Abstract
The Yes Associated Protein 1 (YAP1) is a transcriptional cofactor negatively regulated by Hippo Pathway. The dysregulation of the pathway has been shown to have a role in tumorigenesis and metastasis in several cancers including prostate cancer (PCa). In this study, YAP1 expression was upregulated in the whole cell lysates and cytoplasmic/nuclear extracts of AR negative (PC3) compared to AR positive (LNCaP) prostate cancer cell lines and primary prostate epithelial cells (PrePEC). pYAP1 expression elevated in LNCaP compared to PC3 and PrePEC in whole cell lysates and cytoplasmic extracts. The treatment of LNCaP and PC3 with YAP1-targeting siRNA oligonucleotides (YAP1 siRNA) significantly reduced their proliferation in vitro. Furthermore, treatment with YAP1 siRNA diminished the clonogenicity, anchorage-independent growth on soft agar, migration and invasion of PC3 cells. Co-IP/WB experiments revealed that YAP1 and AR formed a complex and ChIP/PCR results confirmed that YAP1 was bound to androgen response elements (ARE) core region of the prostate specific antigen (PSA) promoter. The loss of function experiments in LNCaP and PC3 revealed that YAP1 regulates proliferation, colony formation as well as anchorage-independent growth and potentially plays an important role in migration and invasion. Finally, analysis of publicly available data sets indicated that LNCaP had no YAP1 copy number alteration whereas PC3 had gain of YAP1 which was also reflected as an increase in the mRNA level. Moreover, YAP1 copy number gain and elevated YAP1 mRNA expression were detected in clinical samples analyzed in publicly available data sets. Taken together, these results suggested that YAP1 has a role in PCa tumorigenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Hippo pathway is a prominent tumor suppressor pathway controlling cell proliferation, cell death and differentiation [1, 2]. The Yes associated protein 1 (YAP1) is a transcription co-factor in the pathway regulating gene expression. Its function is mediated by the core components of the pathway kinases serine threonine kinase 1 and 2 (MST1/2) and the large tumor suppressor serine/threonine protein kinases 1 and 2 (LATS1/2) through phosphorylation. When the Hippo pathway is active, YAP1 is phosphorylated at serine 127 residue (S127). This phosphorylation causes inactivation of YAP1 by sequestration in the cytoplasm through binding to 14–3-3 binding proteins [3]. When the Hippo pathway is inactive, YAP1 translocates to the nucleus and drives the expression of genes together with transcription factors functioning as a transcription co-activator or co-repressor [4]. The best-characterized transcription factors regulated by YAP1 are the TEAD/TEF family transcription factors [5]. Besides TEAD, YAP1 also interacts with other transcription factors such as SMAD [6], p73 [7], ERBB4 [8].
Prostate cancer is the most common solid tumor remaining a leading common cause of death in males despite the early diagnostic efforts [9]. The most common metastatic targets for prostate cancer cells are lymph nodes, bone, lung, and liver [10]. The reason for most of the deaths is the development of distant metastasis of prostate cancer cells to these sites. Androgen deprivation is the main therapy for the patients with metastatic prostate cancer. However, many of the patients develop resistance to androgen blockade resulting in castration resistant prostate cancer (CRPC). The recurrent androgen receptor activation as well as the contribution of transcription factors, oncogenes, and tumor suppressors lead to the progression of prostate cancer to an incurable state [11]. The loss of expression or overexpression of YAP1 has been observed in various cancer types implicating that the protein has a role in carcinogenesis [12,13,14,15,16] . It has also been reported that YAP1 has a role in the metastatic progression of cholangiocarcinoma, colorectal cancer, gastric cancer, head and neck cancers and non-small cell lung cancer [14, 17,18,19,20]. In this study, using loss of function experiments in prostate cancer cell lines followed by functional assays such as cell viability, proliferation, migration, invasion, clonogenic and anchorage-independent growth assays we examined the functional significance of YAP1 in prostate cancer. Furthermore, we investigated YAP1 and AR relationship in protein-protein interaction and genomic level by Co-IP and ChIP experiments respectively.
