Abstract
We investigated the effects of intra-aortic balloon pump (IABP) counterpulsation on left ventricular (LV) contractility, relaxation, and energy consumption and probed the underlying physiologic mechanisms in 12 farm pigs, using an ischemia-reperfusion model of acute heart failure. During both ischemia and reperfusion, IABP support unloaded the LV, decreased LV energy consumption (pressure-volume area, stroke work), and concurrently improved LV mechanical performance (ejection fraction, stroke volume, cardiac output). During reperfusion exclusively, IABP also improved LV relaxation (tau) and contractility (Emax, PRSW). The beneficial effects of IABP support on LV relaxation and contractility correlated with IABP-induced augmentation of coronary blood flow. In conclusion, we find that during both ischemia and reperfusion, IABP support optimizes LV energetic performance (decreases energy consumption and concurrently improves mechanical performance) by LV unloading. During reperfusion exclusively, IABP support also improves LV contractility and active relaxation, possibly due to a synergistic effect of unloading and augmentation of coronary blood flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The intra-aortic balloon pump (IABP) [1] remains the most widely used cardiac assist device, particularly in the setting of cardiogenic shock after myocardial infarction or cardiac surgery [2]. While the effects of IABP support on hemodynamics and traditional indices of left ventricular (LV) mechanical performance in the setting of acute heart failure are well documented [3], its effects on LV contractility, relaxation, and energy consumption have been underinvestigated and results to date have been conflicting [4–7]. In addition, the physiologic mechanisms which underlie the effects of IABP support remain poorly characterized. Despite the fact that there is lack of direct connection between the IABP and the LV itself (since the balloon is placed in the descending aorta and its inflation and deflation occur while the aortic valve is shut), IABP-induced effects on cardiac load, coronary blood flow, and autonomic nervous system could indirectly impact on LV mechanoenergetics. In the present study, we aimed to assess the effects of IABP support on LV contractility, relaxation, and energy consumption, and to elucidate the underlying physiologic mechanisms, in a porcine ischemia-reperfusion model of acute heart failure. We also investigated potential differences of IABP-induced effects on LV mechanoenergetics during the different phases of ischemia and reperfusion.
Methods
Surgical Procedures
The experimental protocol was approved by the Institutional Review Board of Alexandra General Hospital, Athens, Greece. Twelve farm pigs weighing 30–40 kg were studied. For the last 5 days prior to the experimental procedure, pigs were pretreated with ivabradine (1 mg/kg) to decrease heart rate (allowing for better synchronization of the IABP). On the day of the experiment, animals were premedicated with 15 mg/kg of ketamine hydrochloride IM and 0.5 mg/kg of midazolam IV. Anesthesia was induced with 9 mg/kg of thiopental sodium IV and 0.5 mg of fentanyl citrate IV, and maintained by a continuous, IV infusion of 3 mg/kg/h of thiopental sodium, 0.03 mg/kg/h of fentanyl citrate, and 0.25 mg/min of pancuronium bromide. Once anesthetized, animals were intubated and placed on a surgical table in a supine position. Leads were placed on the extremities for continuous ECG monitoring and IABP triggering, and body temperature was maintained with a heating blanket.
Arterial pressure was measured with a catheter placed in the left carotid artery. Midline thoracotomy was performed, the chest was opened, and the heart was suspended in a pericardial cradle. A 5 F Millar pressure-tip catheter (SPR-350S, Millar Instruments, Houston, TX) was placed in the LV through an apical incision. Six piezoelectric crystals (Sonometrics Corporation, London, Canada) were implanted in the LV subepicardium; four crystals were implanted circumferentially in the mid-LV, one was implanted at the LV apex, and one at the LV base. LV volumes were calculated by a three-axis ellipsoid model based on the distance between the LV walls measured by the implanted piezoelectric crystals. The proximal left anterior descending (LAD) artery (proximally to the first diagonal branch) was dissected free, and a ring-shaped Doppler flow probe (ΜediStim Ιnc, PS100022) was placed around the vessel to measure blood flow. A 25-ml intra-aortic balloon (Datascope, Linear) was placed in the descending aorta via the right carotid artery. An elastic tape was placed around the inferior vena cava, allowing for gradual and partial occlusion of the vessel (to decrease cardiac preload). Heparin 100 IU/kg IV was given for anticoagulation, and arrhythmias were suppressed by continuous, IV infusion of 1 mg/min lidocaine. A photograph of the experimental setup is provided in Fig. 1a.
