Abstract
Differential diagnosis is important for clinical management of cases with thyroid diseases. We aimed to find the most useful diagnostic panel including immunohistochemistry markers and BRAF 600E mutation for papillary thyroid carcinoma. The study included 99 papillary thyroid carcinoma, 95 benign thyroid disease and 6 well differentiated tumour of uncertain malignant potential cases. Three groups were compared for immunohistochemistry marker expressions (CK19, Gal-3, HBME-1, p63, CD56). Diagnostic contribution of BRAF V600E mutation and its relationship with the immunohistochemistry and prognostic parameters were evaluated. CK19, Gal-3, HBME-1 and p63 positivity and loss of CD56 expression were significantly higher in papillary thyroid carcinoma than in benign thyroid disease. Gal-3 was the most useful marker to differentiate both papillary thyroid carcinoma and well-differentiated tumour of uncertain malignant potential cases from benign thyroid disease. CK19&HBME-1 and CK19&-Gal-3 dual panels had the highest sensitivities and high specificities for distinguishing papillary thyroid carcinoma from benign thyroid disease. Moreover, CK19&Gal-3 dual panel had the highest sensitivity and specificity to distinguish well-differentiated tumour of uncertain malignant potential from benign thyroid disease. In addition, CK19&Gal-3&HBME-1 was the most sensitive triple panel in differentiating both papillary thyroid carcinoma and well-differentiated tumour of uncertain malignant potential cases from benign thyroid disease. When combined with immunohistochemistry markers, the BRAF V600E reduced sensitivities and increased specificities towards malignancy, and it was not associated with prognostic parameters except for histological subtypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy that represents approximately 85% of all well-differentiated thyroid cancers of follicular origin [1, 2]. Accurate diagnosis of PTC is important for determining the clinical management of the cases. Therefore, the usage of immunohistochemical (IHC) markers for the diagnosis of PTC is of research interest [3].
Cytokeratin 19 (CK19), a member of the keratin family that is an intermediate filament protein in epithelial cells, is highly expressed in PTC [4]. Galectin-3 (Gal-3) is a b-galactoside-binding lectin [5]. Its overexpression was found to be associated with angiogenesis, invasion, apoptosis, metastasis and tumour cell growth in tumours including PTC [6]. Hector Battifora mesothelial-1 (HBME-1), a monoclonal antibody targeting an antigen of mesothelial microvilli, was reported to be expressed abnormally in PTC [7]. P63, a p53 homolog, plays an important role in cell cycle and squamous cell differentiation [8]. CD56 is a neural cell adhesion molecule involved in cell-to-cell adhesion and its expression is downregulated in PTC [9]. It has been reported that the most sensitive marker in the diagnosis of PTC is HBME-1, with a rate of 95.9% for the classical variant PTC (CVPTC) and 81.1% for the follicular variant PTC (FVPTC). On the contrary, CD56 expression was reported as decreased or absent in PTC; this marker is mostly expressed in BTD and follicular carcinomas [10]. Furthermore, PTC harbours point mutations of the BRAF and RAS genes or RET/PTC rearrangements that activate the mitogen-activated protein kinase pathway [11]. BRAF V600E, an activating point mutation, is the most common genetic change in PTC and leads to the conversion of valine to glutamic acid at position 600 [12, 13]. The BRAF V600E mutation is found in approximately 70% of PTC cases [14].
We first aimed to evaluate the role of IHC markers CK19, Gal-3, p63, HBME-1 and CD56 in differentiating PTC, well-differentiated thyroid tumour of uncertain malignant potential (WDT-UMP) and benign thyroid disease (BTD). Our second aim was to investigate the association of IHC expressions and BRAF V600E mutation with clinicopathological prognostic parameters.
Patients and Methods
A total of 200 thyroidectomy materials diagnosed in the pathology department of a university hospital, between 2012 and 2014, were included in this study. Haematoxylin & eosin and IHC-stained slides were re-evaluated together with the pathology reports. The tumours were classified according to recommendations of the World Health Organization (WHO) revealed in 2017 [1]. The patients whose lesions did not have full coverage for the morphologic signs of PTC and did not confirm affirmation of capsular and/or vascular invasion were classified as WDT-UMP [1, 15]. Clinicopathological prognostic parameters were recorded for PTC cases. AJCC/TNM 8th edition was used to determine the stage [16].
