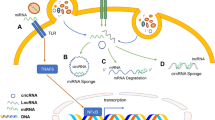
Abstract
Extracellular vesicles (EVs) are secreted by cells and carry diverse components, including proteins, lipids, nucleic acids, and metabolites. EVs could be found in blood and other biofluids. They vary greatly in size, function, cargo, and cellular origin. Accumulating evidence shows that extracellular non-coding RNAs, the dominant extracellular RNAs encapsulated into EVs, function as critical mediators of cell–cell communication and play critical roles in human health and disease. Blood vessels form a dense network that nourishes all of the body’s tissues. These vascular networks’ dysregulated functions contribute to vascular diseases, such as pulmonary arterial hypertension (PAH), hypertension, atherosclerosis, and aneurysm. With the increase in unhealthy lifestyle-associated obesity and metabolic disorders, vascular diseases are becoming serious medical and public health issues with a profound global economic burden. The present review summarizes the latest advances on extracellular non-coding RNAs in pathological vascular remodeling-associated diseases, briefly describing vessel-associated extracellular non-coding RNAs and their mechanisms of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
RNA was once thought to be only found in cells. However, recent studies have debunked this false notion. Extracellular RNAs (exRNAs) were first discovered in plasma [1]. They were later identified to be present in almost all body fluids, including blood, saliva, urine, breast milk, cerebrospinal fluid, amniotic fluid, ascites, bile, and pleural effusion [2]. ExRNAs contain non-coding RNAs other than mRNA, including microRNAs (miRNA), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), small nucleolar RNA (snoRNAs), small nuclear RNAs (snRNAs), transfer RNA (tRNAs), ribosomal RNAs (rRNAs), and PIWI-interacting RNAs (piRNAs) [3]. Emerging evidence shows that the widespread and diverse extracellular non-coding RNAs play critical roles in biological processes, normal growth and development, and human diseases. This review aims to provide a comprehensive overview of extracellular non-coding RNAs that play relevant biological functions in vascular diseases.
Extracellular RNAs
Secreted RNA was first discovered in vitro in the culture medium of 3T3 and SV-40 transformed 3T3 cells [4]. Since then, using the RNA-sequencing technique, increasing evidence demonstrates that exRNAs are widely detected in many human samples, including plasma, urine, and saliva [5,6,7]. ExRNAs play an essential role in cell–cell crosstalk, whether locally or over long distances [8].
The Biogenesis of Extracellular Vesicles
Extracellular vesicles, a diverse group of membrane vesicles from various origins, are highly heterogeneous. Their sizes can range from 50 to 500 nm, but they can also be 1–10 μm. According to the current understanding of their biosynthesis and size, extracellular vesicles can be generally classified into four categories: exosomes, microvesicles, apoptotic bodies, and oncosomes [8]. Among them, exosomes and microvesicles are the two major categories. The common RNA types included in the exosomes and microvesicles are Y RNA, miRNA, tRNA fragments, and snRNA [9, 10].
The production process of exosomes involves the double invagination of the plasma membrane. Firstly, the plasma membrane invaginates and forms a cup-shaped structure containing cell surface proteins as well as soluble proteins associated with the extracellular medium. This process contributes to the de-novo generation of an early-sorting endosome (ESE) [11, 12]. In some cases, the newly formed ESE may fuse with a preexisting ESE. The trans-Golgi network and endoplasmic reticulum can also produce ESEs or be a part of the ESEs. ESEs develop into late-sorting endosomes (LSEs) and subsequently become multivesicular bodies (MVBs), namely multivesicular endosomes (MVEs). Secondly, the endosomal limiting membrane invaginates and generates several intraluminal vesicles (ILVs), which can be released as exosomes upon fusion with the plasma membrane [13, 14]. The detailed mechanisms that mediate exosome biogenesis and release have recently been comprehensively reviewed in ref. [15]. In brief, it involves the canonical stepwise endosomal sorting complex required for transport (ESCRT)-dependent pathway and non-canonical ESCRT-dependent pathways.
