Abstract
Great strides have been made over the last two decades in the management of patients with rhythm disorders. Despite this, however, the remaining critical problems of stroke related to atrial fibrillation or as a result of radiofrequency ablation require innovative solutions to fully realize the potential of these recent advances. Similarly, implanted cardiac devices have revolutionized the care of patients with bradyrhythmias and tachyarrhythmias. Dyssynchronus ventricular pacing associated with present devices; however, results in heart failure, tricuspid regurgitation, and inappropriate device therapy once again create a demand for creative solutions. While not technically an arrhythmia, epilepsy management today is riddled with many of the problems that plagued cardiac arrhythmia management previously, and thus an appreciation of the similarities in requirement for investigative solutions may yield groundbreaking solutions. In this paper, we describe some novel methods to reduce complications associated with rhythm disorders and their treatment and apply the lessons learned from cardiovascular arrhythmia management to the brain. These include: a method to reduce coagulum formation and thus subsequent thromboembolism with indwelling catheters specifically during radiofrequency ablation procedures; a technique to ligate the left atrial appendage through percutaneous subxiphoid pericardial access; development and testing of a novel intramyocardial pace-sense lead, particularly used in a unique anatomic location (the atrioventricular septum) to allow pacing the ventricles in a relatively synchronous manner without crossing the tricuspid valve or entering the coronary sinus; finally, novel modifications of the cardiovascular mapping and ablation techniques used for the management of the central nervous system disorders primarily via the venous drainage of the brain. Innovative and potential solutions to treat the patient with arrhythmia are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electrophysiological studies, radiofrequency ablation, and implantable devices have thoroughly revolutionized the treatment of patients with symptomatic tachyarrhythmia and bradyrhythmia. Benefits range from quality-of-life improvement to preventing sudden death [1–10]. Just three decades ago, patients with symptomatic supraventricular tachycardia that failed drug therapy would have attempted open-heart surgical treatment with high morbidity and with unacceptable morbidity and mortality. Complete endovascular approaches to map and ablate accessory pathways and atrial input to the atrioventricular (AV) node have all but nearly obviated the need for open surgical procedures for these disorders.
In the last 10 years, major strides in the invasive treatment of atrial fibrillation have occurred [1]. The last 10 years have seen major strides in the management of atrial fibrillation including the appreciation of the role of the great thoracic veins including the pulmonary veins and the genesis of atrial fibrillation and thereby presenting a target for ablation [11]. Further and even more recent approaches in managing atrial fibrillation have included targeting the retroatrial ganglionated plexii, the vein of Marshall, and fragmented signals in the atria to improve success rate with invasive procedures.
With each new advance, new opportunities to innovate and find solutions that enhance success rates in each of these subtechniques abound.
The newer methods of treatment have also been associated with unrecognized complications including catastrophic thromboembolism, atrial esophageal fistula [12] formation, and pacemaker-related myocardial dysfunction. Since we need to maintain the success associated with these new vistas of treatment, these complications demand yet further innovation.
Another important requirement for the type of innovation necessitated by the advances, further needs, and complications associated with present solutions for arrhythmia patients is the need for platform technology. Such technology involves not only an enabling invention that allows the use of further multiple creative solutions for the same disorder but also for applying to a completely different organ system.
In this paper, we present the following technologies developed at Mayo Clinic Rochester, MN, USA.
-
1.
A novel circuit and technique to reduce coagulum formation on indwelling catheters and on electrodes including during radiofrequency ablation energy delivery
-
2.
A percutaneous subxiphoid intrapericardial technique to ligate the left atrial appendage to prevent thromboembolism in patients with atrial fibrillation
-
3.
A newly developed intramyocardial bipolar pacing and sensing lead and a novel method to synchronously pace the ventricle without crossing the tricuspid valve or entering the coronary sinus
-
4.
Technique, method, and devices to map and ablate in the central nervous system via the venous drainage of the brain
For each of these inventions, we describe the clinical problem attempting to be solved, results of our experience with the invention, and the scope of the problem solved with the innovation.