Materials and Methods
Cell Culture
PC3, LNCaP cell lines were obtained from DSMZ (German Collection of Microorganisms and Cell Cultures, GmbH, Braunschweig, Germany). These cell lines were cultured in RPMI media (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (Gibco) in a humidified incubator containing 5% CO2 at 37 °C. PrePEC cells were obtained from Lonza (Walkersville, MD, USA). They were cultured in PreEGM bullet kit (Lonza) as recommended by the supplier.
siRNA Mediated Knockdown of YAP1
YAP1 targeted siRNA pool and non-targeting control siRNA were purchased from Dharmacon (GE Healthcare, Lafayette, CO, USA). Non-targeting siRNA was served as control. 20 nM siRNA was used to transfect cells with DharmaFect II (GE Healthcare) or Lipofectamin 2000 (Invitrogen, Carlsbad, CA, USA).
Cell Proliferation Assay
The cell proliferation was measured by cell counting. PC3 or LNCaP cells were seeded in 6 well plates and transfected with control or YAP1 siRNA on the following day. After 72 h of incubation, the cells were harvested and counted with a hemocytometer.
Colony Formation Assay
After 48 h knock-down of YAP1 with siRNAs, second transfection was performed in PC3 cells. Following 24 h of second transfection, the cells were harvested and reseeded to 10 cm cell culture dishes at a density of 2000 cells/plate. The cells were incubated in normal growth medium for 21 days and stained with 0.5% crystal violet (Santa Cruz, Dallas, TX, USA) at the end of incubation period.
Soft Agar Colony Formation Assay
Following 48 h transfection with 20 nM YAP1 siRNAs, transfection was repeated in PC3 cells. 24 h after second transfection cells were harvested and mixed with 0.6% agarose (Invitrogen) in 1:1 ratio. 6 well plates were prepared by applying 0.5% agarose as a supporting matrix. Cells mixed with agarose were layered up to this matrix. After 21 days of incubation, formed colonies were observed under the microscope (Nikon Eclipse LV150N, Amsterdam, Netherlands).
Immunofluorescence (IF)
The cells were seeded on coverslips in 6 well-plates. After 24 h, the cells were fixed with 4% formaldehyde; the cell membrane was permeabilized with 0.2% Triton X-100 detergent. The antibodies (YAP1 or pYAP1) (Cell Signaling Technology, Danvers, MA, USA, 1:50) were applied and incubated for 1 h at 37 °C. DAPI (4′,6-diamidino-2-phenylindole) (Abcam, Cambridge, UK) was used to counterstain the nucleus. The cells were observed under the fluorescence microscope (Zeiss, Oberkochen, Germany).
Cytoplasmic/Nuclear Fractionation and Western Blot Analysis
The cytoplasmic and nuclear fractions were prepared according to the manufacturer’s instructions (Affymetrix, Santa Clara, CA, USA). Whole Cell lysates were prepared using lysis buffer (150 mM NaCl, 20 mM HEPES, 0.5% NP40, 1 mM EDTA, 20 mM Tris-HCl supplemented with the phosphatase and protease inhibitors (Roche, Indianapolis, IN, USA). The protein concentration was determined with DC protein detection kit (Biorad, Hercules, CA, USA). Equal amounts of proteins were separated by SDS polyacrylamide gel electrophoresis and blotted to the nitrocellulose membranes. The membranes were blocked with 5% skimmed milk in PBS. The antibodies and their dilutions used for detection were as follows: anti-YAP1 (1:1000), anti-β-actin (1:1000), anti-pYAPS127 (1:1000) from Cell Signaling Technology and anti-AR (1:1000) from EMD Millipore (Temecula, CA, USA) and Cell Signaling Technology. The signals were detected using SuperSignal® West Pico Chemiluminescent Substrate (Thermoscientific, Rockford, IL, USA) and visualized in Azure c300 imaging system (Azure Biosystems, Dublin, CA, USA).