Microvesicles are formed by the outward budding and fission of the plasma membrane, followed by vesicle secretion into the extracellular milieu [16]. Little is known about the molecular mechanisms that allow microvesicles to be biosynthesized and secreted. Hedgehog (Hh) secretion requires an ESCRT complex in comparison to extracellular vesicles (EVs) [17]. The ESCRT subunits TSG101 and VPS4 are recruited to the plasma membrane by the adaptor protein arrestin domain-containing protein 1 (ARRDC1), which promotes microvesicle release [18]. Other proteins involved in the formation of microvesicles in various cell types include GTPase ADP-ribosylation factor 1 (ARF1) [19], ARF6 [20], small GTPase RhoA [21], and acid sphingomyelinase [22].
EVs can be internalized into the recipient cells via several endocytic pathways, including clathrin-mediated endocytosis, caveolin-dependent endocytosis, raft-dependent endocytosis, phagocytosis, and macropinocytosis [8]. After endocytosis, EVs internalized by target cells can also be degraded through three pathways [23]: (1) Part of EVs can be transported to the endo-/exosome for degradation; (2) another part of EVs localized in the multivesicular body (MVB) can fuse with autophagosomes and form amphisomes, eventually delivered to lysosomal breakdown; and (3) some EVs can also be directly delivered to autophagosome/lysosome for degradation without forming amphisomes.
Carriers of exRNAs
ExRNAs were thought to be unstable due to the RNA degradation enzyme ribonucleases (RNases), which are commonly found in extracellular fluids [24]. However, two pioneering independent studies found exRNAs in microvesicles [25] and exosomes [26]. These extracellular vesicles transport exRNAs from donor cells to recipient cells and may play a role in cell–cell communication. Until now, evidence suggests that exRNAs use two independent mechanisms to avoid degradation. Firstly, RNAs can be encased in various extracellular vesicles (EVs), such as exosomes (< 150 μm), microvesicles (200–500 μm), and oncosomes (1–10 μm) [27, 28]. Secondly, RNAs can tightly bind with Argonaute2 (Ago2) protein complexes [29] or high-density lipoproteins (HDLs) [30], both of which serve as exRNA carriers Fig. 1.
Biogenesis of extracellular RNAs (exRNAs). Early endosomes develop into late endosomes, called the multivesicular bodies (MVBs). The MVB cargo includes complex or individual macromolecules such as RNA, taken up via endocytosis. Captured RNAs on the MVB's external surface undergo inward budding, resulting in intraluminal vesicles (ILVs) containing tiny vesicles. ILVs are produced in both the endosomal sorting complex required for transport (ESCRT)-dependent and -independent ways. Subtypes of MVBs containing RNAs fuse with the plasma membrane and are released into the extracellular milieu (also called exosomes). ExRNAs from the cytoplasm are encapsulated in microvesicles, which are released by outward budding from the plasma membrane. ExRNAs without vesicles can also form ribonucleoprotein (RNP)complexes with proteins. Furthermore, both high-density lipoproteins (HDLs) and low-density lipoproteins (LDLs) have been found to carry different types of RNA
Extracellular Non-Coding RNAs in Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a type of high blood pressure in the lungs (> 25 mmHg) [31]. In individuals with PAH, blood vessels in the lungs are destroyed and remodeled for a variety of reasons. PAH is an aggressive condition and can become life-threatening. Although much progress has been made in recent years to help limit symptoms and improve quality of life, there is still an urgent need to investigate the molecular mechanisms underlying PAH, particularly the potential roles of exRNAs in its pathogenesis.
It has previously been reported that pulmonary arterial endothelial cells (PAECs) promote recruitment, proliferation, and differentiation of pulmonary arterial smooth muscle cells (PASMCs) through the secretion of growth factors such as transforming growth factor β (TGF-β) [32] and platelet-derived growth factor (PDGF) [33]. Apart from growth factors secreted by PAECs, emerging evidence shows that PAEC-generated exosomes promote proliferation while inhibiting apoptosis in PASMCs, implying the involvement of these exosomes in the development of pulmonary hypertension [34]. Furthermore, lipopolysaccharide (LPS) and hypoxia increase the production of exosomes derived from pulmonary arterial endothelial cells (PAECs) [34]. ExRNAs from PASMCs, in turn, influence PAECs’ function. Exosomes enriched in miR-143-3p from pulmonary arterial smooth muscle cells (PASMCs), for example, have a promigratory and pro-angiogenic effect on pulmonary arterial endothelial cells. In contrast, miR-143-3p inhibition ameliorates chronic hypoxia-induced pulmonary hypertension in mice in vivo [35]. Climent et al. also found that in vivo vessel stress mediates miR-143/145 transfer from SMCs to ECs [36].