Reducing Invasive Procedural Risk by Preventing Coagulum Formation
Background
Thromboembolic stroke resulting from coagulum formation on catheters and ablation electrode surfaces is a serious complication seen with radiofrequency ablation procedures [13]. The primary method used by electrophysiologists to minimize this risk is intravenous heparinization prior to ablation in the left atrium and ventricle. Despite documented therapeutic activation clotting times from heparinization, about 2% of complex ablations result in thromboembolism [14, 15]. The reason why coagulum formed despite complete heparinization is likely due to the thrombin-independent nature of this process. Coagulum primarily results from direct heat-related denaturation of fibrinogen to form the fibrin clot, i.e., coagulum. Prior studies have shown that fibrinogen binds to the surface of catheters and sheaths within seconds of exposure [16]. Thus, a critical step in preventing coagulum formation on surfaces is preventing the adherence of fibrinogen itself. At neutral pH, fibrinogen is negatively charged, and this binding is possibly through electrostatic mechanisms [17]. Based on these facts, we hypothesized that maintenance of negative charge on catheters and ablation electrode surfaces during ablation or exposure of blood will reduce the risk of coagulum formation and thus also decrease subsequent thromboembolism.
The Invention
WeFootnote 1 developed a novel circuit (Fig. 1) that allows concurrent application of negative charge onto the ablation and mapping electrodes during radiofrequency ablation [18]. Important variations of the novel circuit and technique include the ability to simultaneously deliver and maintain negative charge on all electrode surfaces as well as the catheter body.
Experimental Results
We first demonstrated, using atomic force microscopy, that fibrinogen molecules were weakly bound to a negatively charged surface. Conversely, with the application of a layer of positively charged nickel ions, fibrinogen adherence to the surface with high affinity resulted [19, 20] (Fig. 2).
Atomic force microscopy images acquired with a Veeco MultiMode AFM (Plainview, NY, USA). Top panel shows an image acquired in liquid with fibrinogen molecules deposited on a surface that attracts fibrinogen with high affinity. The clusters of white are fibrinogen molecules undergoing spontaneous thrombosis. Bottom panel: atomic force microscopy image showing fibrinogen on a negatively charged surface with streaks indicating movement of the fibrinogen molecule being displaced by the atomic force microscope probe
We then assessed [20] the effects of the application of charge during catheter placement without ablation using scanning electron microscopy (Fig. 3). Without negative charge, moderate amounts of coagulum were seen. When positive charge was applied, thick confluent coagulum coating the entire catheter tip was observed. When negative charge was applied, however, markedly decreased coagulum was observed on the catheter tip with large portions of the catheter tip being entirely free of coagulum [20]. Using high-magnification scanning electron microscopy, the classic lace-like pattern of fibrin clot was confirmed on the neutral or positively charged catheter tips. Following this, we assessed efficacy of charge application during ablation. In ex vivo experiments with ablation with negatively charged catheter tips, scanned surface clot coverage was noted similar to the results obtained without energy delivery (Figs. 4 and 5). These experiments were done by deliberately producing variable amounts of charge injection using variable resistors to define a therapeutic range in this model for the beneficial effects of charge delivery.
Close-up photographs of the electrode surface with positive charge (left panel) and negative charge being applied during contact with blood. The bottom panels show the corresponding scanning electron micrographs. Confluent and multilayer coagulum is seen with positive charge being applied, whereas minimal coagulum is seen with negative charge with areas on high-power magnification showing no clot coverage
a–d Similar data as shown in Fig. 4 with radiofrequency energy being delivered through the distal electrode. Marked decreased in surface electrode coverage with coagulum is noted when negative charge was applied (c) when compared to either neutral or positive charge
We then performed a series of in vivo canine experiments to test our technology in the beating heart (Lim et al., manuscript in progress). In these experiments, we have further confirmed the decreased adherence of fibrinogen on ablation electrodes and thus decreased char formation during electrode heating with radiofrequency ablation when negative charge was concurrently applied to the electrode surface (Fig. 6). These experiments have also verified that ablative lesions and electrogram integrity were similar with and without charge delivery. Importantly, the in vivo experiments have allowed us to demonstrate a therapeutic range for concurrent charge application with beneficial effects seen at significantly lower charge delivery than levels at which any arrhythmia, including minimal, ectopy is seen. An additional benefit in preventing coagulum on surfaces during direct current charge application documented in our study is the presence of surface electrolysis possibly acting as a physical “cleansing” agent supplementing the demonstrated fibrinogen-stabilizing effects of negative charge application.