a Experimental preparation. b Schematic depiction of the experimental protocol. c Measured parameters. With transient IVC occlusion, a family of loops is obtained, enabling measurement of load-independent indices. ArtP arterial pressure, CVP central venous pressure, LVP left ventricular pressure, LVV left ventricular volume. d Acute heart failure induction. Ligation of the proximal LAD resulted in induction of acute heart failure, manifested as a dramatic increase in LV end-diastolic pressure and a rightward and upward shift of the pressure-volume loop. Red points mark end diastole
Experimental Protocol
The experimental protocol is depicted schematically in Fig. 1b. After 15 min of hemodynamic stabilization, ventilation was suspended at the end of expiration and baseline measurements of arterial and LV pressures, heart rate, ejection fraction (EF), stroke volume (SV), cardiac output (CO), dP/dT max, dP/dT min, time constant of pressure decay during isovolumic relaxation (tau), and distances between piezoelectric crystals (for LV volume calculation) were recorded using the SonoLab® software (Sonometrics Corporation, London, Canada). Then, the inferior vena cava was gradually and partially occluded, a family of pressure-volume loops (at decreasing preload) was obtained (Fig. 1c), and the end-systolic pressure-volume relationship (ESPVR) and end-diastolic pressure-volume relationship (EDPVR) were recorded. From the slope of the ESPVR, the load-independent index of contractility maximum elastance (Emax) was calculated; from the EDPVR, the LV chamber stiffness constant (Kc) was calculated. From the family of loops (obtained at decreasing preload), the load-independent index of contractility preload-recruitable stroke work (PRSW) was calculated. Myocardial ischemia was then induced by ligation of the proximal LAD artery for 1 h, followed by 2 h of reperfusion. At 20, 40, and 60 min of ischemia and at 20, 40, 60, 80, 100, and 120 min of reperfusion, ventilation was suspended at the end of expiration and the following parameters were measured without IABP support: aortic and LV pressures, heart rate, double product (systolic arterial pressure × heart rate), EF, SV, CO, dP/dT max, dP/dT min, tau, stroke work (external work, the area within the pressure-volume loop), LAD artery blood flow, and distances between piezoelectric crystals (for LV volume calculation). Then, the inferior vena cava was gradually and partially occluded and the ESPVR (from which Emax was calculated) and EDPVR (from which Kc was calculated) were recorded and PRSW was calculated. From the ESPVR and the EDPVR, pressure-volume area (the area contained within the ESPVR, EDPVR, and the systolic segment of the pressure-volume loop) was calculated. Pressure-volume area is an indirect index of total LV energy consumption, as it has been shown to linearly correlate with myocardial oxygen consumption [8]. The ratio of stroke work to pressure-volume area was calculated as an index of efficiency of LV mechanoenergetic performance [9]. Afterwards, the IABP was turned on for 5 min (1:1 assist ratio, synchronization by ECG triggering) and all the aforementioned parameters were measured after 5 min of IABP support (with the IABP on). The experiment was terminated after 2 h of reperfusion by electrical fibrillation of the heart. Data were analyzed with the CardioSoft Pro® software (Sonometrics Corporation, London, Canada).