The expressions of IHC markers were analysed using an IHC panel which included CK19 (CK19 mouse monoclonal antibody, clone A53-B/A2.26; RTU; Cell Marque), Gal-3 (galectin-3, clone 9C4; 1:100; Novocastra), HBME-1 (HBME-1 mouse monoclonal antibody; RTU; Cell Marque), p63 (p63 mouse monoclonal primary antibody, clone 4A4; RTU; Roche) and CD56 (CD56 rabbit monoclonal antibody, clone MRQ-42; RTU; Cell Marque). BRAF V600E mutation was examined by pyrosequencing on paraffin-embedded tumour specimens during the routine practice, and the results were recorded from the pathology reports. The study is in accordance with the Helsinki Declaration. The study protocol was accepted by the Clinical Research Ethics Committee of a local university. Written and signed consent was obtained from all participants.
Statistical analysis was performed with the SPSS version 21 (IBM Corp. NY, USA). Student’s t test was used to compare continuous variables, and chi-square and Fisher’s exact tests were used to compare categorical variables. The receiver operating characteristic (ROC) curve analysis was performed using MedCalc software, Version 20.110 (MedCalc Software Ltd, Ostend, Belgium) in order to determine the diagnostic usefulness of IHC expressions among study groups. The test classification system according to the values that the area under curve (AUC) can take was considered as excellent (0.90–1.00), good (0.80–0.90), bad (0.70–0.80), poor (0.60–0.70) and fail (0.50–0.60) [17]. A p value of < 0.05 was considered statistically significant.
Results
The study included 99 PTC, 95 BTD and 6 WDT-UMP cases. BTD cases were as follows: 26 nodular hyperplasia (NH), 21 Hashimoto thyroiditis (HT), 21 follicular adenoma (FA), 14 Graves disease and 13 lymphocytic thyroiditis. Women: men ratio was 3.3:1 (5.2:1 in PTC, 2.2:1 in BTD, 5:1 in WDT-UMP). The mean age was 47.44 SD12.69 years in PTC, 46.78 SD11.68 years in BTD and 48.67 SD14.02 years in WDT-UMP. There was no significant difference between three groups in terms of the patient’s age and gender. The subtypes were as follows in PTC cases: 40 classical, 49 follicular, 5 diffuse sclerosing, 2 oncocytic, 1 cystic and 2 tall cell variants. Multifocality was present in 25 (25.3%) cases. The mean and median tumour sizes were 1.55 SD1.43 and 1.1 (0.3–8) cm. While 68 (68.7%) cases were non-capsulated, 31 (31.3%) cases were encapsulated. Extrathyroidal extension was present in 11 (11.1%) cases. There was intratumoural and non-tumoural lymphocytic infiltration in 6 (6.1%) and 19 (19.2%) cases, respectively. The tumoural stages were as follows: 77 (77.8%) pT1, 7 (7.1%) pT2, 11 (11.1%) pT3 and 4 (4%) pT4. There was lymph node metastasis in 7 (7.1%) cases, and distant metastasis was in only one (1%) case.
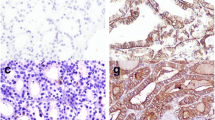
CK19, Gal-3, HBME-1 and p63 expressions were found significantly higher in PTC cases compared to BTD cases (p < 0.0001, p < 0.0001, p < 0.0001, p = 0.009, respectively). CD56 expression was found to be significantly higher in BTD cases than PTC cases (p = 0.001) (Fig. 1). The results are summarized in Table 1. When we analyzed the IHC parameters to distinguish PTC from BTD, Gal-3 (AUC = 0.934, p ≤ 0.0001), CK19 (AUC = 0.879, p ≤ 0.0001) and HBME-1 (AUC = 0.870, p ≤ 0.0001) had the largest AUC with the highest sensitivities which were 88%, 99% and 92%, respectively. Gal-3 was the most specific (99%) marker. AUC values, sensitivities and specificities were very high in double combinations of CK19, Gal-3 and HBME-1. CK19&Gal-3 (AUC = 0.934, p ≤ 0.0001), CK19&HBME-1 (AUC = 0.923, p ≤ 0.0001) and Gal-3&HBME-1 (AUC = 0.899, p ≤ 0.0001) had the largest AUC with the highest sensitivities which were 100%, 94% and 99%, respectively. Their specificities were 87%, 91% and 81%, respectively. Among triple IHC panels, CK19&Gal-3&HBME-1 had the largest AUC (AUC = 0.899, p ≤ 0.0001) with a 80% sensitivity and 100% specificity. AUC, 95% CI, sensitivity, specificity and p values of IHC parameters for differentiating PTC from BTD are given in Table 2. Positive expression of more than one of the CK19, Gal3 and HBME-1 IHC markers favored malignancy (p < 0.001). This was the case in only one patient in BTDs.