Experimental studies have revealed that mesenchymal stromal cell (MSC)-derived exosomes are a promising treatment option for PAH. Ge et al. reported that MSC-derived exosomes suppress the endothelial-mesenchymal transition (EndMT) process and, as a result, attenuate pulmonary vascular remodeling, suggesting a protective role for the MSC-derived exosomes against PAH [37]. EVs secreted by monocrotaline-treated mice exacerbate monocrotaline-induced pulmonary hypertension, most likely by affecting the pulmonary vasculature or causing bone marrow cells to differentiate into endothelial progenitor cells, which cause pulmonary vascular remodeling [38]. According to Lee et al., intravenous administration of mesenchymal stromal cell-derived exosomes (MEX) suppressed vascular remodeling and hypoxic pulmonary hypertension. On the other hand, the fibroblast-derived exosomes had no beneficial effects [39]. MEX inactivates signal transducer and activator of transcription 3 (STAT3) in isolated human pulmonary artery endothelial cells, suggesting a direct role for MEX in hypoxic vascular cells [39] Fig. 2.
Extracellular miRNAs in the pathogenesis of pulmonary arterial hypertension (PAH). Exosomes secreted by pulmonary artery endothelial cells (PAECs) in response to lipopolysaccharide (LPS) and hypoxia promote proliferation but inhibit apoptosis in pulmonary artery smooth muscle cells (PASMCs). In turn, vessel stress facilitates miR-143/145 transfer from SMCs to ECs in vivo and accelerates PAECs’ migration and angiogenesis. Exosomes derived from mesenchymal stromal cells (MSCs) suppress the endothelial-mesenchymal transition (EndMT) process and, as a result, alleviate pulmonary vascular remodeling, indicating a promising strategy for PAH treatment. Monocrotaline-treated mice secrete EVs that aggravate monocrotaline-induced pulmonary hypertension, most likely by triggering the pulmonary vasculature or bone marrow cells to differentiate into endothelial progenitor cells, which cause pulmonary vascular remodeling
Extracellular Non-Coding RNAs in Hypertension
Hypertension, also known as high blood pressure, is defined as blood pressure that is higher than average (a blood pressure ≥ 130/ ≥ 80 mmHg) by the American College of Cardiology and the American Heart Association in 2017 [40]. Hypertension dramatically raises the risk of heart disease and stroke, both of which are leading causes of death in the USA [40].
Platelet-derived growth factor (PDGF) stimulation reduces the enrichment of miR-1246, miR-182, and miR-486 in VSMCs’ exosomes [41]. Exosomes secreted into the biological environment hasten EC migration [41]. Endothelial EVs deliver miR-126-3p to VSMCs, where it stimulates VSMC proliferation and neointima formation by inhibiting LRP6 [42].
Exosomes are secreted by vascular adventitial fibroblasts isolated from spontaneously hypertensive rats (SHR), which mediate the crosstalk between fibroblasts and VSMCs and contribute to VSMC migration [43]. The effect of those exosomes on VSMCs could be blocked by the exosome inhibitor GW4869 [43]. Exosomes from SHR fibroblasts transport angiotensin-converting enzyme (ACE) into VSMCs, increasing angiotensin II and activating AT1R [43].
When brain microvascular pericytes from spontaneously hypertensive rats were compared to normal control rats, they showed a different expression pattern of several EV-derived miRNAs, including miR-21-5p, let-7c-5p, and let-7a-5p [44]. These findings make them promising biomarkers for hypertension diagnosis [44].
Using ncRNA sequencing analysis of exosomes collected from plasma, urine, and total plasma, five lncRNAs, including LINC02614, BAALC-AS1, FAM230B, LOC100505824, and LINC01484, were identified as typical ncRNA signature for urinary albumin excretion in hypertensive patients [45]. A mouse study found that circNr1h4 is downregulated in the kidneys with hypertension-induced injury, suggesting that it may be involved in the occurrence of hypertension-associated kidney impairment [46].
