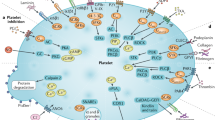
Abstract
Platelets are the primary cell mediator of thrombosis. A deficiency of platelets can result in severe bleeding defects. “Overactive” platelets contribute to life-threatening outcomes in diseases such as heart attack, stroke, and cancer. The use of platelet inhibitors for thrombosis prevention must therefore seek a delicate balance between inhibiting platelet activation and an associated increased bleeding risk. There are currently few platelet inhibitors clinically available, making the search for novel anti-platelet drug targets a major research priority. Several newly identified pathways of platelet activation may hold hope in this area. In addition, important roles for platelets beyond hemostasis have been discovered. Platelets have recently been described as mediators of diverse inflammatory diseases such as arthritis, hepatitis, malaria, and atherosclerosis. This has heightened the need to broaden our understanding of platelet functions and platelet-derived inflammatory mediators. It has also heightened interest in a continued search for new platelet inhibitors and presents new opportunities for platelet inhibitors to be used in a wide array of disease treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Platelets are small (∼1–2 μm), anucleate, megakaryocyte-derived circulating blood cells. Typical human platelet counts are 100,000–200,000/μL, making platelets the second most numerous blood cells. The most well understood function of platelets is its role as the primary cell mediator of thrombosis. A lack of platelets (thrombocytopenia), or defects in platelet function, can result in major life-threatening bleeding. Untoward or unregulated platelet activation can also have severe outcomes by initiating thrombosis and loss of blood supply to major tissue beds. Cardiovascular disease and stroke are leading causes of morbidity and mortality in the Western world, and although many processes play a role in the development of vascular disease, thrombosis is the primary event that precipitates stroke and acute coronary syndromes. There are currently few platelet-specific anti-thrombotic agents available, making direct platelet inhibitors, such as aspirin and clopidogrel (Plavix), mainstays in the long-term treatment of cardiovascular patients.
Thrombosis is the result of a complex set of interactions between platelets, coagulation factors, and the vessel wall. Thrombosis proceeds through what is often described as a stereotypic set of steps: Platelets are first activated, adhere to the vessel wall, and then aggregate with other platelets to form a stable thrombus [1, 2]. There are also many intermediate steps important in efficient thrombus formation, such as loose contacts between platelets before firm adhesion. This creates micro-environments with high local concentrations of pro-thrombotic mediators [3]. Platelets can be activated by vessel wall exposure of extracellular matrix components, by activation of the coagulation cascade generating platelet agonists (e.g., thrombin), or by factors released from activated endothelial cells and platelets (e.g., ADP, thromboxane A2, and vWF) [4–8]. Activation of receptors on resting platelets triggers a variety of intra-platelet signaling pathways, leading to subsequent steps of platelet activation, including conformational changes in receptors (e.g., GPIIb/IIIa), granule exocytosis, and the secretion of vasoactive mediators.
Platelets have an obvious vital function in hemostasis, but they also have an underappreciated immune function. This has been most studied in the context of platelet interactions with innate immune cells where platelets are known to recruit and activate neutrophils and monocytes by contact-dependent and contact-independent mechanisms [9–13]. Platelets also can influence T-cell and B-cell functions, but this is less well explored and is mainly via platelet-derived cytokines and chemokines [14–16]. Many platelet granule constituents and secreted molecules have primary roles as immune mediators (Table 1) [9, 17–19]. These platelet-derived immune mediators recruit and activate leukocytes both at the site of platelet deposition and systemically. Platelets are not only activated by a denuded endothelium, but platelets are also activated by an intact inflamed endothelium and deposit at the site of vascular inflammation without forming a complete obstructive thrombus. We have demonstrated using two separate vascular inflammation based mouse models (a transplant model and a cerebral malaria model) that thrombi are present, lining the lumen of inflamed blood vessels, without occluding the vessels (Fig. 1). Interactions between an inflamed endothelium and platelets may be mediated by increased endothelial adhesion molecule expression (e.g., P-selectin, ICAM, and VCAM) serving to localize platelets and induce platelet secretion. These events perpetuate the cycle of inflammatory interactions between platelets, endothelial cells, and leukocytes.
Activated platelets can also initiate interactions with quiescent endothelial cells and leukocytes through both contact-dependent and contact-independent mechanisms. Platelet inflammatory mediators include surface expressed adhesion molecules (P-selectin and integrins), secreted small molecules (ATP/ADP, serotonin, and glutamate), as well as chemokines, and cytokines (Table 1). Platelet-derived chemokines and cytokines, including platelet factor 4 (PF4/CXCL4), pro-platelet basic protein (ppbp and its breakdown product CXCL7/NAP-2), RANTES/CCL5, IL-1α/β, and TGF-β can recruit and activate leukocytes distant from their site of deposition [20]. Other secreted mediators such as serotonin, ADP, and prostaglandins tend to exert pro-inflammatory effects in the local environment. Exteriorized or activated adhesion molecules on the surface of adherent platelets also assist in arresting leukocytes, providing a contact-dependent mechanism for platelets to exert pro-inflammatory effects.