Transwell Migration and Invasion Assay
The cells were seeded in 6 well-plates and transfected with YAP1 siRNA. After 24 h, the cells were transferred to 8 μm pore size transwells (Corning, Corning, NY, USA) in 1% FBS medium. The medium with 10% and 20% FBS in the lower chamber of transwell were used as chemo attractant for PC3 and LNCaP cells respectively. The transwells were treated with 25% matrigel (Corning,) before seeding the cells for invasion assay. After 48 h of incubation in transwells, the cells in the upper chamber were removed by scraping with cotton swabs. The cells transpassed in the lower part were fixed with methanol and the nucleus of the cells were stained with DAPI (Abcam) and imaged with the fluorescence microscope (Zeiss). The quantification of migrated/invaded cells was performed using ImageJ program.
Chromatin Immunoprecipitation (ChIP)
ChIP was performed using Pierce ChIP kit (Pierce BiotechnologyRockford, IL, USA) by following the manufacturer’s instructions. Briefly, the cells were cross-linked with 1% formaldehyde in cell culture media for 10 min. The crosslinking was terminated with glycine solution. The chromatin was prepared with MNase digestion and precipitated with AR (EMD Millipore and Cell Signaling Technology in 1:1 ratio) or YAP1 (Cell Signaling Technology) antibodies. The precipitated DNA was isolated and the conventional PCR was performed to detect target sequences. ARE in PSA gene promoter region was used to confirm ChIP results. The primer sequences were as follows: AREI F: 5’ AGAACAGCAAGTGCTAGCTC-3′, R: 5’-AGGTGGTAAGCTTGGGGC-3′. The PCR reaction conditions were: 94 °C 5 min, 94 °C 20 s, 61 °C 10 s, 72 °C 30 s total 32 cycles and 72 °C 5 min final extension. The PCR products were separated in 2% agarose gel including ethidium bromide. The gel was visualized in Azure c300 bioimaging system (Azure Biosystems).
Co-Immunoprecipitation (CoIP)
Whole cell lysates were prepared with lysis buffer (100 mM NaCl, 50 mM Tris-HCl, 1%NP40, 0.5%sodium deoxycholate supplemented with the phosphatase and protease inhibitors). The immune complexes were precipitated with AR antibody (EMD Millipore and Cell Signaling Technology in 1:1 ratio) and collected with Protein A/G agarose beads (Thermoscientific). The immunoprecipitates were resolved in 4–12% SDS-PAGE and western blot analysis was performed to detect YAP1 in the immune complexes.
Copy Number Alteration and Gene Expression Analysis Using Publicly Available Datasets
The amplification and mRNA expression of YAP1 gene in prostate cancer cell lines and tissue samples were analyzed by using publicly available datasets. The data and graphs were retrieved from cbioportal database [21, 22] The prostate cancer cell lines were analyzed using MSKCC, Cancer Cell 2010 dataset and the tissue samples were analyzed using TCGA (The Cancer Genome Atlas), PanCancer dataset.
Results
YAP1 Localizes both in the Cytoplasm and Nucleus of Prostate Cancer Cells
In order to determine how YAP1 expression and activity is changed during prostate cancer progression from a less aggressive to more aggressive state, we assessed the protein level and localization in prostate cancer cell model systems. A well differentiated, androgen-dependent LNCaP and less differentiated, androgen-independent PC3 cell lines were chosen for this purpose. A non-cancerous cell model PrePEC was used as a control. Compared to the normal PrePEC cells, YAP1 expression levels were down regulated in LNCaP cells and upregulated in PC3 cells whereas the level of phosphorylation of YAP1 on S127 was increased in LNCaP and was not changed in PC3 cells (Fig. 1a). The cell fractionation assays indicated that PC3 cells (androgen receptor negative) had more YAP1 protein in the cytoplasm and nucleus compared to LNCaP cells (androgen receptor positive) and non-cancerous PrePEC cells (Fig. 1b). The phosphorylation of YAP1 on S127 was higher in the cytoplasm of LNCaP cells compared to PC3 and PrePEC. Immunofluorescence (IF) confirmed the westernblot analysis results (Fig. 2).
