Abstract
Bone marrow stem cells (BMSCs) have been used to treat patient with ST-segment elevation myocardial infarction (STEMI) via intracoronary route. We performed a meta-analysis to evaluate the short-term efficacy and safety of this modality. Seventeen randomized controlled trials (RCTs) of BMSC-based therapy for STEMI, delivered with 9 days of reperfusion and followed up shorter than 12 months, were identified by systematic review. Intracoronary BMSC therapy resulted in an overall significant improvement in left ventricular ejection fraction (LVEF) by 2.74 % (95 % confidence interval (CI) 2.09–3.39, P < 0.00001, I 2 = 84 %) at 3–6-month follow-up and 5.1 % (95 % CI 4.16–6.03, P < 0.00001 and I 2 = 85 %) at 12 months. The left ventricular end-systolic volume (LVESV) and wall motion score index (WMSI) were also reduced at 3–6 months. At 12 months, left ventricular end-diastolic volume (LVEDV), LVESV, and WMSI were significantly reduced in BMSC group. In conclusion, intracoronary BMSC therapy at post-STEMI is safe and effective in patient with acute STEMI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute myocardial infarction (AMI) is a common presentation of coronary artery disease which remains the number one cause of death worldwide [1]. Usually because one of the coronary arteries that supplies blood to the heart develops a blockage due to an unstable buildup of leucocytes, cholesterol, and fat, AMI leads to regional ischemia and subsequent necrosis of myocardial tissues [2, 3]. AMI is classified into ST elevation MI (STEMI) and non-ST elevation MI (NSTEMI). STEMI is the combination of symptoms related to poor oxygenation of the heart with elevation of the ST segments on the electrocardiogram followed by an increase in proteins in bloods related to myocardial death. They make up about 25–40 % of cases [4].
The ideal therapy for myocardial infarction and ischemic heart failure (HF), in which the primary mechanism is cardiomyocyte loss, is to recuperate injured myocytes or regenerate new ones. Since stem cells have the important properties of self-regeneration and differentiation to replace lost tissues or repair the injured vascular and cardiac cells through paracrine effects [5–8], they have been proposed to be an ideal candidate for regeneration of damaged myocardium [9]. Bone marrow stem cell (BMSC) transfer is an emerging therapy for AMI patients. However, the safety and efficacy of this approach for cardiac repair remains controversial. Recent meta-analysis showed intracoronary mononuclear BMSC transfer after AMI improved left ventricular ejection fraction (LVEF) by 2.51 (95 % confidence interval (CI), 1.20–3.83) at a 6-month follow-up [1, 10]. By using a random-effect meta-analysis model, we systematically reviewed the randomized controlled trials (RCTs) that have studied BMSCs for the treatment of acute STEMI.
Methods
Data Source and Search Strategy
We searched PubMed, EMBASE, and the Cochrane Collaboration database till April 2014 for available studies. The search was limited to English language articles. The following search terms were used: bone marrow stem cell, acute myocardial infarction. If the same study had been published in various journals or in different years, our analysis only selected the most recent publication. If different experiments of multiple studies were done and published by same group of researchers, then combined data of these studies with same patient cohort was included in the analysis. Consequently, there was no overlap in patients included in our analysis.
Study Selection
We included randomized controlled studies comparing left ventricular ejection fraction (LVEF) improvement, left ventricular end-diastolic volume (LVEDV) reduction, left ventricular end-systolic volume (LVESV), and wall mean index score (WMIS) in patients treated with intracoronary BMSC transfer. Inclusion criteria were (1) patients with STEMI receiving percutaneous coronary intervention (PCI), (2) BMSC cell dose higher than 108, (3) studies that had a less than 12-month follow-up, and (4) sample size in BMSC transfer group larger than 20. Exclusion criteria were (1) patients with chronic myocardial infarction (CMI) who continued at least more than 1 month after AMI and patients with NSTEMI, (2) reviews, editorial, and abstracts presented at a conference, and (3) duplicate reports and ongoing or unpublished studies.