Validation of Sonomicrometry for Measuring Changes in EF
While multicrustal sonomicrometry has been used for decades for real-time LV volume assessment in pressure-volume analysis [10–12], the three-axis ellipsoid sonomicrometry-based model used in the present study has not been validated for measuring changes in LVEF. We performed a separate study in order to compare sonomicrometry and transthoracic echocardiography for assessment of LVEF in a porcine model of ischemic cardiomyopathy. Detailed methods and results are provided in the supplement. We found that, while sonomicrometry consistently underestimated absolute LVEF values compared to transthoracic echocardiography, the sonomicrometric and echocardiographic measurements of LVEF correlated strongly (R 2 0.959, p < 0.001) (Supp Fig 5). These results validate the use of our sonomicrometry-based approach for measuring dynamic changes in LVEF (but not absolute LVEF values) in response to hemodynamic interventions (e.g., IABP counterpulsation in the present study).
Statistical Analysis
Data are presented as mean ± standard deviation in the text and tables and as mean ± standard error of the mean in the figures. Data obtained from the same recordings (without and after 5 min of IABP support) were compared using paired t test. IABP-induced effects were compared between ischemia and reperfusion using independent-samples t test. Differences between baseline, ischemia, and reperfusion were tested using one-way ANOVA. The coefficient r was calculated to examine the presence of correlations between variables by linear regression analysis. All tests were two sided, and a p value <0.05 was considered statistically significant.
Results
Induction of Acute Heart Failure
Ligation of the proximal LAD resulted in induction of acute heart failure, manifested as a dramatic increase in LV end-diastolic pressure (baseline 12.5 ± 4 mmHg, ischemia 21.4 ± 6 mmHg, reperfusion 21.8 ± 4 mmHg, p < 0.001 [Supp Table 1]) and a shift of the pressure-volume loop to the right and up (Fig. 1d), during both ischemia and reperfusion. Cardiac output, EF, and SV decreased significantly during ischemia and reperfusion compared to baseline [Supp Table 1]. Indices of systolic (dP/dT max, Emax, PRSW) and diastolic function (dP/dT min, tau, Kc stiffness constant) deteriorated significantly during ischemia and reperfusion compared to baseline (Supp Table 1).
IABP Support Optimizes Energetic Performance of the Failing LV
During both ischemia (Table 1) and reperfusion (Table 2), IABP support provided, as expected, mechanical unloading of the failing LV, manifested as a significant decrease in LV afterload (i.e., systolic and end-diastolic arterial pressure [Supp Fig 1A,1B]), LV end-diastolic pressure (Supp Fig 1C), and LV double product (Supp Fig 1D). Supp Fig 2 shows representative arterial and LV pressure waveforms without and with IABP counterpulsation; despite the IABP-induced decrease in systolic and end-diastolic arterial pressure, mean arterial pressure remained unchanged with IABP support during both ischemia (Table 1) and reperfusion (Table 2) due to diastolic arterial pressure augmentation provided by the IABP (Supp Fig 2). IABP support had no effect on heart rate.
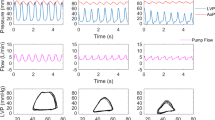
During both ischemia (Table 1) and reperfusion (Table 2), IABP support improved the mechanical performance of the failing LV, manifested as a significant increase in LVEF (Fig. 2a), SV, and cardiac output. Importantly, the IABP-induced enhancement of mechanical performance was accompanied by a concurrent decrease in stroke work (external work, Fig. 2b) and total LV energy consumption (pressure-volume area, Fig. 2d). Figure 2c shows representative pressure-volume loops without and after 5 min of IABP support during reperfusion. The pressure-volume loop shifts to the bottom and left after IABP support, resulting in optimization of LV mechanoenergetics; SV and EF increase and at the same time stroke work (external work) decreases. In addition, during both ischemia (Table 1) and reperfusion (Table 2), IABP support improved the efficiency of LV mechanoenergetic performance, manifested as a significant increase in the ratio of stroke work to pressure-volume area (Fig. 2e). Figure 2f shows representative families of pressure-volume loops (at decreasing preload) without and after 5 min of IABP support during reperfusion. IABP support decreases total LV energy consumption (decreases pressure-volume area) and concurrently optimizes LV mechanoenergetic efficiency (improves the ratio of stroke work to pressure-volume area).