YAP1 expression and localization in prostate cells with western blot analysis. a. YAP1 expression was higher in PC3 cells and lower in LNCaP cells compared to PrePEC cells. pYAP1 expression was higher in LNCaP and lower in PC3 cells compared to PrePEC cells. b. YAP1 was localized both in cytoplasm and nucleus and pYAP1 was mainly cytoplasmic in prostate cells
Loss of YAP1 Expression Inhibited the Proliferation, Migration and Invasion Potential of Prostate Cancer Cells
To study the effect of YAP1 loss in prostate cancer cells, YAP1 mRNA was knocked down with specific siRNA. siRNA application reduced YAP1 protein level both in LNCaP (Fig. 3a) and PC3 (Fig. 4a) cell lines. After 72 h of siRNA treatment, cells were counted. The loss of YAP1 expression decreased cell numbers both in LNCaP and PC3 cell lines (Figs. 3b and 4b). When the transwell assay was performed, YAP1 siRNA treated LNCaP cells could not migrate to the other side of the membrane (Fig. 3c, d). Both migration and invasion ability of PC3 cells diminished upon YAP1 silencing (Fig. 4f, g). The loss of YAP1 in PC3 cells caused a decline in the monolayer colony formation ability of the cells (Fig. 4c, d). In addition, YAP1 siRNA treated PC3 cells formed smaller and fewer colonies in soft agar (Fig. 4e).
Effects of YAP1 knock-down on tumorigenic properties of LNCaP cells. a. YAP1 was silenced in LNCaP cells after 72 h. b. Knock down of YAP1 in LNCaP cells reduced cell proliferation. c. Silencing of YAP1 in LNCaP cells decreased the number of migrated cells. d. Representative DAPI staining of migrated LNCaP cells. All experiments were repeated at least 2 times. Data are presented as means ± SD and analyzed with Student’s t test. Statistical significance denoted as * p < 0.05, ** p < 0.01, *** p < 0.001
Effects of YAP1 knock-down on tumorigenic properties of PC3 cells. a. YAP1 was silenced in PC3 cells after 72 h. b. Knock down of YAP1 inhibited proliferation in PC3 cells. c. Colony forming ability of PC3 cells diminished when YAP1 expression was inhibited. d. Representative images for colony formation. e. YAP1 silenced PC3 cells formed smaller and fewer colonies in 3D. Silencing YAP1 in PC3 cells caused fewer invading (f) and migrating (g) cells in Boyden chambers
YAP1 Forms a Protein Complex with AR and Acts as a Transcriptional Cofactor
YAP1 is a transcriptional cofactor and AR is a well-studied transcription factor in prostate cancer. These facts led us to think that AR and YAP1 might act together to regulate the gene expression in prostate cancer cells. In order to test this hypothesis, firstly, the protein interaction of YAP1 and AR were studied using CoIP. It was shown that AR and YAP1 formed a protein complex in LNCaP cells (Fig. 5a). Secondly, the interaction between ARE in DNA and YAP1 protein was determined by ChIP analysis. After precipitation of chromatin with YAP1 antibody, it was shown that YAP1 resided in ARE in an AR target gene PSA (Fig. 5b). The binding of AR in the same region was also confirmed in our ChIP experiment (Fig. 5b).