Close-up photograph of the distal and proximal electrodes of an ablation catheter. In the left panel, negative charge was applied through our novel system during radiofrequency energy delivery with the catheter placed in the left atrium. In the right panel, negative charge was not delivered with the catheter placed at a similar location with identical power, temperature, and duration settings during radiofrequency energy delivery. Coagulum formed in this instance when negative charge was not being concurrently applied
Potential Application and Impact of Innovation
Thromboembolism remains a catastrophic complication despite present best efforts to prevent it during invasive left heart procedures. Since coagulum formation is not directly prevented with heparinization, our novel system of placing negative charge on the catheter and electrode holds promise for mitigating this risk during clinical ablation.
Heat-denatured fibrinogen (coagulum) once formed can be a nidus for clot propagation and larger soft thrombus formation. Further, even without coagulum statis-enlarged sheaths or catheter in the circulation may form thrombus. Since soft thrombus formation is proven to be inhibited with heparinization, initial use of our system will continue to require heparinization during atrial fibrillation ablation.
We are also evaluating our system [18] by placing charge on the catheter body, various sheaths, and on other nonablation implanted electrode surfaces attempting to prevent the deleterious effects of coagulum and subsequent thrombus on such surfaces.
Reducing Stroke Incidence by Ligating the Left Atrial Appendage
Background
The left atrial appendage is a common site for left atrial thrombus formation and subsequent stroke in patients with atrial fibrillation. Some studies have suggested that greater than 95% of thromboembolism based on TEE studies [21, 22] in patients with atrial fibrillation arise from this structure. Given the widespread prevalence of atrial fibrillation (expected to afflict 16 million by 2050) [23], this becomes significant. At present, the best established therapy for preventing thromboembolism has been warfarin which has been proven to reduce stroke risk in patients eligible for anticoagulation. Multiple problems with warfarin therapy including frequent blood draws, bleeding risk, and drug–dietary interactions result in significant underutilization of this therapy [24].
Because of these difficulties, new strategies have been directed at preventing left atrial appendage thrombus by excluding this structure from the circulation. These include surgical exclusion [25]; however, because of the morbidity associated with invasive surgical procedures, percutaneous approaches have been the nidus around which recent innovation has been based. A self-expanding nitinol cage with permeable fabric covering placed close to the ostium of the left atrial appendage as well as epicardial approaches without thoracotomy are being evaluated [26–28]. An ideal approach, perhaps, is to have closure of the atrial appendage at the level of the ostium but without any portion of the device being exposed to the left-sided circulation.
The Invention
WeFootnote 2 have developed a system that involves ligation of the left atrial appendage via a percutaneous subxiphoid approach using a novel hollow-suture concept [28]. With the aid of local electrograms and fluoroscopy, the appendage is grabbed with an electrode-containing device. The ligator is then placed over the appendage and the hollow suture tightened (Fig. 7) with transesophageal echocardiographic documentation of adequate appendage closure ([29]; Friedman et al., submitted manuscript).
Experimental Results
In four live acute closed-chest canine experiments, we successfully deployed the device ligating the left atrial appendage. In this initial proof of concept study, there was no evidence of trauma to the left atrial appendage or pericardium[30] (Fig. 8). In our experiments, we used transesophageal echocardiography and fluoroscopic visualization to ensure closure of the appendage ostium when the ligature was applied. Additional parameters included the loss of atrial electrograms measured by the electrical mapping plant when closure was achieved. We are presently evaluating the system in closed-chest chronic survival experiments.
Potential Application and Impact of Innovation
If further validated with chronic animal and human data, this minimally invasive percutaneous epicardial approach to ligate the left atrial appendage may eliminate or decrease the risk of thromboembolism in patients with atrial fibrillation. Further, this may be done without having an endovascular structure in the left-sided circulation.
Synchronous Ventricular Pacing Without Crossing the Tricuspid Valve or Entering the Coronary Sinus
Background
Most pacemakers and defibrillator leads are placed near the right ventricular apex. An additional atrial lead may be placed in the right atrial appendage with dual-chamber systems [31]. Despite the clear lifesaving advances that have resulted from endocardial pacing and defibrillation, some problems remain.
