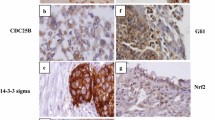
Abstract
The aim of the present study was to investigate the relationship between the intensity of biomarker expression and the response to radiochemotherapy in patients with advanced esophageal squamous cell cancer (ESCC). Ninety-two patients with locally advanced ESCC were examined retrospectively. Pre-treatment tumor samples were stained for proteins SOUL, Hsp 16.2, Growth Hormone-Releasing Hormone Receptor (GHRH-R) and p-Akt using immunhistochemistry methods. Kaplan-Meier curves were used to show the relationship between intensity of expression of biomarkers and clinical parameters and 3-year OS. A significant correlation was found between high intensity staining for Hsp 16.2, p-Akt and SOUL and poor response to NRCT. Application of a higher dose of radiation and higher dose of cisplatin resulted in better clinical and histopathological responses, respectively. Among the clinical parameters, the localization of the tumor in the upper-third of the esophagus and less than 10% weight loss were independent prognostic factors for increased 3-year OS. Hsp16.2, p-Akt and SOUL are predictors of negative response to NRCT, therefore these biomarkers may become promising targets for therapy. Furthermore, level of expression of p-Akt, weight loss and the localization of the tumor are significant factors in the prediction of OS in ESCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is one of the most lethal malignancies and ranks as the eighth most common cancer in the world and the sixth most common cause of death from cancer [1]. The localization of esophageal squamous cell cancer (ESCC) is generally in the upper two-thirds of the esophagus and the development of the ESCC has been linked to nicotine and drug abuse as well as to poor socioeconomic status [2]. Neoadjuvant radiochemotherapy (NRCT) is the accepted modality of therapy for locally advanced ESCC, since preoperative radiochemotherapy has been shown to increase long-term survival [3,4,5]. However, the prognosis for ESCC, especially in advanced stages, remains dismal, despite improvements in multimodal treatment [6].
Response to treatment can be optimized by tailoring the dosage of administered cisplatin, 5-FU and irradiation. According to an earlier study the dose of irradiation, between 30 and 45 Gys, is directly correlated with the complete pathological response of the ESCC at stage II/III ESCC [7]. A meta-analysis involving 1335 patients showed that there is a dose-response relationship between increasing protocol prescribed radiotherapy, 5-fluorouracil, cisplatin dose and pathological complete response to treatment [8]. In the present study we sought to find confirmation of these previous findings in our chosen group of patients. A number of patients receiving NRCT respond poorly or do not respond at all to therapy. There have been numerous studies examining potential markers of response to treatment in order to avoid unnecessary toxicity to patients and to improve their life-quality and survival [9, 10]. The activation of the anti-apoptotic phosphorylated-Akt (p-Akt)-mediated pathways, such as those for PI3K/Akt, Akt/NF-KB and Akt/XIAP, has been shown to correlate with a poor response to NRCT and lower overall survival of patients [11,12,13]. Consequently, the proteins activating the pAkt pathways, for example Heat shock protein 90 (Hsp-90) and protein Aurora-A, have been identified as possible targets of therapy [12, 14]). The levels of Heat shock proteins (Hsps) in tumor specimens have also been correlated with response to treatment. A previous investigation indicated that the expression level of HSP27 may be inversely correlated with the metastatic behavior of ESCC, furthermore another working group found that a higher expression of Hsp-27 was positively correlated with the grade of differentiation of ESCC [15, 16]. Earlier, we reported that small heat-shock protein (sHsp)16,2, Hsp90, heme-binding protein 2 (SOUL) expression as well as Bax/ Bcl-2 ratio correlated with the efficacy of NRCT and could predict outcome in patients with locally advanced ESCC [17]. The identification of proteins that signal poor response to treatment is essential as these can be targets for individualized, more effective therapy. Clinically, the determination of the survival rate of patients is equally important as evaluating the response to treatment, which is measured by the tumor regression grade (TRG) and the clinical downstaging of the tumor.