YAP1 Gene Is Amplified and YAP1 mRNA Transcript is Elevated in some of the Prostate Cancer Cell Lines and Tissue Samples
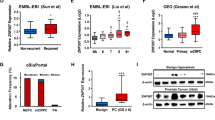
To better elucidate if the overexpression of YAP1 in PC3 was cell model specific, genetic alteration of YAP1 gene was analyzed using publicly available prostate cancer cell line data (MSKCC, 2010). The results indicated that YAP1 was amplified in PC3 cell line but not in LNCaP. This was also reflected in level of YAP1 mRNA. In our experimental work we also observed higher YAP1 protein level in PC3 than in LNCaP. This result may be due to the amplification of YAP1 gene in PC3 (Fig. 6a).
Analysis of YAP1 copy number alterations and mRNA level in prostate cell lines and tissue samples using publicly available datasets. a. LNCaP cell line had no copy number alteration in terms of YAP1 but PC3 had. Therefore, mRNA level of YAP1 was also higher in PC3 (dataset:MSKCC, Cancer Cell 2010). b. In prostate cancer tissue samples, gain of 11q was seen in 3.2% of the cases (14/430). This gain also altered the copy number of YAP1 gene (dataset: TCGA, PanCancer). c. Copy number gain in YAP1 gene also increased the mRNA level of YAP1 (dataset: TCGA, PanCancer). All the data and graphs were retrieved from cbioportal database. (Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1)
To assess if similar results were observed in prostate cancer patients, the tissue samples in TCGA dataset (TCGA, PanCancer dataset) was analyzed. YAP1 gene amplification was detected in 3.2% of cases (Fig. 6b). This proved that YAP1 amplification was not cell line specific but the clinical samples also have the same genetic aberration. YAP1 gene amplification was also reflected as increase in the expression of YAP1 mRNA (Fig. 6c).
Discussion
YAP1 is a member of the Hippo pathway which plays important roles in cellular proliferation, apoptosis and contact inhibition [23]. As the transcriptional effector of the pathway, YAP1 is a crucial member whose role needs to be elucidated clearly. There have been conflicting outcomes in cancer studies suggesting YAP1 as a tumor suppressor or promoter [12, 13]. These results make it difficult to suggest YAP1 as a therapy target. In our study, we aimed to understand the role of YAP1 in cellular proliferation, migration, invasion, monolayer and 3D colony formation abilities in prostate cancer cell models. Furthermore, AR and YAP1 interaction in protein and genomic levels were studied.
One of the basic features of cancer cells is to proliferate fast and in an uncontrolled manner. In our study, we determined that both AR positive LNCaP cells and AR negative PC3 cells proliferated slower when YAP1 expression was silenced. Zhang et al. observed the same effect in LNCaP-C4–2 cells which are castrate resistant prostate cancer cell model. They also showed that culturing cells in growth factor deprived medium instead of whole serum supplemented medium enhanced this effect emphasizing YAP1 as a target in overcoming castrate resistance in prostate cancer [24]. Kuser-Abali et al. confirmed this result even in serum fed conditions [25]. Sheng et al. further analyzed the cell cycle and apoptosis upon YAP1 silencing in PC3 cells and found out that YAP1 knock down arrested cells in G1/S and caused apoptosis [26].
The organ confined stage of prostate cancer is manageable with existing therapies. However, when the disease metastasizes to other organs, few therapy options are available. Many studies have pointed out the role of YAP1 in prostate and other solid tumors metastasis. It was found as a driver for metastasis in medullablastoma by comparing primary and metastatic tumor of the same patient with proteomic analysis [27]. Lee et al. enlightened the role of YAP1 in tumor cell migration in prostate cancer cells mechanistically. The shear stress in lymphatics wall induced ROCK mediated stress fiber formation and this activated YAP1 mediated gene transcription. YAP1 changed the transcription of genes with roles in chemotaxis, formation of lamellipodia/filopodia, invasion and adhesion [28]. Consistent with this explanation, we found out that YAP1 silenced PC3 and LNCaP cells had a decline in their migration ability. Kuser-Abali et al. observed that YAP1 silencing reduced invasion rate of LNCaP C4–2 cells extending our observation to a castration resistant cell model [25]. The role of YAP1 in migration and invasion is evident even in normal prostate cell line RWPE1. Zhang et al. demonstrated that RWPE1 cells could gain migration and invasion potential when YAP1 was overexpressed [24].