A complete appreciation for both the thrombotic and pro-inflammatory functions of platelets, and the concept that platelets initiate, participate in, and sustain inflammation beyond the clot, has been slow to develop. This may be due in part to a misunderstood idea that because platelets are anucleate, they cannot adapt to “environmental changes.” Because of their easily defined role in hemostasis, a long-standing focus of platelet researchers has been on platelet activation signaling pathways and integrin regulation. Recently, the focus of platelet research has broadened to include new signaling mediators and previously unrecognized roles for platelets in vascular inflammatory diseases. We will highlight a few recent discoveries with the potential to lead to the development of new anti-thrombotics and impact the current use of platelet inhibitors. We will also highlight the current state of clinical anti-thrombotic therapy.
Our Changing View of Platelet Functions
Platelets were once viewed as a unique set of cells with their own distinct functions and signaling pathways. We now know that platelets share much in common with other seemingly dissimilar cell types, such as neurons, and share many immune cell functions, presenting new pathways to target for anti-thrombotic drug development. Platelets and the synaptic termini of neurons are surprisingly similar in their composition. On the surface, these are very different cells with very different functions. However, platelets and neurons share many similarities in receptor composition and molecular constituents. Each cell expresses proteins found in few other cell types such as synucleins, ADP/ATP receptors, TPO receptors, serotonin receptors, and glutamate receptors [16, 21–25]. Most platelet dense granule constituents are also neurotransmitters (glutamate, ADP, ATP, serontonin, dopamine, Ca2+, etc.). This similarity may arise from both platelets and synaptic termini having unique functional demands due to their great distance from the “nuclear center”: the megakaryocyte in the bone marrow and the neuron cell body. Our lab has found that platelets, like neurons, express functional ionotropic glutamate receptors [25, 26]. Glutamate is a major neurotransmitter that binds to NMDA, AMPA, and kainate (KA) receptors. Glutamate induces a Ca2+ influx through NMDA receptors and a Na+ influx when glutamate binds AMPAR and KAR. Others have described that platelet-dense granules store and release glutamate, but a role for glutamate in platelet activation had not been described [27, 28]. We found that glutamate is released in large concentrations within a developing thrombus and that AMPAR and KAR signaling amplifies platelet activation. Mice that lack AMPAR and KAR subunits or mice that are treated with receptor inhibitors have prolonged time to thrombus formation in vivo. Importantly, these mice do not have any bleeding diathesis but form a delayed thrombus. This makes glutamate receptors an attractive target for the development of new anti-thrombotics. An important quality for a platelet inhibitor is an ability to blunt, but not totally block, platelet activation so as to not pose a large bleeding risk. We have found that AMPAR and KAR antagonists each prolong bleeding times and delay thrombus formation in mice and also reduce human platelet aggregation [25, 26]. Many glutamate receptor antagonists have gone through clinical trials for treatment of stroke, but none to date has proven particularly effective. It remains to be seen through clinical trial if any of these existing glutamate receptor antagonists with established safety and toxicity data can find a new use as platelet inhibitors.
Another major functional adaptation in common between platelets and neurons is that there are only two known places where pre-mRNA splicing occurs outside the nucleus: in synaptic termini and in platelets [29]. Platelets have stable pre-mRNA, and upon platelet stimulation, this pre-mRNA is spliced into mature mRNA that can be translated into protein. The importance of this discovery goes beyond just the demonstration of RNA splicing outside the nucleus; it provided a clear demonstration that despite the lack of a nucleus platelets have the ability to directly respond to environmental changes by altering their protein composition. Before this discovery, it was known that platelets had RNA, but its significance was not appreciated, and it was largely viewed to be a by-stander effect of platelet budding from the megakaryocyte. With this paper, the group headed by Andrew Weyrich helped to greatly advance a broader understanding that despite their anucleate status, platelets have a clear ability to up- or down-regulate protein expression. The relevance of this finding to health and disease remains to be demonstrated; but nonetheless, it represented a major advance in focusing platelet research in new directions. Recent publications, such as one demonstrating active platelet microRNA, have continued to build on this concept of peripheral platelet translational regulation [30].
The use of mouse models has demonstrated that in addition to forming a coronary artery occlusion at the time of myocardial infarction (MI), platelets may have a prominent role in promoting the pathogenesis of atherosclerosis. Ley and colleagues demonstrated in an ApoE−/− mouse model that platelets deposit RANTES at the site of atherosclerotic lesions, one of the first clear mechanistic descriptions of how platelets may accelerate atherosclerosis [31, 32]. This work and that by others in the mouse model have shown that platelets may be important early in atherosclerotic lesion development and its progression towards an unstable plaque. One of the major issues with mouse models of atherosclerosis is that mice do not form unstable plaques, making an appreciation for the role of platelets in MI risk in humans difficult to extrapolate. However, there have been several human clinical studies indicating that platelets may drive lesion progression and platelets may be activated at sites of a growing atherosclerotic lesion [9, 33], helping to validate this mouse model-based research.