IABP support optimizes left ventricular mechanoenergetics (improves mechanical performance, decreases energy consumption, and optimizes mechanoenergetic efficiency) during ischemia and reperfusion. a Left ventricular ejection fraction without and after 5 min of IABP support during ischemia and reperfusion. b Stroke work (external work) without and after 5 min of IABP support during ischemia and reperfusion. c The pressure-volume loop shifts to the bottom and left after IABP support, resulting in optimization of left ventricular energetic performance; stroke volume and ejection fraction increase and concurrently stroke work decreases. d Pressure-volume area without and after 5 min of IABP support during ischemia and reperfusion. e Ratio of stroke work to pressure-volume area without and after 5 min of IABP support during ischemia and reperfusion. f Representative families of pressure-volume loops (at decreasing preload) without and after 5 min of IABP support during reperfusion. IABP support decreases total LV energy consumption (decreases pressure-volume area) and concurrently optimizes LV mechanoenergetic efficiency (improves the ratio of stroke work to pressure-volume area). SW stroke work, PVA pressure-volume area (*p < 0.05 compared to IABP off)
IABP Support Improves LV Contractility and Relaxation During Reperfusion Exclusively
During both ischemia (Table 1) and reperfusion (Table 2), IABP support induced a significant decrease in dP/dT max (Supp Fig 3A, Supp Fig 4A) and a significant increase in dP/dT min (Supp Fig 3B, Supp Fig 4B). Since (a) dP/dT max and dP/dT min are load-dependent indices (they improve as afterload increases) [13, 14] and (b) IABP support decreased LV afterload during ischemia and reperfusion (Tables 1 and 2), we investigated whether the changes in dP/dT max and dP/dT min observed during IABP support were associated with IABP-induced decreases in cardiac afterload. We found that the changes of dP/dT max and dP/dT min after IABP support correlated significantly with IABP-induced decreases in systolic and end-diastolic arterial pressure (indices of cardiac afterload) (Supp Fig 3C-F, Supp Fig 4C-F), during both ischemia and reperfusion. Thus, the decrease in dP/dT max and the increase in dP/dT min observed during IABP support should be attributed to mechanical unloading (i.e., reduction of cardiac afterload) provided by the IABP, rather than be interpreted as IABP-induced deterioration of LV systolic and diastolic function.
We then investigated the effect of IABP support on load-independent indices of LV contractility (Emax, PRSW) and relaxation (tau, chamber stiffness constant Kc); these indices are relatively impervious to changes in cardiac afterload [15, 16] and therefore enable true assessment of IABP effects on systolic and diastolic function. With regard to indices of contractility, IABP support did not exert any significant effect on Emax or PRSW during ischemia. However, during reperfusion, IABP support induced a significant increase in Emax, manifested as a steeper slope of the ESPVR (Fig. 3a), and in PRSW, suggesting an improvement in LV contractility (Fig. 3b, c). With regard to indices of diastolic function, IABP support improved active relaxation (manifested as a decrease in tau) during reperfusion but not during ischemia (Fig. 3d); chamber stiffness constant Kc (a marker of passive diastolic myocardial properties) did not change significantly after IABP support, neither during ischemia (Table 1) nor during reperfusion (Table 2).
IABP support improves left ventricular contractility and active relaxation exclusively during reperfusion. a Families of pressure-volume loops (at decreasing preload) without and after 5 min of IABP support during reperfusion. Maximum elastance increases, manifested as an increase in the slope of the end-systolic pressure-volume relationship (red IABP off, blue IABP on). The dashed red line on the right denotes the slope of the solid red line on the left (IABP off). Maximum elastance (b), preload-recruitable stroke work (c), and time constant of isovolumic relaxation (d) without and after 5 min of IABP support during ischemia and reperfusion (*p < 0.05 compared to IABP off)
Increased Benefits of IABP During Reperfusion Are Associated with IABP-Induced Augmentation of Coronary Blood Flow
A comparison of IABP-induced effects on hemodynamics and LV mechanoenergetics between the different phases of ischemia and reperfusion is provided in Table 3 and Fig. 4. As highlighted in Figs. 3 and 4, IABP significantly improved LV active relaxation (tau) and contractility (Emax, PRSW) during reperfusion exclusively. In addition, IABP support induced a greater increase in cardiac output and a more profound decrease in stroke work and LV end-diastolic pressure during reperfusion compared to ischemia. Since IABP support also increased mean LAD blood flow during reperfusion (Fig. 5a, b), we hypothesized that the observed increased benefits of IABP support during reperfusion may be associated with augmentation of coronary blood flow. IABP-induced changes in mean LAD blood flow correlated significantly with IABP-induced changes in Emax (Fig. 5c), PRSW (Fig. 5d), and tau (Fig. 5e) during reperfusion; no correlation between changes in blood flow and changes in cardiac output, stroke work, and LVEDP was detected.