In a recent study, Haemmerle et al. studied the effect of platelets in tumor microenvironment on tumor anoikis and metastasis. They disclosed that platelets induced resistance to anoikis and promoted metastasis. When the mechanism of this effect was investigated, it became clear that YAP1 in tumor cells mediated the effect of platelets. Silencing of YAP1 in vivo abolished the platelets induced metastasis. Moreover, overexpression of YAP1 in ovarian cancer cell lines induced anchorage independent growth in soft agar colony formation assay both independent and dependent on platelets [29]. In parallel to this finding we also observed that silencing YAP1 in PC3 cells reduced the colony formation ability both in two dimensions (2D) and three dimensions (3D).
YAP1 does not have the DNA binding ability itself but elucidates its effect by interacting with other transcription factors. The well-known binding partners of YAP1 are TEAD1–4 transcription factors [30]. SMAD1 [6], SMAD2/3 [31], SMAD7 [32], RUNX1/2 [33], ErbB4 [34] are other transcription factors reported to interact with YAP1. AR is a member of nuclear receptor family. It acts as a transcription factor in a ligand dependent manner. AR is crucial for function of normal prostate epithelium and its aberrations such as mutations, amplification and overexpression are common in castration resistant prostate cancer (CRPC) [35]. Therefore, it is a therapy target for prostate cancer especially for CRPC. Several transcriptional cofactors for AR have been reported in the literature [36]. In our study we determined that AR and YAP1 protein interacted in LNCaP cells in full growth medium. Kuser-Abali et al. observed the same result and they also showed that this protein complex formation occurred in the nucleus of LNCaP and LNCaP-C4–2 cells. Furthermore, they also determined that this protein interaction was direct and N terminal domain of AR was bound to the WW/SH3 domain of YAP1 [25]. We also showed that YAP1 resided in the promoter region of AR target gene PSA in LNCaP cells by ChIP experiment. Kuser-Abali et. al uncovered that YAP1 silencing inhibited the transcription of AR target genes PSA, FKBP5, PSMA, TMPRSS2 [25]. Zhang et al. reported that YAP1 knockdown reduced the basal levels of PSA and NKX3.1 mRNA [24]. These results together with our findings revealed that YAP1 can act as a cofactor for AR in AR mediated gene transcription (Fig. 5c).
The present study pointed out that the expression and localization of phosphorylated and unphosphorylated YAP1 proteins were dysregulated in prostate cancer cell lines indicating that YAP1 regulation and interactions with respect to other proteins changed in PCa progression. Nguyen et al. studied YAP1 and ERG interaction in prostate cancer and determined that ERG resided around TEAD binding motifs on DNA and increased histone H3K9/14 acetylation to facilitate YAP1 mediated transcriptional activation of target genes [37, 38].
Although aberrant overexpression of some proteins is a mechanism for tumor development, this is not the sole mechanism [39]. As it was observed in this study, knock-down of YAP1 displayed anti-cancer effect such as decrease in proliferation and migration both in PC3 and LNCaP cell lines. However, only PC3 cells had amplification and overexpression of YAP1 (Fig. 6a). Therefore, the results did not exclude other mechanisms such as change in protein-protein interactions would cause cancer promoting effect of YAP1 rather than the overexpression. In accordance with other published studies, this study demonstrated that YAP1 regulated motility, invasion and proliferation of prostate cancer cells. It could be speculated that this regulation could be mediated by YAP1 and AR protein-protein interactions as observed in AR positive LNCaP cells. Further studies are also needed to explain the mechanism of action of YAP1 in androgen insensitive AR negative cell line PC3.