CK19, Gal-3, HBME-1, p63 and CD56 expressions were significantly higher in WDT-UMP cases compared to those with BTD (p < 0.0001 for all) (Table 1). According to ROC curve analysis, the most sensitive markers to discriminate WDT-UMP from BTD were Gal-3 (AUC = 0.995, p ≤ 0.0001), HBME-1 (AUC = 0.911, p ≤ 0.0001) and CK19 (AUC = 0.884, p ≤ 0.0001) all with a sensitivity of 100% and specificities of 99%, 82% and 77%, respectively. When we analyzed the IHC parameters in a double panel form, CK19&Gal-3 (AUC = 0.999, p ≤ 0.0001), CK19&HBME-1 (AUC = 0.968, p ≤ 0.0001) and Gal-3&HBME-1 (AUC = 0.995, p ≤ 0.0001) had the largest AUC with the highest sensitivities which were all 100%. Their specificities were 100%, 94% and 99%, respectively. When the triple panels were analyzed, CK19&Gal-3&HBME-1 had the largest AUC (AUC = 0.999, p ≤ 0.0001) with 100% sensitivity and specificity (Table 3).
PTC and WDT-UMP cases were statistically similar in terms of IHC panel (Table 1). According to ROC curve analysis, the most sensitive marker was p63 and the most specific markers were CK19, Gal-3 and HBME-1 to differentiate PTC from WDT-UMP. However, IHC panels failed to differentiate PTC from WDT-UMP.
All cases with CVPTC and FVPTC had expressions for at least one of Gal-3, HBME-1 or CK19, but this was not the case for p63 and loss of CD56 expression. None of the markers we analysed were able to be distinctive between CVPTC and FVPTC or other PTC subtypes. Data are not shown.
BRAF V600E mutation was detected in 31 (31.3%) of 99 PTC and in 1 (16.7%) of 6 WDT-UMP cases. In the PTC group, a strong significance was found between BRAF V600E mutation and the histological subtypes. The mutation was higher in CVPTC compared to other subtypes (p < 0.0001). It was present in 21 (52.5%) of 40 CVPTC and 7 (14.3%) of 49 FVPTC cases. It was significantly higher in CVPTC cases than that of FVPTC (X2 = 14.915, p < 0.0001, OR: 0.27, 95% CI: 0.128–0.574). There was no difference between the cases with and without BRAF V600E mutation in terms of the other clinicopathological prognostic parameters (Table 4).
There was no significant association between BRAF V600E mutation and the expression status of each IHC marker (CK19, Gal-3, HBME-1, p63, CD56) for each group (PTC, PTC subtypes and WDT-UMP) where the statistical studies could be performed. When presence of BRAF V600E was combined with single, double or triple IHC panels, the sensitivity of IHC markers decreased, while the specificity increased up to 100% in differentiating PTC from BTD. There was also no statistical difference between PTC and WDT-UMP cases for the presence of BRAF V600E (p = 0.655, Fisher’s exact test). When ROC curves were performed for panels with IHC & BRAF V600E, all single, double and triple panels had sensitivities under 31%, while the specificities were all 100%. Data are not shown.
Discussion
Although the pathological diagnosis of PTC is based on both cytological and histological examination of the typical nuclear morphology [18], additional studies may be required for the differential diagnosis. Therefore, the usage of IHC markers for the diagnosis of PTC remains a research topic. Various IHC markers such as CK19, Gal-3, HBME-1, p63 and CD56 have been reported to be useful for the differential diagnosis of malignant and benign thyroid lesions [19].