Data Extraction
The following information was collected for our analysis: first author and publication year [5]; baseline characteristics (age, sex, number of patients) [11], follow-up period, imaging modalities used to assess the endpoints, type of cell transfused, time from the PCI to cell transfusion [12]; the primary outcome was extracted and tabulated with particular attention to LVEF, LVEDV, LVESV, and WMIS at multiple follow-up time points [13].
Methodological Quality
To determine the quality of the included studies, Guidelines in the Cochrane Handbook for Systematic Reviews of Interventions 5.2 was used to direct the qualitative classification of studies.
Statistical Analysis
The meta-analysis was performed on the start-time point-adjusted changes in LVEF, LVEDV, and LVESV with BMSC therapy using Cochrane Collaboration Review Manager (version 5.2, Cochrane Collaboration, Copenhagen, Denmark) software package. The weighted mean difference (WMD) and 95 % confidence intervals were presented as outcome data. The P values with WMD were calculated from fixed-effect meta-analysis. Heterogeneity across studies was quantified using the I 2 statistic with a scale of 0–100 % (>75 % represented very large between-study inconsistency). We quantitatively assessed publication bias using the Begg adjusted-rank correlation test.
Results
Characteristics of Included Study
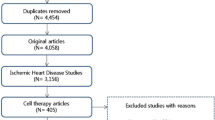
We identified 173 potentially relevant studies as shown in the flow diagram (Fig. 1). Of these studies, 20 studies met the inclusion criteria. Three additional studies were excluded. Sun et al. [14] investigated the effect of autologous bone marrow mononuclear cell transplantation in diabetic patients with STEMI. In this study, the efficacy and proposed mechanism of BMSC transplantation was investigated in diabetic and non-diabetic patients with STEMI. Hamshere et al. performed a randomized double-blind control study on early intracoronary autologous bone marrow cell infusion in AMI (REGENERSTE-AMI trial). However, the detailed results of this trial have not been published yet. Leistner D et al. [15] (TOPCARE-AMI trial) investigated short-term and long-term safety and effects of intracoronary progenitor cell therapy compared with BMSC therapy in AMI patients. So the above three trials were excluded in this meta-analysis study. Thus, a total of 20 studies were included in our systematic review, encompassing patients between 2006 and 2013. The follow-up duration varied between less than 6 and 12 months.
These studies recruited 1393 participants including 710 patients in the treatment arm (ranging from 40 to 204 per study), predominately men, with a mean age of 50–61 years, and a mean follow-up of 3–12 months including 5 studies with a 4-month follow-up [2, 16–19] and 2 studies with a 3-month follow-up [20, 21]. The included studies based on the criteria are shown in Table 1. Of all these trials, STEMI in these patients was diagnosed by cardiologists at each study site by MRI, echocardiography, and angiography and all the patients underwent PCI as a primary intervention. All patients were recruited consecutively during the study periods. BMSCs were prepared by using Ficoll-based methods and suspended with heparinized saline. CD34+ cells were tested in 16 of 20 trials and CD34+ cell numbers were from 0.6 × 106 to 9 × 106. BMSCs were introduced via the intracoronary route in all studies and the dosage ranged from 1 × 108 to 2.46 × 109. The time to BMSC transfer from the onset of AMI ranged from 0.37 to 9 days. The baseline ejection fraction ranged from 34.6 to 60.5 % (mean LVEF 45 %). The characteristics of the included trials are summarized in Table 2.
Quantitative Findings
Pooled analysis of the 17 BMSC trials showed that at 3–6 months of follow-up, the weighed mean difference (WMD) of changes in LVEF (%) in patients treated with BMSCs was a significant increase of 2.74 % (95 % CI 2.09–3.39, P < 0.00001, I 2 = 84 %) compared with controls. In five studies that reported 12-month outcomes, the WMD of change in LVEF at 12 months was 5.1 % with 95 % CI 4.16–6.03; P < 0.00001 and I 2 = 85 % (Figs. 2 and 3).