Atherosclerosis is now seen as an immune-mediated disease, with high cholesterol and oxidized lipid products well established risk factors at the heart of its pathogenesis. Recent work by Podrez et al. has found that not only may platelets be activated at the site of atherosclerotic lesion development but also oxidized lipids may directly activate platelets via platelet CD36 [34]. This study puts platelets in the center of atherothrombosis by indicating that oxidized lipid mediated platelet activation may contribute immune mediators that promote lesion development locally and at sites distant from the actual lesion. Subsequent work by authors from this study has shown that the CD36-dependent platelet activation pathway functions through mitogen activated protein kinase (MAPK) signaling [35]. This knowledge helps to identify two new potential targets for anti-platelet drug development in the prevention and treatment of atherosclerosis: CD36 and MAPK members. However, both CD36 and MAP kinases are expressed by many cells, making the development of platelet-specific antagonists targeting these molecules potentially difficult.
Platelet factor 4 (CXCL4) was the first CXC class chemokine identified and is the most abundant protein found in platelet α-granules. PF4 is prominently known for its role in heparin-induced thrombocytopenia; but through the use of knockout mice, its pro-inflammatory role is becoming better understood. PF4−/− mice on an ApoE−/− background have reduced atherosclerotic lesion development compared with control ApoE−/− mice [36]. This study indicates an important role for this platelet-specific chemokine in the pathogenesis of atherosclerosis. Other studies have taken this knowledge into a potentially clinically relevant therapeutic development direction. PF4 forms a complex with RANTES and together create a highly pro-inflammatory environment. Through the use of peptides that disrupt PF4–RANTES associations, investigators were also able to reduce lesion development in ApoE−/− mice [37].
With a more broad appreciation for platelet functions has come a more broad appreciation of the disease processes in which platelets may have a pathogenic role. Platelets may have a central role in the development of cerebral malaria. A large percentage of children infected with Plasmodium falciparum develop stroke-like symptoms resulting in long-term neurologic complications or death. We have found that platelet depletion of mice after infection with Plasmodium berghei ANKA leads to increased survival compared with control infected mice, and that this is in part mediated by PF4 [15]. Compared with WT mice, PF4−/− mice have improved survival, decreased inflammation, and less cerebral trafficking of T-cells and monocytes [10, 15]. These data, and that of other labs, suggest that platelet antagonists may have potential utility in the treatment and prevention of cerebral malaria. Caution is needed, however, as others have demonstrated that platelets may be able to directly kill intraerythrocytic parasites in uncomplicated infections [38]. Perhaps platelets have differential roles in response to different Plasmodium species or physiologic outcomes of infection.
Rheumatoid arthritis (RA) is a debilitating inflammatory joint disease. The pathogenesis of RA is not well understood and likely multi-factorial. In a recent paper, platelets were shown to help drive the pathogenesis of RA in a mouse model [39]. In particular, platelet-derived microparticles were recovered from RA joints and contributed IL-1 to incite the inflammatory response. Blocking the signaling of IL-1 reduced lesion development. Similarly, transplant rejection begins at the vascular–immune interface and platelets may have a functional role in driving graft rejection. Platelet-derived soluble CD154 in a cardiac allograft model has been shown sufficient to initiate cardiac allograft rejection independent of any cellular source for this molecule [40]. We have also found that platelets sustain vascular inflammation and recruit leukocytes to the transplant vasculature [41]. Can platelet inhibitors be useful in the treatment of RA and as adjunctive therapy for the prolongation of graft survival? More study is needed to address these potential clinical utilities for platelet inhibitors.
Current Clinical State of Anti-platelet Therapies
While investigators have made great strides in identifying new platelet activation pathways and relevant disease processes, clinically, anti-platelet therapy has continued to focus on acute coronary syndromes. As more basic science evidence accumulates on the importance of some of these newly identified platelet signaling pathways and the broad disease implications of platelet functions, we may see new directions in clinical platelet research. The search for agents that interfere with platelet aggregation and thrombus formation is currently driven by percutaneous coronary innovations, with a particular focus on preventing thrombosis following stent implantation. We will briefly discuss the current state of common anti-platelet therapies as well as emerging and investigational agents.
Aspirin
Aspirin is the current cornerstone of anti-platelet therapies. It acts by permanently inactivating prostaglandin H synthase 1 and synthase 2, or cyclooxygenase-1 and cyclooxygenase-2 as they are otherwise known. This inhibition leads to decreased synthesis of thromboxane A2 (TxA2) and subsequently TxA2-induced platelet aggregation [42]. Numerous studies have demonstrated the effectiveness of aspirin in the treatment and prevention of acute coronary syndromes, including myocardial infarction, stroke, and peripheral artery disease [43].
An important issue in aspirin therapy is the concept of “aspirin resistance,” a term coined to describe the lack of a measureable change in platelet function ex vivo, inhibited TxA2 synthesis in vivo, or preventing thrombotic events in individuals [42]. Such patients are at higher risk of ischemic events. It has been suggested that these select patients may require more than the recommended 81 mg of aspirin daily to achieve an ideal protective effect, although it has also been observed that other factors may be at play such as drug interactions and TxA2 production from other sources [42, 43].