References
Harvey KF, Pfleger CM, Hariharan IK (2003) The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 114:457–467
Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso W (2014) The hippo pathway effector yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell 30:137–150
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the hippo pathway is involved in cell contact inhibition and tissue growth control. 2007;2747–61
Ehmer U, Sage J (2016) Control of proliferation and Cancer growth by the hippo signaling pathway. Mol Cancer Res 14:127–140
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22:1962–1971
Alarcon C, Zaromytidou A-I, Xi Q, Gao S, Yu J, Fujisawa S et al (2009) Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF- pathways. Cell. 139:757–769
Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, Oren M, Sudol M, Cesareni G, Blandino G (2001) Physical interaction with yes-associated protein enhances p73 transcriptional activity. J Biol Chem 276:15164–15173
Haskins JW, Nguyen DX, Stern DF (2014) Neuregulin 1-activated ERBB4 interacts with YAP to induce hippo pathway target genes and promote cell migration. Sci Signal 7:ra116
Attard G, Parker C, Eeles RA, Schröder F, Tomlins SA, Tannock I, Drake CG, de Bono JS (2016) Prostate cancer. Lancet. 387:70–82
Disibio G, French SW (2008) Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med 132:931–939
Schrecengost R, Knudsen KE (2013) Molecular pathogenesis and progression of prostate Cancer. Semin Oncol 40:244–258
Hu X, Jia Y, Yu J, Chen J, Fu Q (2015) Loss of YAP protein in prostate cancer is associated with Gleason score increase. Tumori. 101:189–193
Yuan M, Tomlinson V, Lara R, Holliday D, Chelala C, Harada T, Gangeswaran R, Manson-Bishop C, Smith P, Danovi SA, Pardo O, Crook T, Mein CA, Lemoine NR, Jones LJ, Basu S (2008) Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ 15:1752–1759
Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X (2010) Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci 101:1279–1285
Xu MZ, Yao T-J, Lee NPY, Ng IOL, Chan Y-T, Zender L, Lowe SW, Poon RTP, Luk JM (2009) Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 115:4576–4585
Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T, Hilsenbeck SG, Orsulic S, Goode S (2010) Hippo pathway effector yap is an ovarian cancer oncogene. Cancer Res 70:8517–8525
Zhang C, Liang K, Zhou G, Zhang Q, Li J (2014) Expression of hippo pathway in colorectal cancer. Saudi J Gastroenterol 20:188
Zhou G-X, Li X-Y, Zhang Q, Zhao K, Zhang C-P, Xue C-H, Yang K, Tian ZB (2013) Effects of the hippo signaling pathway in human gastric cancer. Asian Pac J Cancer Prev 14:5199–5205
Ge L, Smail M, Meng W, Shyr Y, Ye F, Fan K-H, Li X, Zhou HM, Bhowmick NA Yes-associated protein expression in head and neck squamous cell carcinoma nodal metastasis. Ramqvist T, editor. PLoS One 2011;6:e27529
Pei T, Li Y, Wang J, Wang H, Liang Y, Shi H et al (2015) YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget. 6:17206–17220
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N (2012) The cBio Cancer genomics portal: an open platform for exploring multidimensional Cancer genomics data. Cancer Discov 2(5):401–404
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1
Bao Y, Hata Y, Ikeda M, Withanage K (2011) Mammalian hippo pathway: from development to cancer and beyond. J Biochem 149:361–379
Zhang L, Yang S, Chen X, Stauffer S, Yu F, Lele SM et al (2015) The hippo pathway effector YAP regulates motility , invasion , and castration-resistant growth of. Prostate Cancer Cells 35:1350–1362