Fifteen BMSC studies measured LV volume outcomes at 3–6 months of follow-up. Pooled analysis of these data demonstrated significant reduction in LVESV with BMSCs treatment compared with controls. Weighted mean difference (WMD) of change in left ventricular end-systolic volume (LVESV) was −3.92 ml (95 % CI −5.0 to −2.85, P < 0.00001, I 2 = 62 %). However, no statistic reduction was detected in LVEDV change in BMSC treatment with control. At 12-month follow-up, 5 trials were pooled to analyze the LVESV and LVEDV outcome. The WMD of change in LVESV and LVEDV demonstrated the significant reduction with BMSC treatment compared with controls, which was −11.46 ml (95 % CI −13.69 to −9.23, P < 0.00001, I 2 = 91 %) in LVESV and −7.29 ml (95 % CI −10.19 to −4.38, P < 0.00001, I 2 = 84 %) in LVEDV (Figs. 4, 5, and 6).
With two major imaging modalities for myocardial function measurement (echo and MRI) in the included trials, LVESV demonstrated significant reduction with BMSC treatment compared with controls, which was −2.78 ml (95 % CI −4.10 to −1.45 ml, P < 0.00001, I 2 = 0 %) in 3–6-month follow-up with MRI. With echo measurement, LVESV also showed significant reduction at 3–6-month follow-up, which was −4.25 ml (95 % CI −6.32 to −2.19 ml, P < 0.00001, I 2 = 0 %) (Fig. 5).
Pooled analysis of 6 studies with measurement of wall motion score index (WMSI) at less than 6-month follow-up showed statistic significance with BMSC treatment compared with controls, and the WMD of WMSI change was −0.04 (95 % CI −0.06 to −0.02, P < 0.00001, I 2 = 45 %). Pooled analysis was also performed on 4 studies at 12-month follow-up and the WMD of change was statistically significant, which was −0.06 (95 % CI −0.10 to −0.02, P < 0.002, I 2 = 0 %) in BMSC treatment versus controls.
Assessment for Publication Bias
Begg’s funnel plots test was performed to test qualitative publication bias of the studies for the primary outcome LVEF (Fig. 3) and mortality (Figs. 7, 8, and 9). The funnel plot tests for LVEF at less than 6-month follow-up and at 12-month follow-up showed symmetry. There was no clear evidence that the size of the trials reflect the magnitude of the effects. Begg’s funnel plot for mortality showed no evidence of obvious asymmetry. Therefore, no bias of publications was found.
Adverse Outcome Data
Reporting of adverse outcome was undertaken in most studies assessed. However, some trials provided less detail than others. Events reported include mortality, cardiac death, re-infarction, heart failure hospitalization, revascularization stent thrombosis, and in-stent restenosis. In this analysis, we only analyzed adverse outcome for mortality; there is no difference when comparing BMSC treatment with control.
Discussion
This systemic review is based on the comprehensive search strategy. The selection of trials for meta-analysis was based on the rigorous criteria.
There are several clinical therapy strategies on the treatment of AMI, such as BMSCs (BMMNCs), allogeneic mesenchymal stem cell (MSC) and CD133+/CD34+ [22]. In the published trials on the treatment of AMI, BMSCs have been the most widely used cell populations in clinical AMI practice. In this systemic review, all included clinical trials are BMSC treatment on AMI. BMSC dose play an important role in the efficiency of BMSC therapy in patients with STEMI. BMSC dose administrated is correlated positively with the effect on LVEF. The mean change in LVEF was statistically significant in favor of administrating BMSCs for studies using high doses of BMSCs. Previous trials determined that significant effects on LVEF may only be achieved when infusing doses are higher than 108 BMSCs [1, 12, 23]. So this meta-analysis suggested that a total of more than 108 unfractionated BMSC dose were associated with an increased in LVEF.