Extracellular Non-Coding RNAs in Atherosclerosis
Atherosclerosis is a chronic inflammatory disease that primarily affects the large and middle arteries [47]. Elevated lipid deposition, endothelial dysfunction, inflammatory cell recruitment, coagulation and thrombosis, and vascular cell proliferation and migration all contribute to the initiation and progression of atherosclerosis [48]. In the early stages of atherosclerosis, there are usually no symptoms. However, in advanced stages, atherosclerosis can cause myocardial infarction (MI), stroke, peripheral artery disease, and kidney disease [49]. Recent advances in understanding the role of exRNAs in the development of atherosclerosis may aid in the development of novel diagnostic tools and innovative therapies to treat atherosclerosis-associated diseases.
Atherosclerotic risk factors such as oxidized low-density lipoprotein (oxLDL) and interleukin-6 (IL-6) increase miR-92a-3p levels in parent ECs and promote miR-92a-3p transport into recipient ECs via microvesicles [50]. Angiogenesis is modulated by functional miR-92a-3p in a THBS1-dependent manner [50]. ECs can secrete EVs-encapsulated MiR-92a, which causes an atherogenic phenotype in cocultured macrophages by downregulating KLF4 levels [51]. He et al. also discovered that miR-155-enriched EVs derived from human umbilical vein endothelial cells (HUVECs) mediate the phenotypic switch to proinflammatory M1 macrophages in human THP1 cells [52]. In an atherosclerotic mouse model, the proinflammatory and proatherogenic roles of miR-155-containing EVs were further confirmed [52]. It has also been reported that ox-LDL upregulated miR-505 in HUVECs, most likely via the NF-κB pathway, and that HUVECs-secreted miR-505-enriched exosomes attenuated SIRT3 signaling in neutrophils, increasing neutrophil extracellular traps (NET) release [53]. ApoE−/− mice were shown to have pro-atherogenic roles of miR-505-encapsulated exosomes [53]. Physiological shear stress and cholesterol-lowering statins both upregulate the expression of the miR-143/145 cluster via the shear-responsive transcription factor Krüppel-like factor 2 (KLF2) [54]. Endothelial miR-143/145 is transferred to SMCs by EVs, which induces an atheroprotective phenotype in SMCs [54]. On the other hand, Zhou et al., via Argonaut2 complexes, found that endothelial cells-derived microRNA-126-contained EVs have a pro-atherogenic effect in mice [55].
Nicotine exposure increases the amount of miRNA-21-3p secreted by macrophages via exosomes [56]. EVs containing miRNA-21-3p promote VSMC migration and proliferation by inhibiting PTEN [56]. These findings shed light on new ways nicotine contributes to atherosclerosis and carotid artery diseases. According to Liu et al., oxLDL induces miR-106a-3p expression in THP-1 cells, and miR-106a-3p-enriched exosomes increase VSMC proliferation, which may play a critical role in the development of atherosclerosis [57]. M1 macrophages produced exosomes containing a high level of miR-222, and this M1 macrophage-derived EVs caused neointima formation in mouse carotid artery ligation injury and wire injury models [58]. Mechanistically, miR-222 regulates cyclin-dependent kinase inhibitor 1B (CDKN1B) and cyclin-dependent kinase inhibitor 1C (CDKN1C) to promote VSMC proliferation and migration [58]. Also, exosomes containing VSMCs-secreted MiR-155 break endothelial tight junctions and impair barrier integrity, contributing to the pathogenesis of atherosclerosis [59] Fig. 3.