Present pacing and defibrillator leads sometimes record electrical signals from neighboring structures (far-field signals). With defibrillators, such abnormal sensing can give rise to inappropriate device therapy.
Since most ventricular leads are now placed in the right ventricle, they necessarily cross the tricuspid valve giving rise in some patients with symptomatic tricuspid regurgitation that may subsequently necessitate lead removal, repositioning, or valve surgery[32].
The most significant problem with right ventricular pacing, however, that has recently been described is the detrimental effects on myocardial function as a result of chronic right ventricular apical pacing ([33]; Henz et al., manuscript submitted to Circulation). Cardiac resynchronization therapy (CRT) using a left ventricular pacing lead in addition to the right ventricular lead attempts to overcome the problem of ventricular dyssynchrony and eventual deterioration in cardiac function. While CRT has shown significant benefits in improving quality of life, decreasing hospitalization, and improving symptomatic congestive heart failure[9, 34, 35], despite this major advance (CRT), problems still remain since cannulation of the coronary sinus in placing a left ventricular pacing lead is not straightforward, and a right ventricular lead is used in addition to the left ventricular lead, thus still necessitating crossing and possibly affecting the tricuspid valve [36].
To overcome these present limitations of endocardial pacing (far-field sensing, tricuspid regurgitation, and ventricular dyssynchrony promotion), we hypothesized that a totally intramyocardial bipolar pacing and sensing lead will limit far-field sensing, and, by placing this lead at a novel location (the atrioventricular septum), we would decrease ventricular dyssynchrony without the need to cross the tricuspid valve or enter the coronary sinus.
In the normal heart, the septal leaflet of the tricuspid valve is not at the same level as the mitral valve. This discrepancy results in a portion of the intraventricular septum being proximal to the tricuspid valve (Fig. 9). In this location, the right atrium and left ventricle myocardium are opposed. This unique anatomic location has left ventricular fibers oriented in a manner that would facilitate conduction to the free wall of the left ventricle [37, 38].
Left panel: photograph of the human heart atrioventricular septum. The septal leaflet of the tricuspid valve (arrow) was displaced more apically than the mitral valve (hatched arrow). Thus, a portion of the intraventricular septum is adjacent to and accessed from the right atrium. Right panel: histological section showing the layers of ventricular myocardium clearly present proximal to the insertion of the tricuspid valve leaflet
The Invention
WeFootnote 3 developed an intramyocardial pace-sense lead where both the anode and cathode are located within the myocardium (Henz et al., manuscript submitted to Circulation; [39, 40]; Fig. 10).
Left panel shows illustrations of placement of one iteration of the intramyocardial bipolar pacing lead onto the atrioventricular septum. The inset shows another iteration with a central pin serving as one electrode and an ultra helix serving as the other with both portions placed into the myocardium
The atrioventricular septum is a unique anatomical location (Fig. 9) which can be accessed without crossing the tricuspid valve and essentially has left ventricular myocardium exposed to the atrial endocardium. Since far-field sensing can be a major potential detriment of pacing this site, the intramyocardial bipole is necessary and enables pacing this novel anatomical site. We have designed and tested various bipolar pacing screw designs including housing the anode on a helix and cathode on a central pin, vice versa, two separate helices, and two deployed electrodes on a single screw. Several dimensions of the screw were tested given the variability in atrioventricular septal thickness in humans. If a screw larger than the septal dimension is deployed, a portion of the electrode would be exposed to the left-sided circulation. To avoid this complication and ensure stability of the lead being accurately deployed on the atrioventricular septum, we used a combination of intracardiac ultrasound visualization and fluoroscopy.
Experimental Results
-
1.
We initially performed an open-chest experiment in the canine model verifying the improved sensing capability of intramyocardial bipolar pacing [39].
-
2.