Increased benefits of IABP support during reperfusion compared to ischemia. IABP-induced changes in left ventricular end-diastolic pressure (a), cardiac output (b), maximum elastance (c), preload-recruitable stroke work (d), time constant of isovolumic relaxation (e), and stroke work (f) (*p < 0.05 compared to ischemia)
IABP-induced increase in left ventricular contractility and relaxation is associated with augmentation of coronary blood flow. a LAD blood flow waveforms without and after 5 min of IABP support during reperfusion. b Mean LAD blood flow without and after 5 min of IABP support during reperfusion. IABP-induced changes in mean LAD blood flow correlated significantly with IABP-induced changes in maximum elastance (c), preload-recruitable stroke work (d), and time constant of isovolumic relaxation (e) during reperfusion (*p < 0.05 compared to IABP off)
Discussion
While the effects of IABP support on hemodynamics and traditional indices of LV mechanical performance are well documented [3], few studies have investigated its effects on LV contractility, relaxation, and energy consumption and their results have often been conflicting [4–7]. In addition, the physiologic mechanisms mediating the salutary effects of IABP support remain poorly characterized. In the present study, using a porcine ischemia-reperfusion model of acute heart failure, we (a) performed a comprehensive assessment of the impact of IABP support on LV mechanoenergetics, (b) investigated potential differences of IABP-induced effects during the different phases of ischemia and reperfusion, and (c) probed the physiologic mechanisms underlying the IABP-mediated effects.
Our findings regarding the effects of IABP support on LV afterload, LV mechanical performance, energy consumption, contractility, and relaxation during the different phases of ischemia and reperfusion are depicted schematically in Table 4. We find that during both ischemia and reperfusion, IABP support decreases LV afterload and optimizes LV energetic performance (decreases energy consumption and concurrently improves mechanoenergetic efficiency and mechanical performance). In addition, for the first time to our knowledge, we report that IABP support improves LV contractility and active relaxation exclusively during reperfusion but not during ischemia. Importantly from a physiologic standpoint, we show, for the first time to our knowledge, that the IABP-mediated improvements in LV contractility and active relaxation (observed exclusively during reperfusion) correlate strongly with IABP-induced augmentation of coronary blood flow. The correlation between the IABP-induced improvements in LV contractility and coronary blood flow is consistent with “Gregg’s phenomenon” [17, 18], according to which increased coronary perfusion results in improved cardiac contractility [19], presumably through coronary flow-mediated increases in intracellular calcium of cardiomyocytes [20]. Gregg’s phenomenon may help rationalize why IABP support improved LV contractility exclusively during reperfusion but not during ischemia. The correlation between the IABP-induced improvements in LV relaxation and coronary blood flow is consistent with previous experimental studies, reporting that increased coronary flow is associated with improved LV active relaxation [21]. Since active relaxation occurs in a series of energy-consuming steps [22], it can be postulated that increases in energy supply (as a result of IABP-induced augmentation of coronary blood flow) would result in improved active relaxation of reperfused myocardium post-myocardial infarction.
