Abstract
Ultrasound-guided paravertebral nerve block can provide effective somatic analgesia, which is safe and suitable for surgical patients. In this study, paravertebral nerve block was applied into open unilateral inguinal hernia repair surgery to observe its safety and analgesic effect. A total of 83 patients scheduled for open unilateral inguinal hernia repair surgery were randomly divided into two groups according to computer-generated randomization sequence with different methods of anesthesia: 42 patients with subarachnoid block (group A) and 41 patients with paravertebral nerve block (group B). The two groups were applied with different appropriate anesthesia methods accordingly. The perioperative vital signs, visual analog scale scores, time and dosage of additional analgesics, time to get out of bed, and complications of the two groups were monitored and recorded. In group B, the vital signs were more stable during the operation, the postoperative analgesia time was longer, the time for additional analgesics after the operation was later, the dose was less, the time to get out of bed was earlier, and the perioperative complications were less (P < 0.05). Ultrasound-guided paravertebral nerve block could meet the anesthesia requirements for open unilateral inguinal hernia repair surgery and provide effective postoperative analgesia.
Clinical Trial Identifier: ChiCTR1800017575.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inguinal hernia is formed by internal organs protruding from the abdomen through the weak tissue in the inguinal area [1]. If the inguinal hernia is not treated timely, it is easy to cause serious complications such as intestinal obstruction and intestinal necrosis. Surgery is the only reliable method for the treatment of inguinal hernia. At present, the medically accepted surgical method is tension-free hernia repair, including open surgery and laparoscopic surgery [2]. This study focused on the selection of anesthetic methods for open unilateral inguinal hernia repair surgery (IHRS).
With the increasing application of ultrasound in the department of anesthesiology, ultrasound-guided paravertebral nerve block (PVB) has been successfully used in a variety of surgical anesthesia [3]. In this study, ultrasound-guided PVB was used to observe whether it could meet the anesthesia requirements for open unilateral IHRS and its postoperative analgesic effect.
Patients and Methods
This trail was approved by the Ethics Committee of the Medical Center (NO. LX2018-002), and written informed consent was obtained from all subjects participating in the trial. It has been reported in line with Consolidated Standards of Reporting Trials (CONSORT 2010) Guidelines. It was in accordance with the Helsinki Declaration of 1964 and all subsequent revisions. This study was a randomized controlled single blind clinical trial, and the computer random number table method was used for sampling (Fig. 1).
Patients undergoing open unilateral IHRS in the period from January to December 2020 were randomly allocated into two groups according to computer-generated randomization sequence.
Inclusion criteria included American Society of Anesthesiologists (ASA) I–III; cardiac function 1–2; aged from 30 to 85; stable control of blood pressure; cooperation with nerve block; being conscious; and unilateral inguinal hernia.
Exclusion criteria include tachycardia or bradycardia; infection at the site of puncturing; allergic to ropivacaine; history of substance abuse; with chronic pain; history of lumbar surgery; clotting dysfunction; morbid obesity; and mental dysfunction; intestinal necrosis.
A total of 42 patients were treated with subarachnoid block (group A) and 41 patients with PVB (group B). All patients had the surgery by the same group of general surgeons. The anesthesia was performed by the same anesthesiologist according to the corresponding puncturing procedure. After every patient entered the operating room, oxygen was administered through a nasal catheter (2L/min). ECG, pulse oxygen saturation and non-invasive blood pressure monitoring were performed. After peripheral venipuncture, 1-mg midazolam was injected intravenously before puncturing.
Group A: The patient was placed in the lateral decubitus position with the operating side downward. The back of the patient aligned with the edge of the operating bed, the head bent down, and the hands were folded. After the patient’s skin was disinfected and a sterile sheet was placed, the lumbar vertebrae (L) 3–4 space was selected as the puncturing point. Local anesthesia was performed. The anesthesiologist’s left hand pressed against the patient’s back and fixing the direction of the needle, while the right hand slowly penetrated the needle perpendicular to the skin through the puncturing point. When the cerebrospinal fluid was clear, 15 mg 0.5% ropivacaine was slowly injected into the subarachnoid space.