Extracellular miRNAs in the pathogenesis of atherosclerosis. Oxidized low-density lipoprotein (oxLDL) and interleukin-6 (IL-6) increase miR-92a-3p levels in parent ECs and elevate miR-92a-3p levels in recipient ECs, which promotes angiogenesis by downregulating thrombospondin 1 (THBS1), a negative regulator of EC migration and proliferation. ECs-derived EVs-encapsulated MiR-92a instigates macrophage inflammation by decreasing KLF4 expression. Human umbilical vein endothelial cells (HUVECs)-derived miR-155-enriched EVs promote M1 macrophage polarization and play a pro-atherogenic role in ApoE−/− mice. VSMCs-secreted miR-155 containing exosomes accelerates atherosclerosis by disrupting endothelial tight junctions and impairing barrier integrity. Physiological shear stress and statins upregulate miR-143/145 cluster expression in endothelial cells. Endothelial cell-derived miR-143/145 are transported into vascular smooth muscle cells (VSMCs) and inhibit atherosclerosis. Endothelial cells-derived microRNA-126-contained EVs, on the other hand, promote atherosclerosis. Ox-LDL induces miR-505 expression in HUVECs, and HUVECs-secreted miR-505-enriched exosomes accelerate atherosclerosis by inhibiting SIRT3 signaling and increasing neutrophil extracellular traps (NET) release. Nicotine induces miRNA-21-3p levels in macrophages, and EVs containing miRNA-21-3p promote VSMC migration and proliferation by downregulating PTEN
Exosomal lncRNA LINC01005 secreted from ox-LDL-treated HUVECs promotes the phenotype switch, proliferation, and migration of VSMCs by acting as a sponge of miR-128-3p, leading to the upregulation of KLF4 [60]. OxLDL elevates lncRNA MALAT1 expression in exosomes released from HUVECs [61, 62]. Functionally, exosome-derived lncRNA MALAT1 promotes M2 macrophage polarization [61] and the formation of neutrophil extracellular traps (NETs) [62]. These findings suggest a critical role of exosome-encapsulated lncRNAs in atherosclerosis.
In addition to miRNAs and lncRNAs, circRNAs also play roles in atherosclerosis. For example, circNPHP4 is significantly increased in small extracellular vesicles (sEVs) isolated from monocytes of patients with coronary heart atherosclerotic disease (CAD) [63]. It promotes monocyte adhesion to coronary artery endothelial cells by increasing the expression of ICAM-1 and VCAM-1 [63]. Plasma circRNA‑0006896 [64] and circRNA hsa_circ_0001445 [65] were also correlated with coronary heart diseases, representing the potential of these circRNAs as biomarkers for diagnosis of diagnosis cardiovascular diseases.
Extracellular Non-Coding RNAs in Aneurysm
An aneurysm develops in arteries when the extracellular matrix (ECM) of a blood vessel weakens, resulting in a dilated vessel and vascular rupture [66]. The causes of aneurysms are not fully understood, but aneurysms are usually associated with environmental and hereditary factors, high blood pressure, and smoking. Vascular inflammation, aberrant vascular remodeling, ECM degradation, and shear stress are all part of the pathological process [67]. Aortic aneurysms and cerebral aneurysms are the most common types of aneurysms.
In cerebral aneurysm tissues, MiR-370-3p expression was significantly increased. Ectopic overexpression of miR-370-3p in human vascular smooth muscle cells represses cell proliferation and cell cycle progression, thereby promoting aneurysms [68]. Mechanistically, miR-370-3p inhibits FOXO1 activity and reduces AKT and FOXO1 phosphorylation [68]. In elastase-treated mice, mesenchymal stromal cell (MSC)-derived EVs transfected with miR-147 mimetic mitigates abdominal aortic aneurysm (AAA) by inhibiting inflammation and reducing leukocyte infiltration [69]. In addition, exosome-derived microRNAs (miRNAs) like miR-29a-3p and miRNA-145-5p can be used as biomarkers for the development and progression of intracranial aneurysms [70].
Conclusion and Perspective
A growing body of evidence indicates that extracellular non-coding RNAs play a critical role in vascular diseases. However, many of the studies were performed in cell culture systems. Therefore, there is an urgent need to explore the roles of extracellular non-coding RNAs in vivo using animal models. The experimental methods include genetically overexpressed non-coding RNA sensors or cell/tissue-specific knockdown of non-coding RNAs. However, it is still challenging to control which recipient cells will take up specific extracellular non-coding RNAs in vivo in mammals. A rat model study reported that circulating miRNA profiles correlate with the severity of stroke outcomes in rats of different ages and sex [71]. Moreover, the patients with renal artery stenosis [72] and coronary artery disease [73, 74] show distinct profiles of circulating exRNAs versus those without these vascular diseases. These findings suggest the usefulness of circulating exRNAs as biomarkers for cardiovascular diseases. In conclusion, a deeper understanding of the expression, function, and interaction of the extracellular non-coding RNAs will lead to more strategies for diagnosing and treating vascular diseases.
References
Kamm, R. C., & Smith, A. G. (1972). Nucleic acid concentrations in normal human plasma. Clinical Chemistry, 18(6), 519–522.