Thienopyridines
Along with aspirin, thienopyridines are the other common class of anti-platelet agents in clinical use today. After activation by the cytochrome P450 system, these drugs bond with the P2Y12 receptor on the platelet surface. ADP has an important role in the amplification of platelet aggregation, and thienopyridines block the binding of ADP to the P2Y12 receptor. This class of drugs includes ticlopidine, clopidogrel, and prasugrel [43].
Ticlopidine is the prototype thienopyridine, and its use was shown to be effective in treating stroke and myocardial infarction, particularly in combination with aspirin. However, it was also found to be linked to thrombotic thrombocytopenia purpura, neutropenia, and bone marrow aplasia [43, 44]. Thus, it has since given way to the newer thienopyridine clopidogrel. The Clopidogrel Aspirin Stent Interventional Cooperative Study (CLASSICS) demonstrated clopidogrel to be as effective as ticlopidine with a lower incidence of side effects, and it has since become the first-line thienopyridine for dual anti-platelet therapy with aspirin [45].
As with aspirin, there is a subset of patients who harbor “resistance” or “non-responsiveness” to clopidogrel. The prevalence of clopidogrel resistance has been estimated by various sources to be between 8% and 30%, but depends on dose and time from ingestion. This is associated with insufficient levels of the active clopidogrel metabolite, possibly due to variations in cytochrome P450 (CYP) activity, as well as a genetic predisposition to decreased intestinal absorption [46, 47]. There are polymorphisms in the genes coding for CYP enzymes, with common alleles in CYP2C19 having reduced function resulting in lower clopidogrel active metabolite concentrations [48, 49]. Carriers of a reduced-function CYP2C19 allele have diminished platelet inhibition and a higher rate of adverse cardiovascular events than do noncarriers [50, 51]. There is some evidence that a higher loading and maintenance dose of clopidogrel may be an effective strategy to counter clopidogrel resistance [52, 53].
Prasugrel is a recently FDA-approved third-generation thienopyridine for use in acute coronary syndromes treated with PCI. Phase I and II studies have shown that prasugrel has rapid effect onset and generation of active metabolites superior to clopidogrel with less non-responsiveness and greater ADP inhibition. Prasugrel was approved after the TRITON-TIMI 38 trial revealed that it was superior to clopidogrel when combined with aspirin in reducing the primary endpoints of cardiovascular death, nonfatal MI, and nonfatal stroke [43, 54, 55]. However, it is associated with a significantly increased bleeding risk.
Glycoprotein IIb/IIIa Inhibitors
GPIIb/IIIa is a platelet receptor that has a central role in aggregation by cross-linking platelets via fibrinogen and is the target of a potent class of anti-thrombotic agents. These inhibitors are administered by intravenous route only and are limited to acute settings, typically patients with acute coronary syndromes or who are undergoing PCI. The three drugs in this class are abciximab (a monoclonal antibody against the IIb/IIIa receptor), and eptifibatide and tirofiban (peptide and non-peptide receptor inhibitors, respectively) [56]. All three of these agents have been tested extensively in clinical trials, and meta-analyses have shown benefit versus placebo. The TARGET trial is one of the few studies to directly compare these agents, having compared abciximab and tirofiban directly in patients undergoing PCI. Although abciximab was found superior at 30 days in various composite end points, at 6 months, there was no significant difference. A sub-group analysis found that abciximab’s benefit was seen primarily in patients with acute coronary syndrome undergoing PCI at both 30 days and 6 months, but not at 1 year. This has been attributed to the dosing regimen of abciximab (a large bolus followed by continuous infusion) used in the trial, as well as its high degree of activity in the first hour after initiation, a critical period following PCI [56, 57]. There is no evidence currently that abciximab is superior to tirofiban over the long term, although it does have more data from large clinical trials supporting its use. Eptifibitide’s advantage lies in its cost and its early onset of anti-platelet action similar to abciximab. Bleeding is a major complication with this class of drugs. Thrombocytopenia is the other major problem associated with these drugs, which appears to be more associated with abciximab than tirofiban or eptifibitide [58].
Emerging Therapies
There are currently compounds in development and clinical trials as platelet antagonists. Elinogrel is an investigational P2Y12 receptor inhibitor, which unlike other thienopyridines, is reversible in its blockade of ADP-mediated platelet aggregation with near complete reversal within 24 h of administration [59]. Likewise, ticagrelor is a cyclopentyltriazolopyrimidine derivative currently awaiting FDA approval that also reversibly acts on the P2Y12 receptor [60]. Protease-activated-receptor-1 (PAR-1) is another target of new drug development. In addition to its functions in the coagulation cascade, thrombin activates platelets by cleavage of PAR receptors. There are now two PAR-1 inhibitors under investigation, SCH530348 and E5555. SCH530348 is currently in two large, multi-center phase III trials, while E5555 in currently under phase II study [61, 62]. It remains to be seen if the promises of either compound gain approval for general clinical use.