Then the patient was placed in the supine position, and the sensory regression was measured by acupuncture after 10 min. If the anesthesia level of the patient’s upper abdomen was between thoracic vertebrae (T) 6 and 10, the surgery was performed. Otherwise, it was changed to general anesthesia with the case excluded from the study.
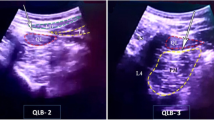
Group B: The patient was placed in the lateral decubitus position with the operating side upward. The GE Logiq E NextGen ultrasonic diagnostic instrument (GE, USA) was used with a low-frequency convex array probe (3–5 MHz). The patient’s skin was disinfected. The ultrasound probe was wrapped in a sterile sleeve, then placed perpendicular to the spine. Once reaching to the transverse process of T12, the inside of the probe was placed on the spinous process of T12 and moving downwards. Applying the in-plane technique, a 10-cm-long 20 G needle (B. Braun GmbH, Germany) was used for puncturing. Bypassing the transverse process, the local anesthetic was injected between the facet and the pleura to make the drug diffusing closer to the intervertebral foramen. Fifteen milliliter 0.5% ropivacaine was injected when the needle reached to the target position and withdrawn without blood or air. During injection, the parietal pleura could be observed sagging. The probe was then moved along the direction of the foot to the transverse process of L1. At a depth of 1.5 cm below the transverse process, between the lateral side of the vertebral body and the psoas major muscle, 15 ml 0.5% ropivacaine was injected once withdrawing the needle without blood or air.
Then the patient was placed in the supine position. The block effect was evaluated by acupuncturing every 5 min, lasting for 20 min. PVB was considered successful if the patient’s sensation disappeared between T10 and L2. Otherwise, PVB was considered failed, and the treatment was changed to general anesthesia with the case excluded from the study.
Anesthesiology residents not involved in the anesthesia were responsible for data recording. These residents were unaware of the method of anesthesia being administered to the patients. Heart rate (HR), mean arterial pressure (MAP), visual analog scale (VAS) scores, total dosage of sufentanil and parecoxib sodium, time of the first use of parecoxib sodium, time to get out of bed, and anaesthetis-related complications were monitored and recorded for both two groups during the perioperative period.
If the patient’s blood pressure was less than 20% of the baseline value after anesthesia, 6-mg ephedrine was given intravenously each time until the blood pressure recovered. If bradycardia occurred, 0.25 mg atropine was injected intravenously each time until HR recovered. The patient was given 10-μg sufentanil intravenously if VAS scores was above 4 during the operation. If the postoperative VAS scores was above 4, 40-mg parecoxib sodium was injected intravenously. The patient with perioperative nausea and vomiting was given 5-mg tolisetron intravenously.
If the patient requested to quit or the anesthesia method was changed at any time during the study, the study was terminated immediately. If the patient developed local anesthetic intoxication or other life-threatening complications, the study should be terminated and an appropriate treatment should be initiated immediately. In this study, one patient in group A was switched to general anesthesia because the block level was below T10. And the study was terminated for this case. In group B, one case was terminated for a patient having bilateral nerve block. It was considered the ropivacaine penetrated into the epidural space caused the epidural anesthesia. There was no case in both groups asking to quit the study. Therefore, 41 patients in group A and 40 patients in group B were included.
Statistical Analysis
The primary endpoint was a postoperative VAS scores higher than four. On the basis of previous studies, it was assumed that when there was a significant difference, 50% of patients in groups A and B had VAS scores above 4 at 2 h and 10 h after surgery, respectively. With a type I error of 0.05 and a power of 0.8, a sample size of 38 patients per group was required. We decided to include 83 patients to take into account possible loss of follow-up at 24-h evaluation. Raw data were entered into a Microsoft Excel Spreadsheet and analyzed using Statistical Package for the Social Sciences (SPSS Inc., version 22.0, Chicago, IL, USA). Continuous data were presented as mean with standard deviation while discrete categorical data were expressed as median (range) and number of patients and/or percentage of cases. Categorical variables were analyzed using Pearson’s Chi-square test, and normally distributed continuous variables were analyzed using the independent sample t test. P < 0.05 was considered statistically significant difference and P < 0.001 as highly significant difference.
