL-6, and TNF-α levels in patients with PAD. These results suggest that EX-induced improvement in vascular endothelial function can be independent of the improvement of systemic inflammation. EX with moderate intensity elicited a greater effect on FMD compared to EX with vigorous intensity, while no significant difference in effect on FMD was found between subgroups training for ≤ 12 weeks and > 12 weeks of EX, < 50 min and ≥ 50 min of session duration, and < 150 min and ≥ 150 min of weekly volume, respectively. These results indicate that vascular dysfunction in PAD patients can be improved by EX with moderate intensity and a relatively lower weekly training volume for a short-term. Future studies are warranted to investigate the association between vascular function and inflammatory cytokines following EX.
Data Availability
The data used and analyzed in the current study are available upon reasonable request.
References
Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109(21_suppl_1):II27–33.
Lerman A, Burnett Jr J. Intact and altered endothelium in regulation of vasomotion. Circulation. 1992;86(6 Suppl):III12–19.
Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168(1):344–51.
Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–40.
Lakshmanan S, Shekar C, Kinninger A, Birudaraju D, Dahal S, Onuegbu A, et al. Association of flow mediated vasodilation and burden of subclinical atherosclerosis by coronary CTA. Atherosclerosis. 2020;302:15–9.
Sorriento D, Iaccarino G. Inflammation and cardiovascular diseases: the most recent findings. MDPI. 2019;20(16):3879.
Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(3):413–20.
Theofilis P, Sagris M, Oikonomou E, Antonopoulos AS, Siasos G, Tsioufis C, et al. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9(7):781.
Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6(12):1198–205.
Shankar A, Li J, Nieto FJ, Klein BE, Klein R. Association between C-reactive protein level and peripheral arterial disease among US adults without cardiovascular disease, diabetes, or hypertension. Am Heart J. 2007;154(3):495–501.
Sprague B, Chesler NC, Magness RR. Shear stress regulation of nitric oxide production in uterine and placental artery endothelial cells: experimental studies and hemodynamic models of shear stress forces on endothelial cells. Int J Dev Biol. 2010;54(2–3):331.
Lee J, Hong J, Umetani M, Lavoy EC, Kim J-H, Park Y. Vascular protection by exercise in obesity: Inflammasome-associated mechanisms. Med Sci Sports Exerc. 2020;52:2538–45.
Hong J, Park E, Lee J, Lee Y, Rooney BV, Park Y. Exercise training mitigates ER stress and UCP2 deficiency-associated coronary vascular dysfunction in atherosclerosis. Sci Rep. 2021;11(1):1–10.
Quarto G, Amato B, Serra R, Benassai G, Monti MG, Salzano A, et al. The effects of Crenotherapy and exercise in peripheral arterial occlusive disease. Ann Ital Chir. 2017;88(6):469–77.
Delaney C, Spark J. A randomised controlled trial of two supervised exercise regimens and their impact on inflammatory burden in patients with intermittent claudication. Vascular. 2016;24(3):264–72.
Dopheide JF, Rubrech J, Trumpp A, Geissler P, Zeller GC, Schnorbus B, et al. Supervised exercise training in peripheral arterial disease increases vascular shear stress and profunda femoral artery diameter. Eur J Prev Cardiol. 2017;24(2):178–91.
Januszek R, Mika P, Konik A, Petriczek T, Nowobilski R, Niżankowski R. The effect of treadmill training on endothelial function and walking abilities in patients with peripheral arterial disease. J Cardiol. 2014;64(2):145–51.
Lin M-L, Fu T-C, Hsu C-C, Huang S-C, Lin Y-T, Wang J-S. Cycling exercise training enhances platelet mitochondrial bioenergetics in patients with peripheral arterial disease: a randomized controlled trial. Thromb Haemost. 2021;121(07):900–12.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons. 2019;10(10):ED000142.
Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, et al. GRADE guidelines: 14 Going from evidence to recommendations: the significance and presentation of recommendations. Journal Clin Epidemiol. 2013;66(7):719–25.
Cochran WG. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10(4):417–51.
Higgin J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–60.
Cohen J. Statistical power analysis for the behavioral sciences Lawrence Earlbaum Associates. Hillsdale, NJ. 1988;6(30):1–567.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Akerman AP, Thomas KN, van Rij AM, Body ED, Alfadhel M, Cotter JD. Heat therapy vs. supervised exercise therapy for peripheral arterial disease: a 12-wk randomized, controlled trial. American Journal of Physiology-Heart and Circulatory Physiology. 2019;316(6):H1495–H1506.
Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, et al. Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3 months of exercise training. Free Radical Biol Med. 2010;49(6):1138–44.
Allen JD, Stabler T, Kenjale AA, Ham KL, Robbins JL, Duscha BD, et al. Diabetes status differentiates endothelial function and plasma nitrite response to exercise stress in peripheral arterial disease following supervised training. J Diabetes Complications. 2014;28(2):219–25.
Andreozzi G, Leone A, Laudani R, Deinite G, Martini R. Acute impairment of the endothelial function by maximal treadmill exercise in patients with intermittent claudication, and its improvement after supervised physical training. Int Angiol. 2007;26(1):12.