The omega-3 fatty acids, eicosapentaenoic acid and docosahexaenoic acid, are found in oily fish and are compounds known to have anti-platelet effects. Although previous research into their potential anti-platelet effects has been inconclusive, a possible synergistic effect with standard dual anti-platelet therapy, namely aspirin and clopidogrel, may exist. Thus far, a few small studies have shown that the addition of omega-3 fatty acids to clopidogrel and aspirin or aspirin alone were able to potentiate platelet response to these agents as measured by ex vivo platelet assays [63–65]. A retrospective study looked at patients treated with high-dose fish oil, aspirin, and clopidogrel and found no statistically significant association with bleeding events compared with matched controls [66]. The approach of combining these omega-3 fatty acids with aspirin and clopidogrel appears to be safe and may represent a simple new means to increase responses to standard anti-platelet therapies without a large increase in bleeding risk.
The major complication of any platelet inhibitor is an increased bleeding risk, making platelet antagonists that can be reversed by a defined antidote a large step forward in clinical therapy. The use of aptamers with complementary antidotes as platelet adhesion antagonists is currently in research development and early clinical trial [67, 68]. Aptamers are single-stranded nucleic acid molecules that directly and specifically inhibit protein functions [69–71]. Rationally designed aptamers that specifically target a part of the VWF A1 domain (the platelet GPIbα binding domain) with an antidote molecule that quickly reverses the aptamer function has shown promise in this area [72].
Summary
Our knowledge of platelet activation pathways and platelet functions has greatly expanded in the past decade. The emerging role of platelets in driving vascular inflammation has likewise seen a great increase in significant publications. These findings will continue to fuel the development of platelet antagonists and the application of these drugs to many new disease processes.
References
Andrews, R. K., & Berndt, M. C. (2004). Platelet physiology and thrombosis. Thrombosis Research, 114(5–6), 447–453.
Buller, H. R., & Ten Cate, T. (1995). Coagulation and platelet activation pathways. A review of the key components and the way in which these can be manipulated. European Heart Journal, 16(Suppl L), 8–10.
Brass, L. F., Zhu, L., & Stalker, T. J. (2005). Minding the gaps to promote thrombus growth and stability. Journal of Clinical Investigation, 115(12), 3385–3392.
Catella-Lawson, F. (2001). Vascular biology of thrombosis: Platelet-vessel wall interactions and aspirin effects. Neurology, 57(5 Suppl 2), S5–S7.
Fitzgerald, D. J. (2001). Vascular biology of thrombosis: The role of platelet-vessel wall adhesion. Neurology, 57(5 Suppl 2), S1–S4.
Fuse, I. (1996). Disorders of platelet function. Critical Reviews in Oncology/Hematology, 22(1), 1–25.
Coughlin, S. R. (1994). Molecular mechanisms of thrombin signaling. Seminars in Hematology, 31(4), 270–277.
Coughlin, S. R. (2005). Protease-activated receptors in hemostasis, thrombosis and vascular biology. Journal of Thrombosis and Haemostasis, 3(8), 1800–1814.
Gawaz, M., Langer, H., & May, A. E. (2005). Platelets in inflammation and atherogenesis. Journal of Clinical Investigation, 115(12), 3378–3384.
Srivastava, K., Field, D. J., Aggrey, A., Yamakuchi, M., & Morrell, C. N. (2010). Platelet factor 4 regulation of monocyte klf4 in experimental cerebral malaria. PLoS ONE, 5(5), e10413. doi:10.1371/journal.pone.0010413.
Weber, C. (2005). Platelets and chemokines in atherosclerosis: Partners in crime. Circulation Research, 96(6), 612–616.
Zarbock, A., Polanowska-Grabowska, R. K., & Ley, K. (2007). Platelet-neutrophil-interactions: Linking hemostasis and inflammation. Blood Reviews, 21(2), 99–111.
Morrell, C. N., Sun, H., Swaim, A. M., & Baldwin, W. M., 3rd. (2007). Platelets an inflammatory force in transplantation. American Journal of Transplantation, 7(11), 2447–2454.
Kirk, A. D., Morrell, C. N., & Baldwin, W. M., 3rd. (2009). Platelets influence vascularized organ transplants from start to finish. American Journal of Transplantation, 9(1), 14–22.
Srivastava, K., Cockburn, I. A., Swaim, A., Thompson, L. E., Tripathi, A., Fletcher, C. A., et al. (2008). Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host & Microbe, 4(2), 179–187.
Lang, P. A., Contaldo, C., Georgiev, P., El-Badry, A. M., Recher, M., Kurrer, M., et al. (2008). Aggravation of viral hepatitis by platelet-derived serotonin. Natural Medicines, 14(7), 756–761. doi:10.1038/nm1780.
Massberg, S., Konrad, I., Schurzinger, K., Lorenz, M., Schneider, S., Zohlnhoefer, D., et al. (2006). Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. The Journal of Experimental Medicine, 203(5), 1221–1233.
Gawaz, M. (2004). Role of platelets in coronary thrombosis and reperfusion of ischemic myocardium. Cardiovascular Research, 61(3), 498–511.
Langer, H., May, A. E., Daub, K., Heinzmann, U., Lang, P., Schumm, M., et al. (2006). Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circulation Research, 98(2), e2–e10.
von Hundelshausen, P., Weber, K. S., Huo, Y., Proudfoot, A. E., Nelson, P. J., Ley, K., et al. (2001). Rantes deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation, 103(13), 1772–1777.