The purpose of this retrospective analysis was therefore 3-fold. We aimed to correlate possible predictive markers of response to NRCT in ESCC as well as their expression to 3-year overall survival. It was also our goal to determine whether dose of NRCT had any effect on the clinical and histological response to NRCT. Finally, we evaluated the association between the clinical parameters (age, Karnowsky index, tumor localization, weight loss) of the patients’ and their 3-year overall survival.
Patients and Methods
Patients and Tumor Specimens
Ninety two consecutive patients with inoperable, loco-regionally advanced (cT3–4, cN0–1, cM0) squamous-cell esophageal cancer received neoadjuvant NRCT from 2006 to 2010. The pre-treatment staging procedures consisted of endoscopy with biopsy, computed tomography (CT) scan of chest and abdomen and bronchoscopy. The patients were treated with external-beam radiotherapy (a total of 36 to 45 Gy, fraction dose: 1.8–2 Gy) and concomitant chemotherapy during the first week of irradiation: cisplatin (60–100 mg/m2 intravenously on day 1) and 5-fluorouracil (750–1000 mg/m2/day, by continuous intravenous infusion through days 1–5) (Table 1). Four weeks after the completion of NRCT, restaging was performed and clinical response to treatment was assessed according to RECIST [18]. Six to nine weeks after neoadjuvant therapy the patients underwent surgical resection, if there was no evidence of disease progression. Pathological response to treatment was determined by histological evaluation of the resected specimen. The histopathological tumor regression grade based on the presence of residual tumor cells and the extent of fibrosis was evaluated. For this purpose the five point tumor regression grading (TRG) system adapted from Mandard et al. was used [19]. The system consists of the following grades: TRG 1 (complete regression) is defined as the absence of residual tumor and fibrosis extending through the different layers of the rectal wall, TRG2 is characterized by the presence of rare residual tumor cells scattered throughout the fibrosis, TRG3 shows an increase in the number of residual tumor cells, but the fibrosis still predominates, TRG4 demonstrates residual tumor outgrowing the fibrosis and TRG5 is characterized by the absence of any tumor regression. Based on the results of previous studies, in order to simplify the statistical analysis, the TRG system was combined into two groups: good responders comprising TRG1–2 and poor responders consisting of TRG 3–5 [19,20,21]. All the patients signed informed consent, which was approved by the Local Ethics Committee.
Preparation of Polyclonal Antibodies Against Hsp 16.2 and SOUL
Rabbits were immunized subcutaneously at multiple sites with 100 pg of recombinant Hsp16.2/ Glutathione S-transferase (GST) or SOUL/GST fusion proteins dissolved in Freund’s complete adjuvant, as described before [22,23,24]. Then four subsequent booster injections of 50 pg doses at 4-week intervals were given. Blood was collected 10 days after the last boosting, and the antisera were stored at −20 C. IgGs were affinity purified from the sera by protein G-Sepharose chromatography (Sigma-Aldrich, Munich, Germany) according to the manufacturer’s protocol.
Immunohistochemistry
Sections of the pre-treatment tumor tissue samples were fixed in formalin and embedded in paraffin. Subsequently, they were incubated with the following primary antibodies: self-developed anti-Hsp 16.2 and anti-SOUL polyclonal primary antibodies, GHRH-R primary antibody purchased from Abcam (Abcam Inc., Cambridge, MA), p-AKT primary antibody purchased from Cell Signaling. GHRH-R antibody detected the presence of both pituitary-type GHRH-R as well as the splice variants of the GHRH-R. Immunohistochemical staining was carried out by the streptavidin-biotin-peroxidase method with hydrogen peroxide/3-amino-9-ethylcarbazole development using the Universal kit. Only secondary IgG was incubated with the control sections. The evaluation of the slides was done with the help of an Olympus BX50 light microscope with incorporated photography system (Olympus Optical Co., Hamburg, Germany). The staining intensity was recorded semiquantitatively as mild (+), moderate (++) or strong (+++), following as described before [25]. For internal positive control, the normal cellular and vascular structures of the samples were used. Positive areas around necrotic fields were excluded due to their probable stress related up-regulation. All slides were assessed by the same experienced pathologist blinded to clinico-pathological data.