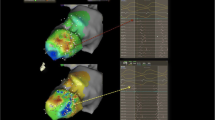
Following this verification, we performed in vivo endocardial deployment of the novel lead on the atrioventricular septum (Fig. 11) using a combination of high-density electroanatomic mapping from the endocardium, electroanatomic mapping from the epicardial surface of both right and left ventricles, electrocardiography, and tissue Doppler imaging via intracardiac ultrasound [41] (Fig. 12). We demonstrated relatively synchronous pacing (shorter QRS duration and relative preexcitation of the left ventricular free wall) when compared to right ventricular apical pacing. These findings were confirmed in three in vivo closed-chest canine experiments (Henz et al., manuscript submitted to Circulation; Fig. 13). This relative synchronous pacing occurred without tricuspid regurgitation, atrioventricular conduction abnormality, or entering the coronary sinus (Figs. 14 and 15).
Isochronal electroanatomic maps obtained during right ventricular apical pacing. The left panel shows endocardial activation viewed in an anterior projection. Note that activation begins (red area) and proceeds first to the septum of the left ventricle with the lateral wall of the left ventricle being activated last of all. The right panel shows similar activation in a posterior view with both endocardial and epicardial activation maps superimposed. Once again, the sequenced and dyssynchronous electrical activation (septum much before lateral wall) is seen
In contrast with Fig. 14, this electroanatomic map was obtained when pacing at the atrioventricular septum. Note the near-simultaneous activation of points on the lateral walls (blue) with a large portion of the anterior wall and septal apex, suggesting more synchronous activation
Potential Application and Impact of Innovation
-
Intramyocardial pacing may decrease far-field sensing that has plagued implanted defibrillators and removed some of the obvious advantages in having an atrial lead to help distinguish between supraventricular and malignant ventricular arrhythmia.
-
Since the atrioventricular septum is proximal to the septal leaflet of the tricuspid valve, tricuspid regurgitation would not occur with ventricular pacing.
-
If further chronic animal and human data confirm the relative ventricular synchrony that we showed in acute experiments by pacing the atrioventricular septum, the deleterious effects of right ventricle apical pacing may be prevented.
Noncardiac Arrhythmias—Different Locations, Similar Solutions: Mapping and Ablation in the Central Nervous System for Seizures via the Venous Drainage of the Brain
Background
Epilepsy is a prevalent disorder with over 50/100,000 persons affected per year [42]. Presently, when drug therapy for this disorder fails and patients have intractable and life-altering symptoms, surgical removal of the epileptogenic cortex is attempted. Although highly successful in eliminating the seizure focus, the procedure is highly invasive associated with significant morbidity. As a result of this, surgical management of seizures is presently uncommon. Adequate localization of the epileptogenic cortex and distinguishing this abnormal tissue from the neighboring eloquent cortex involves intracortical craniotomy-based mapping [12].
The evolution of cardiac arrhythmia management may provide insights into how epilepsy innovations might evolve. Three decades ago, treatment of what is now considered straightforward cardiac rhythm disorders involved open-heart surgery [43, 44]. The revolution in management of cardiac arrhythmia occurred when percutaneous endovascular approaches (mapping and radiofrequency ablation) evolved, initially as an adjunct to surgical therapy and now as a stand-alone curative approach. At the present time, surgical treatment for cardiac rhythm is rarely, if ever, required for disorders such as Wolf–Parkinson–White syndrome and AV node reentry (Fig. 16).
Top panel: endovascular mapping of cardiac arrhythmia to find the earliest site of activation or location of abnormal substrate and then targeted for ablation as revolutionized cardiac rhythm care. Bottom panel: similarly, with seizures, there is often an area of abnormal electrical activity that proceeds (arrow) global abnormalities and can potentially be targeted for focal energy delivery as envisioned in our invention
Since seizure mapping prior to surgical removal of abnormal cortex involves the recording and analysis of electrical signals (electrocorticograms), there may be avenues to use intravenous cardiac electrophysiological approaches and modify and apply them to the central nervous system[45, 46] (Fig. 17).
Further, in the heart, the primary diagnosis of myocardial infarction (a vascular phenomenon) is from the electrocardiogram (electrical parameters). Similarly, with vascular occlusion (stroke), there is a change in the nature of the electrical signals arising from this structure that is ischemic or infarcting in the brain. Whether or not similar approaches (electrical diagnosis for vascular events in the brain) could facilitate early diagnosis of patients with cerebrovascular disease and decrease the present devastating consequences of stroke is not known.