Reheman, A., Tasneem, S., Ni, H., & Hayward, C. P. (2010). Mice with deleted multimerin 1 and alpha-synuclein genes have impaired platelet adhesion and impaired thrombus formation that is corrected by multimerin 1. Thrombosis Research, 125(5), e177–e183. doi:10.1016/j.thromres.2010.01.009.
Michell, A. W., Luheshi, L. M., & Barker, R. A. (2005). Skin and platelet alpha-synuclein as peripheral biomarkers of Parkinson's disease. Neuroscience Letters, 381(3), 294–298. doi:10.1016/j.neulet.2005.02.030.
Ehrenreich, H., Hasselblatt, M., Knerlich, F., von Ahsen, N., Jacob, S., Sperling, S., et al. (2005). A hematopoietic growth factor, thrombopoietin, has a proapoptotic role in the brain. Proceedings of the National Academy of Sciences of the United States of America, 102(3), 862–867. doi:10.1073/pnas.0406008102.
Ferrarese, C., Zoia, C., Pecora, N., Piolti, R., Frigo, M., Bianchi, G., et al. (1999). Reduced platelet glutamate uptake in parkinson's disease. Journal of Neural Transmission, 106(7–8), 685–692.
Morrell, C. N., Sun, H., Ikeda, M., Beique, J. C., Swaim, A. M., Mason, E., et al. (2008). Glutamate mediates platelet activation through the ampa receptor. The Journal of Experimental Medicine, 205(3), 575–584.
Sun, H., Swaim, A., Herrera, J. E., Becker, D., Becker, L., Srivastava, K., et al. (2009). Platelet kainate receptor signaling promotes thrombosis by stimulating cyclooxygenase activation. Circulation Research, 105, 595–603.
Ferrarese, C., Sala, G., Riva, R., Begni, B., Zoia, C., Tremolizzo, L., et al. (2001). Decreased platelet glutamate uptake in patients with amyotrophic lateral sclerosis. Neurology, 56(2), 270–272.
Berk, M., Plein, H., & Ferreira, D. (2001). Platelet glutamate receptor supersensitivity in major depressive disorder. Clinical Neuropharmacology, 24(3), 129–132.
Denis, M. M., Tolley, N. D., Bunting, M., Schwertz, H., Jiang, H., Lindemann, S., et al. (2005). Escaping the nuclear confines: Signal-dependent pre-mrna splicing in anucleate platelets. Cell, 122(3), 379–391.
Landry, P., Plante, I., Ouellet, D. L., Perron, M. P., Rousseau, G., & Provost, P. (2009). Existence of a microrna pathway in anucleate platelets. Nature Structural & Molecular Biology, 16(9), 961–966.
Huo, Y., & Ley, K. F. (2004). Role of platelets in the development of atherosclerosis. Trends in Cardiovascular Medicine, 14(1), 18–22.
Huo, Y., Schober, A., Forlow, S. B., Smith, D. F., Hyman, M. C., Jung, S., et al. (2003). Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein e. Natural Medicines, 9(1), 61–67.
Michiels, J. J., & Gawaz, M. (2007). Preface: platelets in inflammation and atherothrombosis. Seminars in Thrombosis and Hemostasis, 33(2), 119–122.
Podrez, E. A., Byzova, T. V., Febbraio, M., Salomon, R. G., Ma, Y., Valiyaveettil, M., et al. (2007). Platelet cd36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Natural Medicines, 13(9), 1086–1095.
Chen, K., Febbraio, M., Li, W., & Silverstein, R. L. (2008). A specific cd36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circulation Research, 102(12), 1512–1519.
Sachais, B. S., Turrentine, T., Dawicki McKenna, J. M., Rux, A. H., Rader, D., & Kowalska, M. A. (2007). Elimination of platelet factor 4 (pf4) from platelets reduces atherosclerosis in c57bl/6 and apoe−/− mice. Thrombosis and Haemostasis, 98(5), 1108–1113.
Koenen, R. R., von Hundelshausen, P., Nesmelova, I. V., Zernecke, A., Liehn, E. A., Sarabi, A., et al. (2009). Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Natural Medicines, 15(1), 97–103.
McMorran, B. J., Marshall, V. M., de Graaf, C., Drysdale, K. E., Shabbar, M., Smyth, G. K., et al. (2009). Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science, 323(5915), 797–800.
Boilard, E., Nigrovic, P. A., Larabee, K., Watts, G. F., Coblyn, J. S., Weinblatt, M. E., et al. (2010). Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science, 327(5965), 580–583.
Xu, H., Zhang, X., Mannon, R. B., & Kirk, A. D. (2006). Platelet-derived or soluble cd154 induces vascularized allograft rejection independent of cell-bound cd154. Journal of Clinical Investigation, 116(3), 769–774.
Morrell, C. N., Murata, K., Swaim, A. M., Mason, E., Martin, T. V., Thompson, L. E., et al. (2008). In vivo platelet–endothelial cell interactions in response to major histocompatibility complex alloantibody. Circulation Research, 102(7), 777–785.
Patrono, C., Garcia Rodriguez, L. A., Landolfi, R., & Baigent, C. (2005). Low-dose aspirin for the prevention of atherothrombosis. The New England Journal of Medicine, 353(22), 2373–2383. doi:10.1056/NEJMra052717.