Brendle DC, Joseph LJ, Corretti MC, Gardner AW, Katzel LI. Effects of exercise rehabilitation on endothelial reactivity in older patients with peripheral arterial disease. Am J Cardiol. 2001;87(3):324–9.
Correia M A, Oliveira PL, Farah BQ, Vianna LC, Wolosker N, Puech-Leao P, et al. Effects of isometric handgrip training in patients with peripheral artery disease: a randomized controlled trial. J Am Heart Ass. 2020;9(4):e013596.
Domanchuk K, Ferrucci L, Guralnik JM, Criqui MH, Tian L, Liu K, et al. Progenitor cell release plus exercise to improve functional performance in peripheral artery disease: the PROPEL Study. Contemp Clin Trials. 2013;36(2):502–9.
McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301(2):165–74.
Novaković M, Krevel B, Rajkovič U, Cuderman TV, Trontelj KJ, Fras Z, et al. Moderate-pain versus pain-free exercise, walking capacity, and cardiovascular health in patients with peripheral artery disease. J Vasc Surg. 2019;70(1):148–56.
Pasqualini L, Bagaglia F, Ministrini S, Frangione MR, Leli C, Siepi D, et al. Effects of structured home-based exercise training on circulating endothelial progenitor cells and endothelial function in patients with intermittent claudication. Vasc Med. 2021;26(6):633–40.
Payvandi L, Dyer A, McPherson D, Ades P, Stein J, Liu K, et al. Physical activity during daily life and brachial artery flow-mediated dilation in peripheral arterial disease. Vasc Med. 2009;14(3):193–201.
Albaghdadi MS, Wang Z, Gao Y, Mutharasan RK, Wilkins J. High-density lipoprotein subfractions and cholesterol efflux capacity are not affected by supervised exercise but are associated with baseline interleukin-6 in patients with peripheral artery disease. Frontiers in cardiovascular medicine. 2017;4:9.
Collins TC, Twumasi-Ankrah P. A walking intervention to reduce inflammation in patients with diabetes and peripheral arterial/artery disease: a pilot study. SAGE Open Medicine. 2013;1:2050312113505559.
Swardfager W, Herrmann N, Cornish S, Mazereeuw G, Marzolini S, Sham L, et al. Exercise intervention and inflammatory markers in coronary artery disease: a meta-analysis. Am Heart J. 2012;163(4):666-76. e3.
Ploeger HE, Takken T, De Greef M, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: a systematic review. Exerc Immunol Rev. 2009;15(1):6–41.
Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–43.
Cerqueira É, Marinho DA, Neiva HP, Lourenço O. Inflammatory effects of high and moderate intensity exercise—a systematic review. Front Physiol. 2020;10:1550.
Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise? Exerc Sport Sci Rev. 2006;34(4):176–81.
Lancaster GI, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, et al. The physiological regulation of toll-like receptor expression and function in humans. J Physiol. 2005;563(3):945–55.
Li Y, Xu P, Wang Y, Zhang J, Yang M, Chang Y, et al. Different intensity exercise preconditions affect cardiac function of exhausted rats through regulating TXNIP/TRX/NF-ĸBp65/NLRP3 inflammatory pathways. Evidence-Based Complementary and Alternative Medicine. 2020;6(8):58092–8.
Allen JD, Cobb FR, Gow AJ. Regional and whole-body markers of nitric oxide production following hyperemic stimuli. Free Radical Biol Med. 2005;38(9):1164–9.
Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radical Biol Med. 2003;35(7):790–6.
Lee J-H, Lee R, Hwang M-H, Hamilton MT, Park Y. The effects of exercise on vascular endothelial function in type 2 diabetes: a systematic review and meta-analysis. Diabetol Metab Syndr. 2018;10(1):1–14.
Lähteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ Res. 2012;110(9):1252–64.
Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120(9):357–75.
Early KS, Stewart A, Johannsen N, Lavie CJ, Thomas JR, Welsch M. The effects of exercise training on brachial artery flow-mediated dilation: a meta-analysis. J Cardiopulm Rehabil Prev. 2017;37(2):77–89.
Joris PJ, Zeegers MP, Mensink RP. Weight loss improves fasting flow-mediated vasodilation in adults: a meta-analysis of intervention studies. Atherosclerosis. 2015;239(1):21–30.
Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342(25):1878–86.
Author information
Authors and Affiliations
Contributions
JL, EL, MM, and YP conducted a study design. JL, AZ, and YP conducted literature review and data extraction. JL and YP performed statistical analysis. JL, EL, MM, and YP drafted the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
No human studies were carried out by the authors for this article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J., Zarezadehmehrizi, A., LaVoy, E.C. et al. Exercise Training Improves Brachial Artery Endothelial Function, but Does Not Alter Inflammatory Biomarkers in Patients with Peripheral Artery Disease: a Systematic Review and Meta-analysis. J. of Cardiovasc. Trans. Res. 17, 585–597 (2024). https://doi.org/10.1007/s12265-023-10451-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-023-10451-0