The Invention
WeFootnote 4 have developed a system that includes sheaths and specifically designed mapping and ablation catheters to access proximate cortical brain tissue via the central nervous system venous network [47]. This novel technique involves endovascular mapping using the superficial and deep-penetrating cortical veins along with the petrosal sinus, other sinuses, and the vein of Galen. These new techniques needed to cater to the unique structure of the brain, tortuosity of the vessels, and high impedance from brain tissue with delivering radiofrequency energy.
We further have developed a technique to detect via venous-based mapping early electrogram changes occurring as a result of cortical ischemia (Fig. 18).
Experimental Results
-
Ex vivo cadaveric experiments were performed to successfully assess the feasibility of placing catheters via the jugular vein to reach the petrosal sinus and various branches up to and including the cortical veins.
-
Using fluoroscopic and angiographic guidance (Fig. 19), we performed an in vivo experiments with an entirely venous-based approach in the swine model and successfully mapped and placed histologically confirmed ablative lesions in the cortex of the pig [48] (Fig. 20).
-
We performed an open craniotomy experiment in the canine model with simultaneous venous, intracortical, and surface cortical electrogram recordings in the baseline state and with pacing-induced seizure activity and confirmed with a mathematical transform model the essential identical results obtained with venous-based mapping and plunge electrode cortical mapping.
-
We are presently assessing specifically designed catheters in other animal models to map and ablate cortical tissue and assess electrogram characteristics associated with early cerebral ischemia.
Fluoroscopic images showing venography performed to help access the venous system in the swing model. Catheters were placed through the vein to successfully map and create ablation lesions [48]
Potential Application and Impact of Innovation
If further validated in other animal and seizure models, this technology may change the way in which patients with drug refractory seizures are treated. Further validation alongside present surgical mapping is required prior to fulfilling this promise.
The venous-based approach may provide a platform for placement of deep-brain-stimulating electrodes, other indwelling electrodes, and electrodes used to detect early ischemia and allow prompt treatment for impending stroke.
Summary
Despite the major strides made in the management of patients with rhythm disorders over the last few decades, innovative solutions continue to be required to improve the quality of these patients’ lives. We have briefly reviewed our group’s work attempting to reduce thromboembolism during invasive procedures [20] and decreasing stroke from atrial fibrillation with a percutaneous epicardial appendage ligation system. We have also described our work with a novel intramyocardial pacing lead placed in a unique anatomical position (atrioventricular septum) to synchronously pace the ventricles without crossing the tricuspid valve or needing to cannulate the coronary sinus. Finally, we discuss a novel technique to modify and use our knowledge obtained from endovascular approaches to cardiac arrhythmia management to the central nervous system to treat and detect common disorders such as seizures and stroke.
Notes
Inventors of this technology are Dr. Bernard C. Lim, MD, Ph.D.; Dr. Kalpathi Venkatachalam, MD; Dr. Arshad Jahangir, MD; Dr. Samuel J. Asirvatham, MD; Susan B. Johnson, BS.
Inventors of this technology are Dr. Paul A. Friedman, MD; Dr. Charles Bruce, MD; Dr. Samuel J. Asirvatham, MD. Acknowledgment for development—Aegis Medical, Trevor McCaw, Elliot Hong, Steve Berhow, Randy Beyreis, and Andy Danielsen.
Inventors of this technology are Dr. Paul A. Friedman, MD; Dr. Charles Bruce, MD; Dr. Samuel J. Asirvatham, MD; Susan B. Johnson, BA. Acknowledgment for development—Boston Scientific (Natick, MA, USA) for lead development, Andy Danielsen, Steve Berhow, and Randy Beyreis.
Inventors of this technology are Dr. Paul A. Friedman, MD; Dr. Charles Bruce, MD; Dr. Samuel J. Asirvatham, MD; David R. Holmes, Jr., MD. Acknowledgment for development—Gregory Worrell, MD; S. Matthew Stead, MD; Andy Danielsen; and Susan B. Johnson.
References
Haissaguerre, M., Jais, P., & Shah, D. (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. New England Journal of Medicine, 339, 659.
Kindermann, M., Hennen, B., & Jung, J. (2006). Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: The Homburg Biventricular Pacing Evaluation (HOBIPACE. Journal of the American College of Cardiology, 47, 1927.