Despite the plethora of salutary effects induced by the IABP in the experimental setting, the clinical effectiveness of short-term IABP support in patients with cardiogenic shock post-myocardial infarction has been called into question by the results of the IABP-SHOCK II trial [23, 24]. While the reasons underlying the discordance between the pathophysiological/hemodynamic benefits of IABP support observed in experimental studies and the absence of hard evidence regarding clinical benefits [23–25] are unclear, relevant considerations include (a) the hemodynamic support provided by the IABP (increase in EF by ∼2.7 %, increase in SV by ∼2.6 ml in our study) may be insufficient to achieve clinical benefits in the setting of cardiogenic shock [26] and (b) inherent methodological difficulties in the design/execution of randomized trials in gravely ill patients suffering from cardiogenic shock (e.g., rescue LV assist device insertion or rescue IABP insertion in patients randomized to the non-IABP group) may complicate the interpretation of the trials’ results [26, 27]. In any case, given that mortality in cardiogenic shock remains high [23, 24], new appropriately powered and carefully designed clinical studies are warranted in order to clarify the proper indications of IABP use in this setting.
From a clinical standpoint, the fact that IABP support decreases LV energy consumption and concurrently optimizes LV mechanoenergetic efficiency and improves LV mechanical performance, contractility, and relaxation could theoretically rationalize the expansion of the indications of IABP use, beyond that of short-term hemodynamic stabilization. New potential indications could include use of long-term IABP counterpulsation as a bridge to decision making (cardiac surgery, LV assist device implantation, or transplantation), as a bridge to transplantation, or even as a bridge to myocardial recovery. Long-term counterpulsation, if proven safe and effective, would be attractive given the wide availability, percutaneous insertion, and acceptable cost of the IABP compared to LV or biventricular assist devices. To that end, converging data suggest safety and cost-effectiveness (and possibly efficacy) of long-term circulatory support with IABP in patients with end-stage heart failure [28–31]. The potential of long-term counterpulsation could possibly be enhanced by implementation of novel, fully implantable counterpulsation devices [32–37].
Limitations
Our study has several limitations. First, we used a model of iatrogenic myocardial infarction caused by LAD ligation, which differs from acute coronary syndromes with regard to influence of micro-embolization derived from atherosclerotic plaque or thrombus. Second, myocardial infarction was performed in otherwise healthy, young animals. This differs from the clinical situation which usually involves older patients with multiple comorbidities. Third, the animals used in the study weighted 30–40 kg. While we tried to account for the decreased animal weight (relative to humans) by using a 25-ml intra-aortic balloon (instead of the 40-ml intra-aortic balloon typically used clinically), we cannot exclude the possibility that increased body weight and increased balloon volume may alter the hemodynamic efficacy of IABP counterpulsation in clinical applications. Fourth, our sonomicrometry-based approach, while valid for measuring dynamic changes in LVEF, underestimates absolute LVEF values (see supplemental methods and results and Supp Fig 5).
Conclusions
In acute heart failure, IABP support optimizes LV energetic performance (decreases energy consumption and concurrently improves mechanical performance and mechanoenergetic efficiency) by LV unloading, during both ischemia and reperfusion. During reperfusion exclusively, IABP support also improves LV contractility and active relaxation, possibly due to a synergistic effect of unloading and augmentation of coronary blood flow. These findings theoretically rationalize the use of long-term IABP counterpulsation as a bridge to decision making, as a bridge to transplantation, or even as a bridge to myocardial recovery.
References
Moulopoulos, S. D., Topaz, S., & Kolff, W. J. (1962). Diastolic balloon pumping (with carbon dioxide) in the aorta: a mechanical assistance to the failing circulation. American Heart Journal, 63, 669–675.
Anderson, R. D., Ohman, E. M., Holmes, D. R., et al. (1997). Use of intraaortic balloon counterpulsation in patients presenting with cardiogenic shock: observations from the GUSTO-1 study. Journal of the American College of Cardiology, 30, 708–715.
Nanas, J. N., & Moulopoulos, S. D. (1994). Counterpulsation: historical background, technical improvements, hemodynamic and metabolic effects. Cardiology, 84, 156–167.