We excluded the timing of BMSC transfer after primary PCI. Huang RC et al. and Jeevanantham et al. investigated the timing of cell delivery and showed that the time of cell transfer does not have a BMSC treatment effect in AMI patients [1, 24]. In REPAIR-AMI trial and SWISS-AMI trials, the beneficial effects of BMSCs appeared to be more pronounced with cell application at 5–7 days [13, 19]. Zimmet et al. suggested that the time between AMI and cell delivery, over the 9-day post-infarct time frame, did not appear to be associated with changes in LVEF [25]. A larger randomized study suggested that BMSCs should ideally be administrated more than 4 days after STEMI to obtain the best benefit from this therapy [18].
This present systematic review aimed to assess data from RCTs relevant to the clinical practice of BMSC therapy for AMI. This meta-analysis suggests that intracoronary BMSC therapy post-STEMI produces improvements in absolute LVEF values of the order of 2.74 % at 3–6-month follow-up. In terms of LV volumes, statistically significant reduction in LVESV and LVEVD was seen with intracoronary BMSC treatment at 3–6 months with −3.92 ml (95 % CI −5.00 to −2.85) and 1.69 ml (95 % CI 3.47–0.09), respectively.
Wall motion score index is another powerful prognostic information of LV function after AMI [26, 27]. In this meta-analysis, only 6 studies reported WMSI at 3–6 months and 4 studies reported WMSI at 12 months. Statistically significant reduction was observed with BMSC treatment. Even though fewer trials were included in this analysis for WMSI than analysis for LVEF, heterogeneity is low to 45 %. So WMSI is suggested to be assessed and reported in the trials of BMSC therapy in STEMI.
To assess the durability of these improvements in LVEF, LV volumes, and WMSI, observed with intracoronary BMSC treatment, we made them at 3–6 months and 12 months. In these studies, improvements in LVEF observed at 3–6 months increased at 12 months with 5.10 % (95 % CI 4.16–6.03). Moreover reduction in LVESV, LVEDV, and WMSI observed at 3-6 months reduced at 12 months with −11.46 ml (95 % CI −13.69 to −9.23), −7.29 ml (95 % CI −10.19 to −4.38), and −0.06 ml (96 % CI −0.10 to −0.02). In this analysis, BMSC group exhibited a significant attenuation of LVEDV compared with control at 12-month follow-up, instead of in 3–6 months of follow-up. The occurrence of cell treatment-related LVEF improvement go parallel to LVESV but appeared to be earlier than the beneficial changes in LVEDV. This result is consistent with Zhang et al.’s study that the increase in LVEF was observed at 3 months post cell treatment or earlier [28], whereas the benefits on LVEDV might begin to emerge up to 12 months after procedure [29]. However, the potential mechanism responsibility for the time-related clinical benefits was still uncertain.
MRI is currently considered the gold standard for primary endpoint measures of efficacy and volumes. In this meta-analysis, 50 % of all trials used MRI and 30 % of all trials used echo. LVESV in treatment group showed significant changes in 3–6-month follow-up compared with control group by both MRI-based and echo analysis. This result supports that imaging modalities have no effect on the assessment on the treatment.
With regard to the safety of BMSC therapy, the low mortality in the intervention arms of all studies assessed in this analysis indicated that BMSC therapy after PCI in post-STEMI is safe and feasible. It has been reported that primary PCI after AMI can improve changes in LVEF and eventually effects on mortality [30]. In this analysis, PCI after AMI was set up as one of the criteria on trials collection. The results showed here confirmed that autologous BMSC therapy may be a safe administration to treat patients with STEMI. Moreover the relative risk of mortality was not significantly increased in participants who received BMSC therapy compared with control, consistent with the earlier meta-analysis results [25]. Further adverse outcome, revascularization, rehospitalization, re-infarction, and stent thrombosis are suggested to be included in the assessment of safety and feasibility of BMSC therapy. Earlier meta-analysis have revealed significantly lower revascularization rate with intracoronary BMSC compared with controls [25].