Results
The primary indicators for outcome measurement were the perioperative VAS scores and time of the first use of parecoxib sodium. The secondary indicators were the time to get out of bed, analgesic dose, vital signs (HR and MAP), and complications.
Data of 81 patients were analyzed: 41 patients in group A and 40 patients in group B. The two groups were comparable in terms of demographic data, i.e.,: ASA, age, BMI, gender, duration of operation, and hospitalization days (P > 0.05) (Table 1).
There was no significant difference in HR and MAP between two groups in pre-operation, and at 10 h, 12 h, and 24 h after operation (P > 0.05). During operation, at 4 h, 6 h, and 8 h after operation, the HR and MAP of group A were significantly higher than group B (P < 0.05). After anesthesia, the MAP decrease in group A was significantly more than group B. The more stable vital signs after operation in group B were possibly related to the longer analgesia effect (Figs. 2 and 3).
There was no significant difference in VAS scores between the two groups in pre-operation, intraoperative stitching, and at 10 h, 12 h, and 24 h after operation (P > 0.05). VAS scores of group A was significantly higher than group B at 2 h, 4 h, 6 h, and 8 h after operation (P < 0.05). This suggested that the postoperative analgesic effect of group A only stayed for less than 2 h, while that of group B lasted for 8–10 h (Fig. 4).
The dosage of ephedrine in group A was significantly higher than group B (P < 0.05). It suggested that subarachnoid block could cause a drop in blood pressure, while PVB had little effect on blood pressure. There was no significant difference in the intraoperative dosage of sufentanil between the two groups; PVB could achieve the same analgesic effect as that by subarachnoid block during surgery (P > 0.05). Parecoxib sodium was added for the first time in group A at 2 h and group B at 9 h after surgery. PVB provided postoperative analgesia for a longer time than subarachnoid block after operation (P < 0.05). The total dosage of parecoxib sodium supplemental in group A was significantly higher than group B (P < 0.05). The postoperative time to get out of bed in group A was significantly later than group B (P < 0.05) (Table 2).
The complications of group A were significantly more than group B (P = 0.0130) (Table 3).
Discussion
IHRS is a common operation in general surgery [4]. In recent years, with the aggravation of aging society, the incidence of inguinal hernia is increasing [5]. The most common type of anesthesia for IHRS is intraspinal anesthesia. However, many patients suffer from diseases of the lumbar spine, such as spinous process hyperplasia and narrowing of lumbar space. Anatomical signs on the body surface of these patients are not clear, causing difficulties in anesthesia puncture [6]. General anesthesia is often used when intraspinal anesthesia is contraindicated or intraspinal anesthesia fails. However, the incidence of pulmonary infection is significantly higher after general anesthesia, especially for elderly patients [7]. Many patients have complications with cardiovascular and respiratory diseases, which increase the risk of anesthesia [8, 9]. Searching for the optimum anesthesia method to improve the safety of patients during perioperative period has always been a concern of anesthesiologists.
The open IHRS’s incision was 2 cm above the inguinal ligament [10]. The nerves in this area are mainly innervated by the iliohypoabdominal, ilioinguinal, and genital femoral nerves. These three nerves rise with the most from the anterior branches of spinal nerves of T12 and L1, and a small part from T11. With the in-depth research on thoracic paravertebral space, the technique of PVB has become mature with safety and effectiveness confirmed. PVB can block these three nerves simultaneously [11, 12].
With the increasing use of ultrasound-guided technology in anesthesia, visual and accurate PVB becomes feasible [13]. Ultrasound can locate the anatomy of the spine and monitoring the exact location of the nerves and the important structures surrounding them [14]. In the course of puncturing, the route of puncturing needle and the diffusion of local anesthetic can be observed through the whole process, which greatly improves the effect of PVB and avoids serious complications. Based on previous reports and our experience with PVB, 15 ml 0.5% ropivacaine injected at one level can spread to at least two levels above and below [15]. In this study, 15 ml 0.5% ropivacaine was injected at T12 and L1 each, which could be diffused to T10 to L2, covering the sensory block at the sites involved in IHRS. This reduced the number of spinal segments and the time needed for puncturing, which has also been demonstrated in this study. Patients with ultrasound-guided PVB experienced no intraoperative pain or discomfort of pulling.