Schreuder, J. J., Maisano, F., Donelli, A., et al. (2005). Beat-to-beat effects of intraaortic balloon pump timing on left ventricular performance in patients with low ejection fraction. Annals of Thoracic Surgery, 79, 872–880.
Kawaguchi, O., Pae, W. E., Daily, B. B., & Pierce, W. S. (1999). Ventriculoarterial coupling with intra-aortic balloon pump in acute ischemic heart failure. Journal of Thoracic and Cardiovascular Surgery, 117, 164–171.
Khir, A. W., Price, S., Henein, M. Y., Parker, K. H., & Pepper, J. R. (2003). Intra-aortic balloon pumping: effects on left ventricular diastolic function. European Journal of Cardio-Thoracic Surgery, 24, 277–282.
Bavaria, J. E., Furukawa, S., Kreiner, G., Gupta, K. B., Streicher, J., & Edmunds, L. H., Jr. (1990). Effect of circulatory assist devices on stunned myocardium. Annals of Thoracic Surgery, 49, 123–128.
Suga, H. (2003). Global cardiac function: mechano-energetico-informatics. Journal of Biomechanics, 36, 713–720.
Kameyama, T., Asanoi, H., Ishizaka, S., & Sasayama, S. (1991). Ventricular load optimization by unloading therapy in patients with heart failure. Journal of the American College of Cardiology, 17, 199–207.
Sodums, M. T., Badke, F. R., Starling, M. R., et al. (1984). Evaluation of left ventricular contractile performance utilizing end-systolic pressure-volume relationships in conscious dogs. Circulation Research, 54, 731–739.
Glower, D. D., Spratt, J. A., Snow, N. D., et al. (1985). Linearity of the frank-starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation, 71, 994–1009.
Little, W. C., Freeman, G. L., & O’Rourke, R. A. (1985). Simultaneous determination of left ventricular end-systolic pressure-volume and pressure-dimension relationships in closed-chest dogs. Circulation, 71, 1301–1308.
Konishi, T., Nakamura, Y., Kato, I., & Kawai, C. (1992). Dependence of peak dP/dt and mean ejection rate on load and effect of inotropic agents on the relationship between peak dP/dt and left ventricular developed pressure—assessed in the isolated working rat heart and cardiac muscles. International Journal of Cardiology, 35, 333–341.
Leeuwenburgh, B. P., Steendijk, P., Helbing, W. A., & Baan, J. (2002). Indexes of diastolic RV function: load dependence and changes after chronic RV pressure overload in lambs. American Journal of Physiology. Heart and Circulatory Physiology, 282, H1350–H1358.
Burkhoff, D., Mirsky, I., & Suga, H. (2005). Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. American Journal of Physiology. Heart and Circulatory Physiology, 289, H501–H512.
Starling, M. R., Montgomery, D. G., Mancini, G. B., & Walsh, R. A. (1987). Load independence of the rate of isovolumic relaxation in man. Circulation, 76, 1274–1281.
Feigl, E. O. (1983). Coronary physiology. Physiological Reviews, 63, 1–205.
Gregg, D. E. (1963). Effect of coronary perfusion pressure or coronary flow on oxygen usage of the myocardium. Circulation Research, 13, 497–500.
Schouten, J. A., Allaart, C. P., & Westerhof, N. (1992). Effect of perfusion on force of contraction in thin papillary muscles and trabeculae from rat heart. Journal of Physiology, 451, 585–604.
Kitakaze, M., & Marbán, E. (1989). Cellular mechanism of the modulation of contractile function by coronary perfusion pressure in ferret hearts. Journal of Physiology, 414, 455–472.
Farhi, E. R., Canty, J. M., Jr., & Klocke, F. J. (1989). Effects of graded reductions in coronary perfusion pressure on the diastolic pressure-segment length relation and the rate of isovolumic relaxation in the resting conscious dog. Circulation, 80, 1458–1468.
Zile, M. R., & Brutsaert, D. L. (2002). New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation, 105, 1387–1393.