Lässer, C. (2019). Mapping extracellular RNA sheds lights on distinct carriers. Cell, 177(2), 228–230.
Kim, K. M., et al. (2017). RNA in extracellular vesicles. Wiley Interdisciplinary Reviews: RNA, 8(4), e1413.
Kolodny, G. M., Culp, L. A., & Rosenthal, L. J. (1972). Secretion of RNA by normal and transformed cells. Experimental Cell Research, 73(1), 65–72.
Freedman, J. E., et al. (2016). Diverse human extracellular RNAs are widely detected in human plasma. Nature Communications, 7, 11106.
Max, K. E. A., et al. (2018). Human plasma and serum extracellular small RNA reference profiles and their clinical utility. Proceedings of the National Academy of Sciences of the United States of America, 115(23), E5334–E5343.
Godoy, P. M., et al. (2018). Large Differences in small RNA composition between human biofluids. Cell Reports, 25(5), 1346–1358.
Gruner, H. N., & McManus, M. T. (2021). Examining the evidence for extracellular RNA function in mammals. Nature Reviews Genetics, 22(7), 448–458.
Jeppesen, D. K., et al. (2019). Reassessment of exosome composition. Cell, 177(2), 428-445 e18.
Witwer, K. W., & Thery, C. (2019). Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. Journal of Extracell Vesicles, 8(1), 1648167.
Kalluri, R. (2016). The biology and function of exosomes in cancer. The Journal of Clinical Investigation, 126(4), 1208–1215.
McAndrews, K. M., & Kalluri, R. (2019). Mechanisms associated with biogenesis of exosomes in cancer. Molecular Cancer, 18(1), 52.
Kahlert, C., & Kalluri, R. (2013). Exosomes in tumor microenvironment influence cancer progression and metastasis. Journal of Molecular Medicine (Berlin, Germany), 91(4), 431–437.
van Niel, G., D’Angelo, G., & Raposo, G. (2018). Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology, 19(4), 213–228.
Teng, F., & Fussenegger, M. (2020). Shedding light on extracellular vesicle biogenesis and bioengineering. Advance Science (Weinheim, Baden-Wurttemberg, Germany), 8(1), 2003505.
Tricarico, C., Clancy, J., & D’Souza-Schorey, C. (2017). Biology and biogenesis of shed microvesicles. Small GTPases, 8(4), 220–232.
Matusek, T., et al. (2014). The ESCRT machinery regulates the secretion and long-range activity of Hedgehog. Nature, 516(7529), 99–103.
Nabhan, J. F., et al. (2012). Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proceedings of the National Academy of Sciences of the United States of America, 109(11), 4146–4151.
Schlienger, S., Campbell, S., & Claing, A. (2014). ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Molecular Biology of the Cell, 25(1), 17–29.
Muralidharan-Chari, V., et al. (2009). ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Current Biology, 19(22), 1875–1885.
Li, B., et al. (2012). RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene, 31(45), 4740–4749.
Bianco, F., et al. (2009). Acid sphingomyelinase activity triggers microparticle release from glial cells. The EMBO Journal, 28(8), 1043–1054.
Zheng, J., et al. (2019). Extracellular vesicles degradation pathway based autophagy lysosome pathway. American Journal of Translational Research, 11(3), 1170–1183.
Sorrentino, S. (1998). Human extracellular ribonucleases: Multiplicity, molecular diversity and catalytic properties of the major RNase types. Cellular and Molecular Life Sciences, 54(8), 785–794.
Ratajczak, J., et al. (2006). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: Evidence for horizontal transfer of mRNA and protein delivery. Leukemia, 20(5), 847–856.
Valadi, H., et al. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology, 9(6), 654–659.
Di Vizio, D., et al. (2012). Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. The American Journal of Pathology, 181(5), 1573–1584.
Minciacchi, V. R., Freeman, M. R., & Di Vizio, D. (2015). Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Seminars in Cell & Developmental Biology, 40, 41–51.
Turchinovich, A., et al. (2011). Characterization of extracellular circulating microRNA. Nucleic Acids Research, 39(16), 7223–7233.
Vickers, K. C., et al. (2011). MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature Cell Biology, 13(4), 423–433.