Oral, H., Chugh, A., & Ozaydin, M. (2006). Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation, 114, 759.
Pappone, C., Oral, H., & Santinelli, V. (2004). Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation, 109, 2724.
Pappone, C., Oreto, G., & Lamberti, F. (1999). Catheter ablation of paroxysmal atrial fibrillation using a 3D mapping system. Circulation, 100, 1203.
Saxon, L., et al. (1993). Increased risk of progressive hemodynamic deterioration in advanced heart failure patients requiring permanent pacemakers. American Heart Journal, 125, 1306.
Scanavacca, M., et al. (2004). Left atrial-esophageal fistula following radiofrequency catheter ablation of atrial fibrillation. Journal of Cardiovascular Electrophysiology, 15, 960.
Buxton, A., et al. (1993). Prevention of sudden death in patients with coronary artery disease: The multicenter unsustained tachycardia trail (MUSTT). Progress in Cardiovascular Diseases, 36, 215–226.
Cazeau, S., et al. (2001). Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. New England Journal of Medicine, 344(12), 873–880.
Daubert, J., et al. (2008). Inappropriate implantable cardioverter-defibrillator shocks in MADIT II. Journal of the American College of Cardiology, 51(14), 1357–1365.
Packer, D., et al. (2008). Imaging of the cardiac and thoracic veins. In S. Chen, M. Haissaguerre, & D. Zipes (Eds.), Thoracic vein arrhythmias: Mechanisms and treatments (pp. 77–98). Malden: Blackwell Futura.
Asztely, F., et al. (2007). Long term follow-up of the first 70 operated adults in the Goteborg Epilepsy Surgery Series with respect to seizures, psychosocial outcome and use of antiepileptic drugs. Journal of Neurology, Neurosurgery and Psychiatry, 78(6), 605–609.
Asirvatham, S. J. (2007). Ablation for atrial fibrillation: Can we decrease thromboembolism without increasing the risk for bleeding? Circulation, 116(22), 2517–2519.
Asirvatham, S. J., & Friedman, P. A. (2006). Silent cerebral thromboembolism with left atrial ablation: A lurking danger. Journal of Cardiovascular Electrophysiology, 17(1), 8–10.
Zhou, L., et al. (1999). Thromboembolic complications of cardiac radiofrequency catheter ablation: A review of the reported incidence, pathogenesis and current research directions. Journal of Cardiovascular Electrophysiology, 10(4), 611–620.
Baier, R. E., & Dutton, R. C. (1969). Initial events in interactions of blood with a foreign surface. Journal of Biomedical Materials Research, 3(1), 191–206.
Hemmerle, J., et al. (1999). Direct observation of the anchoring process during the adsorption of fibrinogen on a solid surface by force-spectroscopy mode atomic force microscopy. Proceedings of the National Academy of Sciences of the United States of America, 96(12), 6705–6710.
Lim, B., et al. (2007). Novel catheter design to reduce ablation-related thromboembolism. Europace, 9, 096.
Lim, B. (2005). Atomic force microscopy imaging of SXIII in its native state. Biophysics Journal, 88(Suppl), 541.
Lim, B., et al. (2008). Concurrent application of charge using a novel circuit prevents heat-related coagulum formation during radiofrequency ablation. Journal of Cardiovascular Electrophysiology, 19, 843–850.
Blackshear, J. L., et al. (2003). Thoracoscopic extracardiac obliteration of the left atrial appendage for stroke risk reduction in atrial fibrillation. Journal of the American College of Cardiology, 42(7), 1249–1252.
Blackshear, J. L., & Odell, J. A. (1996). Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Annals of Thoracic Surgery, 61(2), 755–759.
Miyasaka, Y., et al. (2006). Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation, 114(2), 119–125.
Anonymous. (1990). Preliminary report of the Stroke Prevention in Atrial Fibrillation Study. The New England Journal of Medicine, 322(12), 863–868.
Healey, J. S., et al. (2005). Left Atrial Appendage Occlusion Study (LAAOS): Results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. American Heart Journal, 150(2), 288–293.
Fountain, R. B., et al. (2006). The protect af (WATCHMAN left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. American Heart Journal, 151(5), 956–961.