Thiele, H., Zeymer, U., Neumann, F. J., et al. (2012). Intraaortic balloon support for myocardial infarction with cardiogenic shock. New England Journal of Medicine, 367, 1287–1296.
Thiele, H., Zeymer, U., Neumann, F. J., et al. (2013). Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet, 38, 1638–1645.
Sjauw, K. D., Engström, A. E., Vis, M. M., et al. (2009). A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? European Heart Journal, 30, 459–468.
Perera, D., Lumley, M., Pijls, N., & Patel, M. R. (2013). Intra-aortic balloon pump trials: questions, answers, and unresolved issues. Circulation. Cardiovascular Interventions, 6, 317–321.
Kapelios, C. J., Terrovitis, J. V., & Nanas, J. N. (2014). Current and future applications of the intra-aortic balloon pump. Current Opinion in Cardiology, 29, 258–265.
Gjesdal, O., Gude, E., Arora, S., et al. (2009). Intra-aortic balloon counterpulsation as a bridge to heart transplantation does not impair long-term survival. European Journal of Heart Failure, 11, 709–714.
Cochran, R. P., Starkey, T. D., Panos, A. L., & Kunzelman, K. S. (2002). Ambulatory intraaortic balloon pump use as bridge to heart transplant. Annals of Thoracic Surgery, 74, 746–751.
Mark, J., Russo, M. J., Jeevanandam, V., et al. (2012). Intra-aortic balloon pump inserted through the subclavian artery: a minimally invasive approach to mechanical support in the ambulatory end-stage heart failure patient. Journal of Thoracic and Cardiovascular Surgery, 144, 951–955.
Terrovitis, J., Ntalianis, A., Kaldara, E., et al. (2012). The role of prolonged mechanical support with counterpulsation in patients with idiopathic dilated cardiomyopathy and advanced stage heart failure. European Journal of Heart Failure, 11, S87 [abstract].
Nanas, J. N., Nanas, S. N., Charitos, C. E., et al. (1987). Effectiveness of a counterpulsation device implanted on the ascending aorta. ASAIO Transactions, 10, 203–206.
Jeevanandam, V., Jayakar, D., Anderson, A. S., et al. (2002). Circulatory assistance with a permanent implantable IABP: initial human experience. Circulation, 106, I183–I188.
Koenig, S. C., Litwak, K. N., Giridharan, G. A., et al. (2008). Acute hemodynamic efficacy of a 32-ml subcutaneous counterpulsation device in a calf model of diminished cardiac function. ASAIO Journal, 54, 578–584.
Mitnovetski, S., Almeida, A. A., Barr, A., et al. (2008). Extra-aortic implantable counterpulsation pump in chronic heart failure. Annals of Thoracic Surgery, 85, 2122–2125.
Hayward, C. S., Peters, W. S., Merry, A. F., et al. (2010). Chronic extra aortic balloon counterpulsation: first-in-human pilot study in end-stage heart failure. Journal of Heart and Lung Transplantation, 29, 1427–1432.
Solanki, P. (2014). Aortic counterpulsation: C-pulse and other devices for cardiac support. Journal of Cardiovascular Translational Research, 7, 292–300.
Conflict of Interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Craig Stolen oversaw the review of this article
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees. No human studies were carried out by the authors of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 37 kb)
Supplemental Table 1
(DOCX 43 kb)
Supplemental Figure 1
(DOCX 1324 kb)
Supplemental Figure 2
(DOCX 303 kb)
Supplemental Figure 3
(DOCX 584 kb)
Supplemental Figure 4
(DOCX 558 kb)
Supplemental Figure 5
(DOCX 676 kb)
Rights and permissions
About this article
Cite this article
Malliaras, K., Charitos, E., Diakos, N. et al. Effects of Intra-aortic Balloon Pump Counterpulsation on Left Ventricular Mechanoenergetics in a Porcine Model of Acute Ischemic Heart Failure. J. of Cardiovasc. Trans. Res. 7, 810–820 (2014). https://doi.org/10.1007/s12265-014-9600-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-014-9600-6