Kuser-Abali G, Alptekin A, Lewis M, Garraway IP, Cinar B (2015) YAP1 and AR interactions contribute to the switch from androgen-dependent to castration-resistant growth in prostate cancer. Nat Commun 6:8126
Sheng X, Bin LW, Wang DL, Chen KH, Cao JJ, Luo Z et al (2015) YAP is closely correlated with castration-resistant prostate cancer, and downregulation of YAP reduces proliferation and induces apoptosis of PC-3 cells. Mol Med Rep 12:4867–4876
Gu S, Chen K, Yin M, Wu Z, Wu Y (2016) Proteomic profiling of isogenic primary and metastatic medulloblastoma cell lines reveals differential expression of key metastatic factors. J Proteome 160:55–63
Lee HJ, Diaz MF, Price KM, Ozuna JA, Zhang S, Sevick-muraca EM, et al. Fluid shear stress activates YAP1 to promote cancer cell motility. 2017; 8:14122
Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, Lyons YM, Nagaraja AS, Dood RL, Wen Y, Mangala LS, Hansen JM, Rupaimoole R, Gharpure KM, Rodriguez-Aguayo C, Yim SY, Lee JS, Ivan C, Hu W, Lopez-Berestein G, Wong ST, Karlan BY, Levine DA, Liu J, Afshar-Kharghan V, Sood AK (2017) Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat Commun 8:310
Chai J, Xu S, Guo F (2017) TEAD1 mediates the oncogenic activities of hippo-YAP1 signaling in osteosarcoma. Biochem Biophys Res Commun 488:297–302
Grannas K, Arngården L, Lönn P, Mazurkiewicz M, Blokzijl A, Zieba A et al (2015) Crosstalk between hippo and TGFβ: subcellular localization of YAP/TAZ/Smad complexes. J Mol Biol 427:3407–3415
Ferrigno O, Lallemand F, Verrecchia F, L’Hoste S, Camonis J, Atfi A et al (2002) Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene 21:4879–4884
Vitolo MI, Anglin IE, Mahoney WM, Renoud KJ, Gartenhaus RB, Bachman KE, Passaniti A (2007) The RUNX2 transcription factor cooperates with the YES-associated protein, YAP65, to promote cell transformation. Cancer Biol Ther 6:856–863
Omerovic J, Puggioni EM, Napoletano S, Visco V, Fraioli R, Frati L et al (2004) Ligand-regulated association of ErbB-4 to the transcriptional co-activator YAP65 controls transcription at the nuclear level. Exp Cell Res 294:469–479
Hoang DT, Iczkowski KA, Kilari D, See W, Nevalainen MT (2017) Androgen receptor-dependent and -independent mechanisms driving prostate cancer progression: opportunities for therapeutic targeting from multiple angles. Oncotarget 8:3724–3745
Shiota M, Yokomizo A, Fujimoto N, Naito S (2011) Androgen receptor cofactors in prostate Cancer: potential therapeutic targets of castration-resistant prostate Cancer. Curr Cancer Drug Targets 11:870–881
Nguyen, Liem T.l. Nguyen LT, Tretiakova MS, Silvis MR et al. EA the YTP and I the D of A-RPTCC 2015;27(6):797–808. doi:10. 1016/j. ccell. 2015. 05. 005.
Tretiakova MS, Silvis MR, Lucas J, Klezovitch O, Coleman I et al (2015) ERG Activates the YAP1 Transcriptional Program and Induces the Development of Age-Related Prostate Tumors. Cancer Cell 27:797–808
Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP, Brabletz T ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun ; 2016;7:10498
Acknowledgements
This work is financially supported by the The Scientific and Technological Research Council of Turkey (TUBITAK) (P.No:114S419) and Istanbul Medeniyet University Scientific Research Grants (FBA-2014-293).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest. This article does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Collak, F.K., Demir, U. & Sagir, F. YAP1 Is Involved in Tumorigenic Properties of Prostate Cancer Cells. Pathol. Oncol. Res. 26, 867–876 (2020). https://doi.org/10.1007/s12253-019-00634-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00634-z