Statistical Analysis
All statistical analyses were carried out using the SPSS 15.0 statistical program (SPSS, Chicago). Univariate chi-square test was used to compare clinical parameters and biological markers for clinical response and tumor regression grade. To increase the number of patients per group, the categories of the various variables were combined for these analyses: age over 60 years vs. 60 years or below, cT2 vs. cT3-cT4, cN0 vs. cN1–2, tumor localization, radiotherapy dose of higher than 40 Gy vs 40 Gy or below, dose of ciplatin over 75 mg/m2 vs 75 mg/m2 or below, 5-FU dose over 750 mg/m2 vs. 750 mg/m2 or below. For tsting statistical intensity, values of immunohistochemistry were dichotomised into low (0, +) and high (++, +++) intensity categories. All parameters were analyzed afterwards in a logistic regression multivariate analysis. A p value of less than 0.05 was considered statistically significant. The effect of the clinical parameters and the biological markers on overall survival (OS) was demonstrated using Kaplan-Meier curves and the level of significance was determined using the log-rank test. The survival functions were computed from the date of the first symptoms/the start of neoadjuvant RCT by using Kaplan-Meier estimates, and the log-rank test was used to assess the equality of survival functions. The univariate and multivariate Cox regression analyses were performed to test for the independent influence of potential prognostic factors on overall survival (OS). Probability (p) values <0.05 were considered statistically significant, and statistical tests were based on a two-sided significance level. Statistical analyses were performed with use of Statistical Package for the Social Sciences software (SPSS, Chicago, IL).
Results
Clinical Outcome
Patients underwent restaging, whereas 2 patients died during the treatment and 2 patients refused control examinations. Clinical evaluation found that 36 (39%) tumors showed clinical response to neoadjuvant CRT, 4 (4%) patients had complete remission, 32 (35%) patients had partial remission, 42 patients had stable disease (46%), 14 patients had progressive disease (15%). Resection was performed in 42 (46%) cases, with R0 resection rate of 47%. Histopathological evaluation of response to preoperative CRT in resected oesophageal specimens revealed a complete response (TRG1) in 6 of 42 cases (14%) and significant response (TRG2) in 16 of 42 cases (38%). Hence, good responders accounted for 52% of the patients, while poor responders represented the remaining 48% of the patients.
The Association Between Protein Expression and Response to NRCT in ESCC
Tumor samples taken before initiation of treatment were stained for four molecular markers (SOUL, Hsp 16.2, GHRH-R) and p-Akt), then the intensity of staining was evaluated in both the responding and non-responding groups. Responsiveness to NCRT was also determined according to clinical downstaging and TRG classification. Among the markers evaluated, expression of GHRH was low in 90% of the tumor specimens and GHRH-R staining did not show a significant association with tumor response to CRT. However, high expression levels of Hsp16.2 in the pre-treatment tumor biopsies were significantly correlated with poor clinical and histopathological response (p = 0.001, p = 0.000 respectively). High intensity staining for p-AKT was also associated with significantly lower rate of good clinical and histopathological response (p = 0.02, p = 0.032 respectively). Low expression of SOUL resulted in twice as many clinically responding patients (p = 0.037) and four times as many histopathologically responding patients (p = 0.001). The relationship between the expression of the proteins and response to NRCT is shown in (Table 2).
The Association Between Treatment Parameters and Response to NCRT in ESCC
We investigated whether the dosage of chemotherapy (Cisplatin and 5-Fluorouracil) and irradiation affected the clinical downstaging and TRG of the tumors. A higher dose of irradiation (41–45 Gy) resulted in a significantly higher number of clinical responders (p = 0.009), while the dosage didn’t significantly affect TRG. A higher dose of Cisplatin (above 75 mg/m2), on the other hand, significantly increased the number of TRG responders (p = 0.004) but did not significantly affect clinical response. In our study the administered dose 5-Fluorouracil (5-FU) did not significantly affect TRG and clinical response (Table 3).