Gurbel, P. A., & Tantry, U. S. (2010). Combination antithrombotic therapies. Circulation, 121(4), 569–583. doi:10.1161/CIRCULATIONAHA.109.853085.
Symeonidis, A., Kouraklis-Symeonidis, A., Seimeni, U., Galani, A., Giannakoulas, N., Fragopanagou, E., et al. (2002). Ticlopidine-induced aplastic anemia: Two new case reports, review, and meta-analysis of 55 additional cases. American Journal of Hematology, 71(1), 24–32. doi:10.1002/ajh.10150.
Bertrand, M. E., Rupprecht, H. J., Urban, P., & Gershlick, A. H. (2000). Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: The clopidogrel aspirin stent international cooperative study (classics). Circulation, 102(6), 624–629.
Staritz, P., Kurz, K., Stoll, M., Giannitsis, E., Katus, H. A., & Ivandic, B. T. (2009). Platelet reactivity and clopidogrel resistance are associated with the h2 haplotype of the p2y12-adp receptor gene. International Journal of Cardiology, 133(3), 341–345. doi:10.1016/j.ijcard.2007.12.118.
De Miguel, A., Ibanez, B., & Badimon, J. J. (2008). Clinical implications of clopidogrel resistance. Thrombosis and Haemostasis, 100(2), 196–203.
Hulot, J. S., Bura, A., Villard, E., Azizi, M., Remones, V., Goyenvalle, C., et al. (2006). Cytochrome p450 2c19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood, 108(7), 2244–2247. doi:10.1182/blood-2006-04-013052.
Brandt, J. T., Close, S. L., Iturria, S. J., Payne, C. D., Farid, N. A., Ernest, C. S., 2nd, et al. (2007). Common polymorphisms of cyp2c19 and cyp2c9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. Journal of Thrombosis and Haemostasis, 5(12), 2429–2436. doi:10.1111/j.1538-7836.2007.02775.x.
Mega, J. L., Close, S. L., Wiviott, S. D., Shen, L., Hockett, R. D., Brandt, J. T., et al. (2009). Cytochrome p-450 polymorphisms and response to clopidogrel. The New England Journal of Medicine, 360(4), 354–362. doi:10.1056/NEJMoa0809171.
Simon, T., Verstuyft, C., Mary-Krause, M., Quteineh, L., Drouet, E., Meneveau, N., et al. (2009). Genetic determinants of response to clopidogrel and cardiovascular events. The New England Journal of Medicine, 360(4), 363–375. doi:10.1056/NEJMoa0808227.
Bonello, L., Camoin-Jau, L., Armero, S., Com, O., Arques, S., Burignat-Bonello, C., et al. (2009). Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. The American Journal of Cardiology, 103(1), 5–10. doi:10.1016/j.amjcard.2008.08.048.
Tavassoli, N., Voisin, S., Carrie, D., Lapeyre-Mestre, M., Galinier, M., Montastruc, J. L., et al. (2010). High maintenance dosage of clopidogrel is associated with a reduced risk of stent thrombosis in clopidogrel-resistant patients. American Journal of Cardiovascular Drugs, 10(1), 29–35. doi:10.2165/11318260-000000000-00000/4.
Wiviott, S. D., Braunwald, E., McCabe, C. H., Horvath, I., Keltai, M., Herrman, J. P., et al. (2008). Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the triton-timi 38 trial: A subanalysis of a randomised trial. Lancet, 371(9621), 1353–1363. doi:10.1016/S0140-6736(08)60422-5.
Michelson, A. D., Frelinger, A. L., 3rd, Braunwald, E., Downey, W. E., Angiolillo, D. J., Xenopoulos, N. P., et al. (2009). Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the triton-timi 38 trial. European Heart Journal, 30(14), 1753–1763. doi:10.1093/eurheartj/ehp159.
Moliterno, D. J. (2008). Advances in antiplatelet therapy for acs and pci. Journal of Interventional Cardiology, 21(Suppl 1), S18–S24. doi:10.1111/j.1540-8183.2008.00409.x.
Roffi, M., Moliterno, D. J., Meier, B., Powers, E. R., Grines, C. L., DiBattiste, P. M., et al. (2002). Impact of different platelet glycoprotein iib/iiia receptor inhibitors among diabetic patients undergoing percutaneous coronary intervention: Do tirofiban and reopro give similar efficacy outcomes trial (target) 1-year follow-up. Circulation, 105(23), 2730–2736.
Tuhta, A. G., Yesildag, O., & Koprulu, D. (2006). Tirofiban-associated acute thrombocytopenia. Acta Cardiologica, 61(5), 577–579.
Berger, J. S., Roe, M. T., Gibson, C. M., Kilaru, R., Green, C. L., Melton, L., et al. (2009). Safety and feasibility of adjunctive antiplatelet therapy with intravenous elinogrel, a direct-acting and reversible p2y12 adp-receptor antagonist, before primary percutaneous intervention in patients with st-elevation myocardial infarction: The early rapid reversal of platelet thrombosis with intravenous elinogrel before pci to optimize reperfusion in acute myocardial infarction (erase mi) pilot trial. American Heart Journal, 158(6), 998–1004. e1001.