In summary, this study showed evaluated RCT evidence for BMSC therapy after AMI. Although long-time follow-up and larger size of recruited patients are not included in this study, this analysis supports safety and efficiency of BMSC therapy in STEMI patients.
References
Jeevanantham, V., & Butler, M.-L.-S. (2012). Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation, 126(5), 551–68.
Janssens, S., & Dubois, C. (2006). Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet, 367, 117–121.
Ren, G., Micheal, L., Entman, M., & Frangogiannis, N. G. (2002). Morphological characteristics of the microvasculature in healing myocardial infarcts. The Journal of Histochemistry and Cytochemistry, 50(1), 71–79.
O'Gara, P., Kushner, F., Ascheim, D., Casey Jr, D. E., Chung, M., de Lemos, J., et al. (2013). 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Founda. Circulation, 127(4), e362–425.
1.Allgower, M. (1956). In M. Allgower, The cellular basis of woun repair. Charles C. Thomas.
15.Jiang, Y., Jahagirdar, B., Reinhardt, R., Schwartz, R., Keene, C., Ortiz-Gonzalez, X., et al. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. 418 (6893), 41-49.
Krause, D., Theise, N., Collector, M., Henegariu, O., Hwang, S., Gardner, R., et al. (2001). Multi-organ, multi- lineage engraftment by a single bone marrow-derived stem cell. Cell, 105(3), 369–377.
29.Quaini, F., Urbanek, K., Graiani, G., Lagrasta, C., Maestri, R., Monica, M., et al. (2004). The regenerative potential of the human heart. 95 (1), S26-S28.
Goodell, M., Jackson, K., Majka, S., Mi, T., Wang, H., Pocius, J., et al. (2001). Stem cell plasticity in muscle and bone marrow. Annals of the New York Academy of Sciences, 938, 208–218.
Jeong, H., & Yim, H. (2013). The effect of rigorous study design in the research of autologous bone marrow-derived mononuclear cell transfer in patients with acute myocardial infarction. Stem Cell Research & Therapy, 4(4), 82.
Cao, F., & Sun, D. (2009). Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. European Heart Journal, 30(16), 1986–94.
Clifford, D. M., & Fisher, S.-R. (2012). Long-term effects of autologous bone marrow stem cell treatment in acute myocardial infarction: factors that may influence outcomes. PloS One, 7(5), e37373.
Dill, T., & Schächinger, V. (2009). Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the reinfusion of enriched progenitor cells and infarct remodeling in acute myocardial infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. American Heart Journal, 157(3), 541–7.
Sun, D., & Narsinh, K. (2013). Effect of autologous bone marrow mononuclear cells transplantation in diabetic patients with ST-segment elevation myocardial infarction. International Journal of Cardiology, 167(2), 537–47.
Leistner, D. M., & Fischer-Rasokat, U. (2011). Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): final 5-year results suggest long-term safety and efficacy. Clinical Research in Cardiology, 100(10), 925–34.
Herbots, L., & D’hooge, J. (2009). Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. European Heart Journal, 30(6), 662–70.
Hirsch, A., Nijveldt, R., & Investigators, H. (2011). Intracoronary infusion of mononuclear cells from bone marrow or peripheral blood compared with standard therapy in patients after acute myocardial infarction treated by primary percutaneous coronary intervention: results of the randomized controlled HEBE. European Heart Journal, 32(14), 1736–47.
Schachinger, V., Erbs, S., & REPAIR-AMI Investigators. (2006). Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. The New England Journal of Medicine, 4, 1210–1221.
Sürder, D., & Manka, R. (2013). Intracoronary injection of bone marrow-derived mononuclear cells early or late after acute myocardial infarction: effects on global left ventricular function. Circulation, 127(19), 1968–79.
Meluzín, J., & Janousek, S. (2008). Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. International Journal of Cardiology, 128(2), 185–92.
Roncalli, J., & Mouquet, F. (2011). Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: results of the randomized multicenter BONAMI trial. European Heart Journal, 32(14), 1748–57.
de Jong, R., Houtagraaf, J. H., Samiei, S., Boersma, E., & Duckers, H. J. (2014). Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circulation. Cardiovascular Interventions, 7(2), 156–67.