The Relationship Between Expression of Pre-treatment Proteins (SOUL, Hsp 16.2, GHRH-R and p-Akt) and 3-year Overall Survival (OS)
It was our aim to examine whether there was a correlation between pre-treatment protein expression and 3-year OS. The intensity of GHRH-R (Fig. 1a) staining did not affect 3-year OS significantly (p = 0,891). Low expression of Hsp 16.2 and SOUL (Fig. 1b and c) did not significantly increase 3-year OS (p = 0.19 and p = 0.63 respectively), however, a non-significant improvement after about 8 months in the 3-year OS was apparent. Interestingly, low intensity staining for p-Akt (Fig. 1d) increased the 3-year OS significantly (p = 0.00) (Table 4.).
The relationship between pre-treatment proteins GHRH-R ap = 0.891, Hsp 16.2 bp = 0.19, SOUL cp = 0.63, and p-Akt dp = 0.00 staining and 3-year OS. The effect of biological markers on overall survival was demonstrated using Kaplan-Meier curves and the level of significance was determined using the log-rank test. Probability (p) values <0.05 were considered statistically significant
The Relationship Between Clinical Parameters (Age, Karnofsky Score, Pre-treatment Weight-Loss, Tumor Localization,) and 3-year OS
We evaluated the effect of the individual clinical parameters on 3-year OS. The cutoff value for age was 60 years, for weight-loss (between initial symptoms and beginning of NCRT) was 10% of original body mass, and for Karnofsky score 80%. We could not detect a significant difference in 3-year OS among our patients in the two age groups (Fig. 2a) or in the groups assigned according to their Karnofsky score (Fig. 2b), although there was a non-significant improvement in the OS of younger patients after 12 months. However, there was a significant decrease in 3-year OS in patients whose pre-treatment weight-loss (Fig. 2c) exceeded 10% of their body mass (p = 0.045). The localization of the tumor affected 3-year OS greatly (Fig. 2d). Patients with upper-third ESCC had a significantly higher 3-year OS, than patients with middle and lower third tumors (p = 0.002)(Table 4.).
The relationship between clinical parameters age ap = 0.875, Karnofsky score b p = 0.6, pre-treatment weight-loss cp = 0.045, tumor localization dp = 0.002 and 3-year OS. The effect of the clinical parameters on overall survival was demonstrated using Kaplan-Meier curves and the level of significance was determined using the log-rank test. Probability (p) values <0.05 were considered statistically significant
Discussion
The accepted standard treatment modality for locally advanced ESCC is NRCT followed by surgery. NRCT consists of irradiation and concomitant chemotherapy, based on the administration of cisplatin and 5-FU [3, 4]. An earlier study showed that there was a positive correlation between the administration of higher doses of radiotherapy, 5-FU,cisplatin and complete pathological remission [8]. Later, a number of other studies found evidence that application of higher cisplatin and irradiation doses resulted in a significantly increased rate of complete responses and improved 5-year OS [7, 26, 27]. In accordance with these studies we found that higher radiation doses (over 40Gy) led to an increased number of clinical responders, and that application of higher cisplatin doses (over 75 mg/m2) resulted in more histopathological responders. Although advances have been made in therapy, due to the poor prognosis of ESCC, it is of great importance, that responders be identified before initiating treatment [28]. In our previous investigation we identified possible novel biomarkers of response to NCRT. We showed that overexpression of Hsp 16.2 and Hsp 90 in tumor samples was associated with poor response to CRT. Hsp-s are chaperones, that have a major role in cytoprotection through the prevention of the aggregation of stress-accumulated misfolded proteins [29]. Hsp-s have also been implicated in the increased survival of tumor cells [12, 30]. A study by Ui et al. showed that the inhibitor of Hsp 90 (17-AAG) synergized with cisplatin and helped induce apoptosis in cisplatin –resistant ESCC [12]. This effect was shown to be modulated through the the Akt/Xiap pathway [12]. Hsp 16.2 is a member of the small heat shock family [22, 23]. In accordance with our earlier findings, we found that tumors that stained high for Hsp 16.2 had a significantly lower rate of clinical and