Wallentin, L., Becker, R. C., Budaj, A., Cannon, C. P., Emanuelsson, H., Held, C., et al. (2009). Ticagrelor versus clopidogrel in patients with acute coronary syndromes. The New England Journal of Medicine, 361(11), 1045–1057. doi:10.1056/NEJMoa0904327.
Goto, S., Yamaguchi, T., Ikeda, Y., Kato, K., Yamaguchi, H., & Jensen, P. (2010). Safety and exploratory efficacy of the novel thrombin receptor (par-1) antagonist sch530348 for non-st-segment elevation acute coronary syndrome. Journal of Atherosclerosis and Thrombosis, 17(2), 156–164.
Serebruany, V. L., Kogushi, M., Dastros-Pitei, D., Flather, M., & Bhatt, D. L. (2009). The in-vitro effects of e5555, a protease-activated receptor (par)-1 antagonist, on platelet biomarkers in healthy volunteers and patients with coronary artery disease. Thrombosis and Haemostasis, 102(1), 111–119. doi:10.1160/TH08-12-0805.
Gajos, G., Rostoff, P., Undas, A., & Piwowarska, W. (2010). Effects of polyunsaturated omega-3 fatty acids on responsiveness to dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: The omega-pci (omega-3 fatty acids after pci to modify responsiveness to dual antiplatelet therapy) study. Journal of the American College of Cardiology, 55(16), 1671–1678. doi:10.1016/j.jacc.2009.11.080.
Lev, E. I., Leshem-Lev, D., Mager, A., Vaknin-Assa, H., Harel, N., Zimra, Y., et al. (2010). Circulating endothelial progenitor cell levels and function in patients who experienced late coronary stent thrombosis. European Heart Journal. doi:10.1093/eurheartj/ehq184.
Lev, E. I., Solodky, A., Harel, N., Mager, A., Brosh, D., Assali, A., et al. (2010). Treatment of aspirin-resistant patients with omega-3 fatty acids versus aspirin dose escalation. Journal of the American College of Cardiology, 55(2), 114–121. doi:10.1016/j.jacc.2009.08.039.
Watson, P. D., Joy, P. S., Nkonde, C., Hessen, S. E., & Karalis, D. G. (2009). Comparison of bleeding complications with omega-3 fatty acids + aspirin + clopidogrel-versus-aspirin + clopidogrel in patients with cardiovascular disease. The American Journal of Cardiology, 104(8), 1052–1054. doi:10.1016/j.amjcard.2009.05.055.
Oney, S., Nimjee, S. M., Layzer, J., Que-Gewirth, N., Ginsburg, D., Becker, R. C., et al. (2007). Antidote-controlled platelet inhibition targeting von willebrand factor with aptamers. Oligonucleotides, 17(3), 265–274. doi:10.1089/oli.2007.0089.
Dyke, C. K., Steinhubl, S. R., Kleiman, N. S., Cannon, R. O., Aberle, L. G., Lin, M., et al. (2006). First-in-human experience of an antidote-controlled anticoagulant using rna aptamer technology: A phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor ixa activity. Circulation, 114(23), 2490–2497. doi:10.1161/CIRCULATIONAHA.106.668434.
Nimjee, S. M., Rusconi, C. P., Harrington, R. A., & Sullenger, B. A. (2005). The potential of aptamers as anticoagulants. Trends in Cardiovascular Medicine, 15(1), 41–45. doi:10.1016/j.tcm.2005.01.002.
Nimjee, S. M., Rusconi, C. P., & Sullenger, B. A. (2005). Aptamers: An emerging class of therapeutics. Annual Review of Medicine, 56, 555–583. doi:10.1146/annurev.med.56.062904.144915.
Rusconi, C. P., Roberts, J. D., Pitoc, G. A., Nimjee, S. M., White, R. R., Quick, G., Jr., et al. (2004). Antidote-mediated control of an anticoagulant aptamer in vivo. Nature Biotechnology, 22(11), 1423–1428. doi:10.1038/nbt1023.
Becker, R. C., Oney, S., Becker, K. C., & Sullenger, B. (2009). Antidote-controlled antithrombotic therapy targeting factor ixa and von willebrand factor. Annals of the New York Academy of Sciences, 1175, 61–70. doi:10.1111/j.1749-6632.2009.05017.x.
Acknowledgements
Craig N. Morrell is supported by National Institutes of Health grants HL094547, HL093179, and R01HL093179-02S109. Dr. Block is supported by National Institutes of Health grant 1R21HL102582-01 and a research grant from GlaxoSmithKline. Dr. Ombrello is supported by 2T32HL007937-11 through the National Heart, Lung, and Blood Institute. This publication was also made possible by Grant Number KL2 RR 024136 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ombrello, C., Block, R.C. & Morrell, C.N. Our Expanding View of Platelet Functions and Its Clinical Implications. J. of Cardiovasc. Trans. Res. 3, 538–546 (2010). https://doi.org/10.1007/s12265-010-9213-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-010-9213-7