Dunderovic et al. reported HBME-1 as the best balanced marker in terms of sensitivity and specificity and Gal-3 as the most sensitive marker, while Nechifor-Boila et al. reported HBME-1 as the most sensitive marker in the diagnosis of PTC [10, 20]. In another study, CK19 was reported as the most specific marker for PTC, but it should not be used alone because of its expression in 40% in NH cases [19]. In a study, CK19 expression was diffuse and intensively positive in PTC, but a serious number of benign cases also had focal and weak expression of CK19 [20]. Gal-3 was reported as a useful marker for malignant thyroid lesions particularly non-follicular ones from BTD. P63 expression was reported between 6.9 and 74% in thyroid carcinomas [19]. Unlike the other markers, loss of CD56 was very specific for the malignancy [10, 20]. In a meta-analysis study, loss of CD56 expression was significantly higher in PTC and follicular carcinoma, than in FA, NH and HT, and CD56 can be a useful marker in differing FVPTC from FA [9]. In our study, CK19, Gal-3, HBME-1 and p63 expressions were significantly higher in PTC, while CD56 expression was significantly higher in BTD cases, as consistent with the literature. When used alone, CK19 was the most sensitive and Gal-3 was the most specific marker to differentiate PTC from BTD. Although most of them were focal and weak, CK19 expression was present in 23% of the BTD cases. However, Gal-3 was not expressed in 99% of BTD cases. Therefore, we think that Gal-3 is more useful as a single marker.
Archolia et al. reported that CK19, Gal-3 and HBME-1 combinations had high sensitivity and specificity [21]. Nechifor-Boila et al. found that both CK19&HBME-1&Gal-3 and CK19&HBME-1&CD56 panels had high sensitivities [10]. Dunderovic et al. showed that the best combinations for differentiating PTC from FA and non-neoplastic lesions were CD56&Gal-3 and CK19&Gal-3, respectively. They also reported that co-expression for combination of markers in the diagnosis of follicular lesions decreases sensitivity, but increases specificity for malignancy [20]. We found that CK19&HBME-1 co-expression was the most balanced double panel to differentiate PTC from BTD. CK19&Gal-3 was another useful panel with a little less sensitivity. Double panels including p63 with CK19 or Gal-3 had 100% specificity, but their sensitivities were very low. In addition, CK19&Gal-3&HBME-1 was the most sensitive triple panel. It was reported that HBME-1 expression was higher in CVPTC than in FVPTC. Taştekin et al. found that HBME-1&Gal-3 panel was useful particularly for CVPTC [19]. In our study, there was expression of at least one of CK19, Gal-3 or HBME-1 in all CVPTC and FVPTC cases. However, there was no difference between two variants for expression of each marker.
In a study including 22 WDT-UMP cases, the presence of HBME-1 and the absence of CD56 were found to be the most frequent association. They stated that WDT-UMP trended towards the IHC panel of PTC, suggesting a possible pathogenetic link between the two entities [15]. We had only six cases of WDT-UMP in this study. Although the number of WDT-UMP cases is small, when we compared the WDT-UMP and PTC groups, we found that each IHC marker had statistically similar expression. According to ROC curve analysis, the most sensitive marker was p63 and the most specific markers were CK19, Gal-3 and HBME-1 to differentiate PTC from WDT-UMP. However, since the AUC’s were in the range considered ‘poor/fail’, none of the markers can be considered distinctive. Expressions of four markers were found significantly higher in WDT-UMP than in BTD cases. Gal-3, HBME-1 and CK19 alone had high sensitivity and specificity to differentiate WDT-UMP from BTD. CK19&Gal-3, CK19&HBME-1 and Gal-3&HBME-1 as double panels, and CK19&Gal-3&HBME-1 as a triple panel all had 100% sensitivity and up to 100% specificity.
The frequency of BRAF V600E mutation in PTC in the Turkish population was reported as 52.8% [22]. In this study, the frequency of BRAF V600E mutation was 31.3% and 16.7% in PTC and WDT-UMP groups, respectively. However, there was no statistical difference between the two groups in terms of the presence of BRAF V600E mutation. It has been reported that BRAF V600E mutation is associated with aggressive clinicopathological parameters and poor prognosis in PTC cases [23,24,25]. In our study, there was no correlation between BRAF V600E mutation and clinicopathologic prognostic parameters including multifocality, tumour size, capsule status, extrathyroidal extension, intratumoural and non-tumoural lymphocytic infiltration, tumoural stage, lymph node metastasis and distant metastasis. However, a strong significance was found in terms of histological variants. BRAF V600E mutation was significantly higher in CVPTC compared to other subtypes similar to the literature [24].
Ramkumar et al. found a direct correlation between BRAF V600E mutation and HBME-1 and Gal-3 expression and reported that the combined usage of HBME-1, Gal-3 and BRAF V600E mutation could help differentiating FVPTC, FA and solitary nodules with papillary-like nuclear features [26]. In our study, we also evaluated the relationship between BRAF V600E mutation status and IHC markers, in PTC and WDT-UMP groups. However, we couldn’t find any correlation. When we added the BRAF V600E mutation to the CK19&HBME-1 combination, which is the most balanced IHC panel in terms of sensitivity and specificity, the sensitivity to distinguish PTC and BTD decreased dramatically and the specificity increased to 100%.