Prins, K. W., & Thenappan, T. (2016). World Health Organization Group I Pulmonary Hypertension: Epidemiology and Pathophysiology. Cardiology Clinics, 34(3), 363–374.
Antonelli-Orlidge, A., et al. (1989). An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proceedings of the National Academy of Sciences of the United States of America, 86(12), 4544–4548.
Asakawa, H., & Kobayashi, T. (1999). The effect of coculture with human smooth muscle cells on the proliferation, the IL-1 beta secretion, the PDGF production and tube formation of human aortic endothelial cells. Cell Biochemistry and Function, 17(2), 123–130.
Zhao, L., et al. (2017). Exosomes derived from human pulmonary artery endothelial cells shift the balance between proliferation and apoptosis of smooth muscle cells. Cardiology, 137(1), 43–53.
Deng, L., et al. (2015). MicroRNA-143 Activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circulation Research, 117(10), 870–883.
Climent, M., et al. (2015). TGFbeta Triggers miR-143/145 Transfer From Smooth Muscle Cells to Endothelial Cells, Thereby Modulating Vessel Stabilization. Circulation Research, 116(11), 1753–1764.
Ge, L., et al. (2021). Mesenchymal stromal cell-derived exosomes attenuate experimental pulmonary arterial hypertension. Current Pharmaceutical Biotechnology, 22(12), 1654–1662.
Aliotta, J. M., et al. (2013). Induction of pulmonary hypertensive changes by extracellular vesicles from monocrotaline-treated mice. Cardiovascular Research, 100(3), 354–362.
Lee, C., et al. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation, 126(22), 2601–2611.
(2018). Correction to: 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 71(6):e140-e144.
Heo, J., et al. (2020). Vascular smooth muscle cell-derived exosomal microRNAs regulate endothelial cell migration under PDGF stimulation. Cells, 9(3), 639.
Jansen, F., et al. (2017). Intercellular transfer of miR-126-3p by endothelial microparticles reduces vascular smooth muscle cell proliferation and limits neointima formation by inhibiting LRP6. Journal of Molecular and Cellular Cardiology, 104, 43–52.
Tong, Y., et al. (2018). Exosome-mediated transfer of ACE (angiotensin-converting enzyme) from adventitial fibroblasts of spontaneously hypertensive rats promotes vascular smooth muscle cell migration. Hypertension, 72(4), 881–888.
Wu, Q., et al. (2020). Differential miRNA expression analysis of extracellular vesicles from brain microvascular pericytes in spontaneous hypertensive rats. Biotechnology Letters, 42(3), 389–401.
Riffo-Campos, A. L., et al. (2022). Exosomal and plasma non-coding RNA signature associated with urinary albumin excretion in hypertension. International Journal of Molecular Sciences, 23(2), 823.
Lu, C., et al. (2020). CircNr1h4 regulates the pathological process of renal injury in salt-sensitive hypertensive mice by targeting miR-155-5p. Journal of Cellular and Molecular Medicine, 24(2), 1700–1712.
Lusis, A. J. (2000). Atherosclerosis. Nature, 407(6801), 233–241.
Libby, P., Ridker, P. M., & Hansson, G. K. (2011). Progress and challenges in translating the biology of atherosclerosis. Nature, 473(7347), 317–325.
Wang, Y., et al. (2019). Exosomes: An emerging factor in atherosclerosis. Biomedicine & Pharmacotherapy, 115, 108951.
Liu, Y., et al. (2019). Atherosclerotic conditions promote the packaging of functional microRNA-92a-3p into endothelial microvesicles. Circulation Research, 124(4), 575–587.
Chang, Y. J., et al. (2019). Extracellular microRNA-92a mediates endothelial cell-macrophage communication. Arteriosclerosis, Thrombosis, and Vascular Biology, 39(12), 2492–2504.
He, S., et al. (2018). Endothelial extracellular vesicles modulate the macrophage phenotype: Potential implications in atherosclerosis. Scandinavian Journal of Immunology, 87(4), e12648.
Chen, L., et al. (2019). Exosome-encapsulated miR-505 from ox-LDL-treated vascular endothelial cells aggravates atherosclerosis by inducing NET formation. Acta Biochimica et Biophysica Sinica (Shanghai), 51(12), 1233–1241.