Bakhtiary, F., et al. (2008). Simplified technique for surgical ligation of the left atrial appendage in high-risk patients. Journal of Thoracic and Cardiovascular Surgery, 135(2), 430–431.
Sosa, E., et al. (1996). A new technique to perform epicardial mapping in the electrophysiology laboratory. Journal of Cardiovascular Electrophysiology, 7(6), 531–536.
Friedman, P. A., Asirvatham, S., & Bruce, C. J. (2008). Device and methods for ligating anatomical structures. US Patent WO/2008/036408.
Bunch, T. J., et al. (2005). Outcomes after cardiac perforation during radiofrequency ablation of the atrium. Journal of Cardiovascular Electrophysiology, 16(11), 1172–1179.
Friedman, P. A., et al. (2006). Dual-chamber versus single-chamber detection enhancements for implantable defibrillator rhythm diagnosis: The detect supraventricular tachycardia study. Circulation, 113(25), 2871–2879.
Lin, G., et al. (2005). Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. Journal of the American College of Cardiology, 45(10), 1672–1675.
Wilkoff, B. L., et al. (2002). Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. Journal of the American Medical Association, 288(24), 3115–3123.
Abraham, W. T. (2002). Cardiac resynchronization therapy for heart failure: Biventricular pacing and beyond. Current Opinion in Cardiology, 17(4), 346–352.
Rivero-Ayerza, M., et al. (2006). Effects of cardiac resynchronization therapy on overall mortality and mode of death: A meta-analysis of randomized controlled trials. European Heart Journal, 27(22), 2682–2688.
Asirvatham, S. (2004). Biventricular device implantation. In D. L. Hayes (Ed.), Resynchronization and defibrillation for heart failure: A practical approach (pp. 99–137). Oxford: Blackwell/Futura.
Sealy, W. C., & Gallagher, J. J. (1980). The surgical approach to the septal area of the heart based on experiences with 45 patients with Kent bundles. Journal of Thoracic and Cardiovascular Surgery, 79(4), 542–551.
Torrent-Guasp, F., et al. (2005). Towards new understanding of the heart structure and function. European Journal of Cardio-Thoracic Surgery, 27(2), 191–201.
Asirvatham, S. J., et al. (2007). Intramyocardial pacing and sensing for the enhancement of cardiac stimulation and sensing specificity. Pacing and Clinical Electrophysiology, 30(6), 748–754.
Friedman, P. A., Bruce, C. J., & Asirvatham, S. (2007). Helical electrodes for intramyocardial pacing and sensing. US Patent WO/2007/073435.
Asirvatham, S. J., Bruce, C. J., & Friedman, P. A. (2003). Advances in imaging for cardiac electrophysiology. Coronary Artery Disease, 14(1), 3–13.
Zarrelli, M. M., et al. (1999). Incidence of epileptic syndromes in Rochester, Minnesota: 1980–1984. Epilepsia, 40(12), 1708–1714.
Asirvatham, S. (2004). Anatomy of the vena cava: An electrophysiological perspective. In D. Zipes (Ed.), Thoracic vein arrhythmias: Mechanisms and treatment. Oxford: Blackwell Futura.
Jackman, W. M., et al. (1992). Treatment of supraventricular tachycardia due to atrioventricular nodal reentry, by radiofrequency catheter ablation of slow-pathway conduction. New England Journal of Medicine, 327(5), 313–318.
Guenot, M., et al. (2004). SEEG-guided RF thermocoagulation of epileptic foci: Feasibility, safety, and preliminary results. Epilepsia, 45(11), 1368–1374.
Parrent, A. G., & Blume, W. T. (1999). Stereotactic amygdalohippocampotomy for the treatment of medial temporal lobe epilepsy. Epilepsia, 40(10), 1408–1416.
Friedman, P. A., et al. (2008). Detecting and treating nervous system disorders. US Patent WO/2007/061982.
Henz, B. D., et al. (2008). Successful radiofrequency ablation of the cerebral cortex in pigs using the venous system: Possible implications for treating CNS disorders. Epilepsy Research, 80, 213–218.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asirvatham, S.J. Innovation Focus: the Patient with Arrhythmia. J. of Cardiovasc. Trans. Res. 1, 258–272 (2008). https://doi.org/10.1007/s12265-008-9061-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-008-9061-x