The limitation of the current study is the small number of WDT-UMP patients; however, they are rarely encountered in the clinic. Furthermore, there are other studies investigating the IHC markers and BRAF V600E mutation in thyroid diseases. However, the number of studies evaluating these markers as panels is limited.
Conclusion
In conclusion, we suggest Gal-3, CK19 and HBME-1 and their dual and triple panels as precious markers in distinguishing both PTC and WDT-UMP from BTD. When BRAF V600E was combined with these IHC markers/panels, the sensitivity decreased dramatically and the specificity for malignancy increased. Further studies with larger groups are needed to identify WDT-UMP cases at risk for PTC transformation.
References
Lloyd RW, Osamura RY, Klöppel G, Rosai J (eds) (2017) 4th edn. IARC, Lyon, France
Clark OH (2011) Thyroid cancer and lymph node metastases. J Surg Oncol 103(6):615–618. https://doi.org/10.1002/jso.21804
Nga ME, Lim GS, Soh CH, Kumarasinghe MP (2008) HBME-1 and CK19 are highly discriminatory in the cytological diagnosis of papillary thyroid carcinoma. Diagn Cytopathol 36(8):550–556. https://doi.org/10.1002/dc.20841
Song Q, Wang D, Lou Y, Li C, Fang C, He X, Li J (2011) Diagnostic significance of CK19, TG, Ki67 and galectin-3 expression for papillary thyroid carcinoma in the northeastern region of China. Diagn Pathol 6:126. https://doi.org/10.1186/1746-1596-6-126
Chiu CG, Strugnell SS, Griffith OL, Jones SJ, Gown AM, Walker B, Nabi IR, Wiseman SM (2010) Diagnostic utility of galectin-3 in thyroid cancer. Am J Pathol 176(5):2067–2081. https://doi.org/10.2353/ajpath.2010.090353
Cho H, Kim JY, Oh YL (2018) Diagnostic value of HBME-1, CK19, galectin 3, and CD56 in the subtypes of follicular variant of papillary thyroid carcinoma. Pathol Int 68(11):605–613. https://doi.org/10.1111/pin.12729
Sack MJ, Astengo-Osuna C, Lin BT, Battifora H, LiVolsi VA (1997) HBME-1 immunostaining in thyroid fine-needle aspirations: a useful marker in the diagnosis of carcinoma. Mod Pathol 10(7):668–674
Hosny Mohammed K, Ewaz A, Cohen C, Siddiqui MT (2017) Double staining: diagnostic utility in non-small cell lung carcinoma in the era of tissue conservation. J Am Soc Cytopathol 6(4):170–175. https://doi.org/10.1016/j.jasc.2017.05.003
Pyo JS, Kim DH, Yang J (2018) Diagnostic value of CD56 immunohistochemistry in thyroid lesions. Int J Biol Markers 33(2):161–167. https://doi.org/10.1177/1724600817748538
Nechifor-Boila A, Borda A, Sassolas G, Hafdi-Nejjari Z, Borson-Chazot F, Lifante JC, Sturm N, Lavérriere MH, Berger N, Decaussin-Petrucci M (2013) Immunohistochemical markers in the diagnosis of papillary thyroid carcinomas: the promising role of combined immunostaining using HBME-1 and CD56. Pathol Res Pract 209(9):585–592. https://doi.org/10.1016/j.prp.2013.06.012
Witt RL, Ferris RL, Pribitkin EA, Sherman SI, Steward DL, Nikiforov YE (2013) Diagnosis and management of differentiated thyroid cancer using molecular biology. Laryngoscope 123(4):1059–1064. https://doi.org/10.1002/lary.23838
Duesbery NS, Webb CP, VandeWoude GF (1999) MEK wars, a new front in the battle against cancer. Nat Med 5(7):736–737. https://doi.org/10.1038/10457
Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA (2003) High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 63(7):1454–1457
Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, Sponziello M (2020) Update on fundamental mechanisms of thyroid cancer. Front Endocrinol (Lausanne) 11:102. https://doi.org/10.3389/fendo.2020.00102
Nechifor-Boila A, Borda A, Sassolas G, Hafdi-Nejjari Z, Cătană R, Borson-Chazot F, Berger N, Decaussin-Petrucci M (2015) Thyroid tumors of uncertain malignant potential: morphologic and imunohistochemical analysis of 29 cases. Pathol Res Pract 211(4):320–325. https://doi.org/10.1016/j.prp.2014.12.005