Hergenreider, E., et al. (2012). Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature Cell Biology, 14(3), 249–256.
Zhou, J., et al. (2013). Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: Role of shear stress. Circulation Research, 113(1), 40–51.
Zhu, J., et al. (2019). Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics, 9(23), 6901–6919.
Liu, Y., et al. (2020). Exosome-mediated miR-106a-3p derived from ox-LDL exposed macrophages accelerated cell proliferation and repressed cell apoptosis of human vascular smooth muscle cells. European Review for Medical and Pharmacological Sciences, 24(12), 7039–7050.
Wang, Z., et al. (2019). Exosomes derived from M1 macrophages aggravate neointimal hyperplasia following carotid artery injuries in mice through miR-222/CDKN1B/CDKN1C pathway. Cell Death & Disease, 10(6), 422.
Zheng, B., et al. (2017). Exosome-mediated miR-155 transfer from smooth muscle cells to Endothelial cells induces endothelial injury and promotes atherosclerosis. Molecular Therapy, 25(6), 1279–1294.
Zhang, Z., et al. (2020). Exosomal LINC01005 derived from oxidized low-density lipoprotein-treated endothelial cells regulates vascular smooth muscle cell phenotypic switch. BioFactors, 46(5), 743–753.
Huang, C., et al. (2018). Exosomal MALAT1 derived from oxidized low-density lipoprotein-treated endothelial cells promotes M2 macrophage polarization. Molecular Medicine Reports, 18(1), 509–515.
Gao, H., et al. (2020). Exosomal MALAT1 derived from ox-LDL-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biological Chemistry, 401(3), 367–376.
Xiong, F., et al. (2021). CircNPHP4 in monocyte-derived small extracellular vesicles controls heterogeneous adhesion in coronary heart atherosclerotic disease. Cell Death & Disease, 12(10), 948.
Wen, Y., et al. (2021). circRNA0006896miR1264DNMT1 axis plays an important role in carotid plaque destabilization by regulating the behavior of endothelial cells in atherosclerosis. Molecular Medicine Reports, 23(5).
Vilades, D., et al. (2020). Plasma circular RNA hsa_circ_0001445 and coronary artery disease: Performance as a biomarker. The FASEB Journal, 34(3), 4403–4414.
Petsophonsakul, P., et al. (2019). Role of vascular smooth muscle cell phenotypic switching and calcification in aortic aneurysm formation. Arteriosclerosis, Thrombosis, and Vascular Biology, 39(7), 1351–1368.
Kuivaniemi, H., et al. (2015). Understanding the pathogenesis of abdominal aortic aneurysms. Expert Review of Cardiovascular Therapy, 13(9), 975–987.
Hou, W. Z., et al. (2017). MicroRNA-370-3p inhibits human vascular smooth muscle cell proliferation via targeting KDR/AKT signaling pathway in cerebral aneurysm. European Review for Medical and Pharmacological Sciences, 21(5), 1080–1087.
Spinosa, M., et al. (2018). Human mesenchymal stromal cell-derived extracellular vesicles attenuate aortic aneurysm formation and macrophage activation via microRNA-147. The FASEB Journal 32(11), fj201701138RR.
Liao, B., et al. (2020). Exosome-derived MiRNAs as biomarkers of the development and progression of intracranial aneurysms. Journal of Atherosclerosis and Thrombosis, 27(6), 545–610.
Selvamani, A., et al. (2014). Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clinical Science (London, England), 127(2), 77–89.
Park, M. Y., et al. (2015). Circulating and renal vein levels of microRNAs in patients with renal artery stenosis. Nephrology, Dialysis, Transplantation, 30(3), 480–490.
Hosen, M. R., et al. (2021). CAD increases the long noncoding RNA PUNISHER in small extracellular vesicles and regulates endothelial cell function via vesicular shuttling. Molecular Theraphy-Nucleic Acids, 25, 388–405.
Zhao, Z., et al. (2017). Peripheral blood circular RNA hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. Science and Reports, 7, 39918.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81974046 and 82170467).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, Y., Dai, X. Emerging Roles of Extracellular Non-Coding RNAs in Vascular Diseases. J. of Cardiovasc. Trans. Res. 15, 492–499 (2022). https://doi.org/10.1007/s12265-022-10237-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10237-w