It could be seen from this study that the duration of postoperative analgesia of PVB was significantly longer than that of subarachnoid block. But the lower limb motor nerves of group B were not blocked, allowing an earlier time to get out of bed and avoiding the discomfort and related complications caused by long-time in bed. The number of patients with postoperative uroschesis in group A was significantly larger than that in group B. We considered the following reasons: First, PVB did not block the sacral nerve with little effect on the bladder pressure reflex and the bladder detrusor contraction. Second, for patients in group B getting out of bed earlier, the abdominal pressure increased the pressure of the bladder and helping the bladder urination function to recover faster [16]. The blood pressure of group A decreased significantly during operation, while that of group B was stable. The dosage of ephedrine in group B was lower than group A. The reason was that subarachnoid blocked the efferent fibers of the sympathetic nerve. It caused the dilation of arteries and veins at the same time, reducing the amount of returned blood and causing a drop in blood pressure. This also increased the incidence of nausea and vomiting, and limiting the patient’s ability to get out of bed early. The biggest advantage of PVB was that its postoperative analgesic effect was significantly better than subarachnoid block [17]. In this study, VAS scores of group B was significantly lower than group A at 2 h, 4 h, 6 h, and 8,h after surgery.
PVB is not completely safe and can be associated with a number of complications [11], including local anesthetic intoxication, pneumothorax, kidney injury, bilateral nerve block, and nerve damage. In this study, ultrasound-guided PVB was used to complete puncturing and injection under visual conditions, which could avoid most serious complications. Bilateral nerve block occurred on one patient in group B due to the local anesthetic diffusing into the epidural space through the intervertebral foramen. It did not cause discomfort to the patient.
Conclusion
Ultrasound-guided paravertebral nerve block could satisfy the anesthesia for open unilateral inguinal hernia repair surgery. It had little effect on circulation and fewer complications, providing a long postoperative analgesia time and a quicker recovery for patients. Paravertebral nerve block was especially suitable for patients with poor cardiopulmonary function because it had fewer side effects and has little impact on patients’ respiratory and circulatory functions. It opened up the possibility of surgery for patients who might otherwise had been unable to have subarachniod anesthesia or general anesthesia. Ultrasound-guided paravertebral nerve block is well worth promoting. There were still some deficiencies in this study, such as no long-term follow-up visits with patients to observe the effect of paravertebral nerve block on postoperative chronic pain after inguinal hernia repair surgery. In addition, it is difficult for beginners to puncture via ultrasound-guided paravertebral nerve block which is a new technique, and considering the location of the paravertebral nerve is deep. It requires experienced anesthesiologists.
Materials and/or Code Availability
Not applicable.
References
Miller HJ (2018) Inguinal hernia: mastering the anatomy. Surg Clin North Am 98(3):607–621. https://doi.org/10.1016/j.suc.2018.02.005
Bullen NL, Massey LH, Antoniou SA, Smart NJ, Fortelny RH (2019) Open versus laparoscopic mesh repair of primary unilateral uncomplicated inguinal hernia: a systematic review with meta-analysis and trial sequential analysis. Hernia 23(3):461–472. https://doi.org/10.1007/s10029-019-01989-7
Kulhari S, Bharti N, Bala I, Arora S, Singh G (2016) Efficacy of pectoral nerve block versus thoracic paravertebral block for postoperative analgesia after radical mastectomy: a randomized controlled trial. Br J Anaesth 117(3):382–386. https://doi.org/10.1093/bja/aew223
Towfigh S (2018) Inguinal hernia: four open approaches. Surg Clin North Am 98(3):623–636. https://doi.org/10.1016/j.suc.2018.02.004