Martin-Rendon, E., & Brunskill, S. (2008). Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. European Heart Journal, 29(15), 1807–18.
Huang, R. C., & Yao, K. (2007). Evaluation of myocardial viability with 201Tl/18F-FDG DISA-SPECT technique in patients with acute myocardial infarction after emergent intracoronary. Zhonghua Xin Xue Guan Bing Za Zhi, 35(6), 497–9.
Zimmet, H., & Porapakkham, P. (2012). Short- and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. European Journal of Heart Failure, 14(1), 91–105.
Lebeau, R., & Serri, K. (2012). Assessment of left ventricular ejection fraction using the wall motion score index in cardiac magnetic resonance imaging. Archives of Cardiovascular Diseases, 105(2), 91–8.
Møller, J. E., & Hillis, G. (2006). Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. American Heart Journal, 151(2), 419–25.
Zhang SN, S. A., Ge, J. B., Yao, K., Huang, Z. Y., Wang, K. Q., et al. (2009). Intracoronary autologous bone marrow stem cell transfer for patients with acute myocardial infarction: a meta-analysis of randomized controlled trials. International Journal of Cardiology, 136, 178–85.
Zhang, C., Sun, A., Zhang, S., Yao, K., Wu, C., Fu, M., et al. (2010). Efficiency and safety of intracoronary autologous bone marrow-derrived cell transplantation in patients with acute myocardial infarction: insights from randomized controlled trials with 12 or more months follow-up. Clinical Cardiology, 33, 353–60.
Halkin, A., Mandeep, S., Mikolsky, E., Grines, C. L., Tcheng, J. E., Garcia, E., Cox, D. A., et al. (2005). Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADLLAC risk score. Journal of the American College of Cardiology, 45, 1397–1405.
Meyer, G. P., & Wollert, K. (2006). Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation, 113(10), 1287–94.
Huikuri, H. V., Kervinen, K., & Investigators, F. (2008). Effects of intracoronary injection of mononuclear bone marrow cells on left ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. European Heart Journal, 29(22), 2723–32.
Tendera, M., Wojakowski, W., & Investigators, R. (2009). Intracoronary infusion of bone marrow-derived selected CD34+ CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre myocardial regeneration by Intracor. European Heart Journal, 30(11), 1313–21.
Lunde, K., & Solheim, S. (2006). Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. The New England Journal of Medicine, 355(12), 1199–209.
Plewka, M., & Krzemińska-Pakula, M.-D. (2009). Effect of intracoronary injection of mononuclear bone marrow stem cells on left ventricular function in patients with acute myocardial infarction. The American Journal of Cardiology, 104(10), 1336–42.
Grajek, S. P. M.-M.-S. (2010). Influence of bone marrow stem cells on left ventricle perfusion and ejection fraction in patients with acute myocardial infarction of anterior wall: randomized clinical trial: Impact of bone marrow stem cell intracoronary infusion on improvement of microc. European Heart Journal, 31(6), 691–702.
Traverse, J. H., & McKenna, D. (2010). Results of a phase 1, randomized, double-blind, placebo-controlled trial of bone marrow mononuclear stem cell administration in patients following ST-elevation myocardial infarction. American Heart Journal, 160(3), 428–34.
Wöhrle, J., & Merkle, N. (2010). Results of intracoronary stem cell therapy after acute myocardial infarction. The American Journal of Cardiology, 105(6), 804–12.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Enrique Lara-Pezzi oversaw the review of this article
Rights and permissions
About this article
Cite this article
Cong, Xq., Li, Y., Zhao, X. et al. Short-Term Effect of Autologous Bone Marrow Stem Cells to Treat Acute Myocardial Infarction: A Meta-Analysis of Randomized Controlled Clinical Trials. J. of Cardiovasc. Trans. Res. 8, 221–231 (2015). https://doi.org/10.1007/s12265-015-9621-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-015-9621-9