Tuttle M, Morris LF, Haugen B et al (2017) Thyroid-differentiated and anaplastic carcinoma (chapter 73). In: Amin MB, Edge SB, Greene F et al (eds) AJCC Cancer Staging Manual. Springer International Publishing, 8th New York City
Grzybowski M, Younger JG (1997) Statistical methodology: III. Receiver operating characteristic (ROC) curves. Acad Emerg Med 4(8):818–26. https://doi.org/10.1111/j.1553-2712.1997.tb03793.x
Yashaswini R, Suresh TN, Sagayaraj A (2017) Cytological evaluation of thyroid lesions by nuclear morphology and nuclear morphometry. J Cytol 34(4):197–202. https://doi.org/10.4103/JOC.JOC_87_16
Tastekin E, Keskin E, Can N, Canberk S, Mut A, Erdogan E, Asa N, Güldiken S, Sezer A, Azatcam M (2019) CD56, CD57, HBME1, CK19, Galectin-3 and p63 immunohistochemical stains in differentiating diagnosis of thyroid benign/malign lesions and NIFTP. Pol J Pathol 70(4):286–294. https://doi.org/10.5114/pjp.2019.93131
Dunđerović D, Lipkovski JM, Boričic I, Soldatović I, Božic V, Cvejić D, Tatić S (2015) Defining the value of CD56, CK19, galectin 3 and HBME-1 in diagnosis of follicular cell derived lesions of thyroid with systematic review of literature. Diagn Pathol 10:196. https://doi.org/10.1186/s13000-015-0428-4
Arcolia V, Journe F, Renaud F, Leteurtre E, Gabius HJ, Remmelink M, Saussez S (2017) Combination of galectin-3, CK19 and HBME-1 immunostaining improves the diagnosis of thyroid cancer. Oncol Lett 14(4):4183–4189. https://doi.org/10.3892/ol.2017.6719
Dağlar-Aday A, Toptaş B, Oztürk T, Seyhan F, Saygili N, Eronat AP, Akadam-Teker B, Yilmaz-Aydoğan H, Aksoy F, Oztürk O (2013) Investigation of BRAF V600E mutation in papillary thyroid carcinoma and tumor-surrounding nontumoral tissues. DNA Cell Biol 32(1):13–18. https://doi.org/10.1089/dna.2012.1776
Liu C, Chen T, Liu Z (2016) Associations between BRAF(V600E) and prognostic factors and poor outcomes in papillary thyroid carcinoma: a meta-analysis. World J Surg Oncol 14(1):241. https://doi.org/10.1186/s12957-016-0979-1
Tabriz N, Grone J, Uslar V, Tannapfel A, Weyhe D (2020) BRAF V600E mutation correlates with aggressive clinico-pathological features but does not influence tumor recurrence in papillary thyroid carcinoma-10-year single-center results. Gland Surg 9(6):1902–1913. https://doi.org/10.21037/gs-20-244
Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O’Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V (2013) Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309(14):1493–1501. https://doi.org/10.1001/jama.2013.3190
Ramkumar S, Sivanandham S (2021) The combined utility of HBME-1 and galectin-3 immunohistochemistry and BRAF V600E mutations in the diagnosis of papillary thyroid carcinoma. Cureus 13(12):e20339. https://doi.org/10.7759/cureus.20339
Acknowledgements
We would like to thank Professor Oğuz Öztürk and Professor Hulya Yilmaz Aydogan for their great support in statistical analysis and critical reading.
Author information
Authors and Affiliations
Contributions
A.D.A.: Methodology, analysis/interpretation, writing. H.D.: Methodology, writing. T.Ö.: Conceptualization, review and editing, supervision. All authors have read and approved the final draft.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study is in accordance with the Helsinki Declaration. The study protocol was accepted by the Clinical Research Ethics Committee of the Faculty of Medicine of Amasya University (Decision no:139, Date: 07/10/2021). Written and signed consent was obtained from all participants.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dağlar Aday, A., Demir, H. & Öztürk, T. Utility of Immunohistochemistry Markers CK19, Gal-3, HBME-1 and Their Combinations for Differential Diagnosis of Thyroid Diseases. Indian J Surg 85, 1203–1211 (2023). https://doi.org/10.1007/s12262-023-03760-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-023-03760-w