Pawlak M, Tulloh B, de Beaux A (2020) Current trends in hernia surgery in NHS England. Ann R Coll Surg Engl 102(1):25–27. https://doi.org/10.1308/rcsann.2019.0118
Park SK, Bae J, Yoo S, Kim WH, Lim YJ, Bahk JH, Kim JT (2020) Ultrasound-assisted versus landmark-guided spinal anesthesia in patients with abnormal spinal anatomy: a randomized controlled trial. Anesth Analg 130(3):787–795. https://doi.org/10.1213/ANE.0000000000004600
Miskovic A, Lumb AB (2017) Postoperative pulmonary complications. Br J Anaesth 118(3):317–334. https://doi.org/10.1093/bja/aex002
Bayrak M, Altıntas Y (2018) Comparing laparoscopic cholecystectomy in patients with chronic obstructive pulmonary disease under spinal anesthesia and general anesthesia. BMC Surg 18(1):65. https://doi.org/10.1186/s12893-018-0396-1
Saraswat V (2015) Effects of anaesthesia techniques and drugs on pulmonary function. Indian J Anaesth 59(9):557–564. https://doi.org/10.4103/0019-5049.165850
Abdulhai S, Glenn IC, Ponsky TA (2017) Inguinal hernia. Clin Perinatol 44(4):865–877. https://doi.org/10.1016/j.clp.2017.08.005
Ardon AE, Lee J, Franco CD, Riutort KT, Greengrass RA (2020) Paravertebral block: anatomy and relevant safety issues. Korean J Anesthesiol 73(5):394–400. https://doi.org/10.4097/kja.20065
Pushpanathan E, Pawa A (2016) Paravertebral block and access to the paravertebral space. Anaesthesia 71(11):1372–1373. https://doi.org/10.1111/anae.13657
D’Ercole F, Arora H, Kumar PA (2018) Paravertebral block for thoracic surgery. J Cardiothorac Vasc Anesth 32(2):915–927. https://doi.org/10.1053/j.jvca.2017.10.003
Tripathy S, Mandal I, Rao PB, Panda A, Mishra T, Kar M (2019) Opioid-free anesthesia for breast cancer surgery: a comparison of ultrasound guided paravertebral and pectoral nerve blocks. A randomized controlled trial. J Anaesthesiol Clin Pharmacol 35(4):475–480. https://doi.org/10.4103/joacp.JOACP_364_18
Krediet AC, Moayeri N, van Geffen GJ, Bruhn J, Renes S, Bigeleisen PE, Groen GJ (2015) Different approaches to ultrasound-guided thoracic paravertebral block: an illustrated review. Anesthesiology 123(2):459–474. https://doi.org/10.1097/ALN.0000000000000747
Bojaxhi E, Lee J, Bowers S, Frank RD, Pak SH, Rosales A, Padron S, Greengrass RA (2018) Paravertebral blocks reduce the risk of postoperative urinary retention in inguinal hernia repair. Hernia 22(5):871–879. https://doi.org/10.1007/s10029-018-1792-2
Fusco P, Cofini V, Petrucci E, Scimia P, Paladini G, Behr AU, Gobbi F, Pozone T, Danelli G, Di Marco M, Vicentini R, Necozione S, Marinangeli F (2016) Unilateral paravertebral block compared with subarachnoid anesthesia for the management of postoperative pain syndrome after inguinal herniorrhaphy: a randomized controlled clinical trial. Pain 157(5):1105–1113. https://doi.org/10.1097/j.pain.0000000000000487
Author information
Authors and Affiliations
Contributions
Pengcheng Xie: literature search; experimental studies; manuscript preparation; manuscript editing; manuscript review.
Yinglie Xu: concept; data acquisition; data analysis.
Yiming Wu: definition of intellectual content; data acquisition.
Xiang Ao: statistical analysis; design.
Corresponding authors
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Fudan University Pudong Medical Center (No. LX2018-002).
Consent to Participate
Informed consent was obtained from all individual participants included in the study. Patients signed informed consent regarding publishing their data.
Consent for Publication
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 1, 2, 3, and 4. This manuscript is approved by all authors for publication. I hereby declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Competing Interests
The authors declare no competing interests.
Data Sharing
No additional data available.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pengcheng Xie and Yinglie Xu are both first authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xie, P., Xu, Y., Wu, Y. et al. Randomised Controlled Clinical Trial of Spinal Anesthesia Versus Paravertrebral Block for Hernia Surgery. Indian J Surg (2023). https://doi.org/10.1007/s12262-023-03686-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12262-023-03686-3