histopathological response than those that expressed Hsp 16.2 at lower levels. We were curious to determine how the levels of p-Akt were associated with the response to CRT. As expected, mostly, tumors expressing a higher amount of p-Akt proved to be poor responders to CRT. This finding could be in part explained by a previous report, that Hsp 16.2 inhibits cell death by binding to Hsp 90 and through the activation of the PI-3kinase/Akt cytoprotective pathway [22]. Our detection of a significant correlation between high staining of proteins p-Akt, Hsp 16.2 and poor response could be observed in both clinical and histopathological (TRG) responsiveness. This indicates the potential of these proteins as markers of response. Besides the response to therapy, the length of survival is also important when assessing the efficacy of treatment. It was of particular interest that we found that patients whose tumors showed high staining for Hsp 16.2 and p-Akt had a worse 3-year OS than patients whose tumors stained low. The inverse correlation between length of OS and intensity of staining for the protein, however, was found to be significant only for p-Akt but not for Hsp 16.2. This latter result could be explained by the relatively small number of patients in our group. Since the role of the activation of the p-Akt pathways in ESCC has been reported by a number of studies, the possibility of using p-Akt pathway as a target in the treatment of cancer has emerged [13, 14, 31]. Our evidence suggests that the selective targeting of Hsp 16.2, and by thus, inhibiting the PI-3kinase/Akt pathway, could be a promising tool in the treatment of ESCC. Unlike in our previous study, where the inverse correlation existed, but was not significant, we now found that low SOUL staining in tumor samples was associated with significantly improved clinical and histopathological response. SOUL is a heme-binding protein which has been shown to promote necrotic cell death by inducing mitochondrial permeability [24]. It could be expected, that a higher intensity of necrosis would allow the decrease of the tumor. Surprisingly, a non-significant but negative correlation between the intensity of staining for SOUL and 3-year OS could be detected. In recent studies, we found evidence that tumor necrosis factor alfa (TNF-alfa) could be implicated in increased resistance to chemotherapy in prostate cancer [32]. Another investigation showed that patients with elevated transmembrane TNF-α expression were more likely to have a worse prognosis than patients with low tmTNF-α expression in colorectal cancer [33]. Therefore, we hypothesized that by generating a higher grade of necrosis inside the tumor, SOUL could make tumor cells less sensitive to chemotherapy. However, the precise mechanism behind the negative effect of SOUL on response and OS needs to be further elucidated. Various cancers have been found to express GHRH-R and/or its splice variants and GHRH has been shown to act as an autocrine growth factor for many malignancies [34,35,36,37,38,39]. From the four proteins that were examined, staining for GHRH-R showed no significant correlation with the response to therapy, neither could a difference be detected between patients whose tumors expressed GHRH-R at different levels in the 3-year OS. Moreover, 90% of tumor specimens stained low for GHRH-R. These results are supported by the previous finding that squamous cell carcinoma of the oesophagus was negative for GHRH-R and SV-1, while adenocarcinomas of the oesophagus showed a strong expression of both receptors [40]. Numerous studies have examined the effect of individual clinical parameters on the OS of patients. Old age, male gender, low hemoglobin content, low Karnofsky index and low socioeconomic staus have all been correlated to poor OS [41,42,43,44]. In our study, we found no significant correlation between patients over or below 60 years and 3-year OS, but an improvement it the younger age group was apparent after 12 months. Similarly, the Karnofsky score of the patients did not significantly affect the 3-year OS. Since these parameters have been investigated by a number of studies earlier on a large number of patients, it is rational to assume that our results failed to show the expected significant correlation due to the smaller number of patients in our study. Nutritional status has been proven to be predictive of OS. Di Fiore et al. found that a BMI over 18 kg/m2 was an independent prognostic factor of survival in patients with locally advanced esophageal cancer [45] and another investigation showed that a weight-loss above 9.7% from the onset of the disease until the start of the therapy had a significantly unfavorable impact on survival [46]. Accordingly, we found that those patients who lost more than 10% of their body weight between the appearance of the first symptoms of the illness and the start of NRCT, had a significantly shorter 3-year OS, than those patients who lost less than 10%. Before the introduction of NRCT, tumors in the upper-third of the esophagus were considered to have a worse prognosis, than middle and lower-third ESCC. In an earlier study, we reported that a higher rate of response could be observed in patients with upper-third ESCC, compared to patients middle third ESCC [47, 48]. As a continuation of our previous investigation, the degree of 3-year OS of patients with differing localization of tumors was evaluated. We found, that not only the response to NCRT-but the 3-year OS was also significantly better in patients with upper-third tumors than patients with middle or lower third tumors. This study provides an extensive evaluation of the correlation between possible biomarkers and response to NRCT and OS. We showed that high levels of Hsp 16.2, p-Akt and SOUL were negative prognostic factors in response to therapy and that a high level of these proteins was correlated with decreased 3-year overall survival. These findings underline the significance of these markers as potential predictors of response which possibly can be applied in clinical practice. We also found that patients with tumors in the upper-third region of the esophagus had an improved 3-year OS. This finding implicates that the efficacy of NRCT is also greatly dependent on the localization of the tumors in ESCC.
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386. https://doi.org/10.1002/ijc.29210
Keighley MR (2003) Gastrointestinal cancers in Europe. Aliment Pharmacol Ther 18(Suppl 3):7–30
Mariette C, Piessen G, Triboulet JP (2007) Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. The Lancet Oncology 8(6):545–553. https://doi.org/10.1016/S1470-2045(07)70172-9
Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J, Australasian Gastro-Intestinal Trials G (2007) Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. The Lancet Oncology 8(3):226–234. https://doi.org/10.1016/S1470-2045(07)70039-6
Hanna A, Birla R, Iosif C, Boeriu M, Constantinoiu S (2016) Benefits and Disadvantages of Neoadjuvant Radiochemotherapy (RCT) in the Multimodal Therapy of Squamous Esophageal Cancer (ESC). Chirurg 111(1):12–25
Nakajima M, Kato H (2013) Treatment options for esophageal squamous cell carcinoma. Expert Opin Pharmacother 14(10):1345–1354. https://doi.org/10.1517/14656566.2013.801454
Ordu AD, Nieder C, Geinitz H, Scherer V, Kup PG, Schuster T, Combs SE, Fakhrian K (2014) Association between radiation dose and pathological complete response after preoperative radiochemotherapy in esophageal squamous cell cancer. Anticancer Res 34(12):7255–7261
Geh JI, Bond SJ, Bentzen SM, Glynne-Jones R (2006) Systematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: evidence of a radiation and chemotherapy dose response. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 78(3):236–244. https://doi.org/10.1016/j.radonc.2006.01.009
Gillham CM, Reynolds J, Hollywood D (2007) Predicting the response of localised oesophageal cancer to neo-adjuvant chemoradiation. World Journal of Surgical Oncology 5:97. https://doi.org/10.1186/1477-7819-5-97
Sarbia M, Ott N, Puhringer-Oppermann F, Brucher BL (2007) The predictive value of molecular markers (p53, EGFR, ATM, CHK2) in multimodally treated squamous cell carcinoma of the oesophagus. Br J Cancer 97(10):1404–1408. https://doi.org/10.1038/sj.bjc.6604037
Zhu Z, Yu W, Fu X, Sun M, Wei Q, Li D, Chen H, Xiang J, Li H, Zhang Y, Zhao W, Zhao K (2015) Phosphorylated AKT1 is associated with poor prognosis in esophageal squamous cell carcinoma. Journal of experimental & clinical cancer research : CR 34:95. https://doi.org/10.1186/s13046-015-0212-z
Ui T, Morishima K, Saito S, Sakuma Y, Fujii H, Hosoya Y, Ishikawa S, Aburatani H, Fukayama M, Niki T, Yasuda Y (2014) The HSP90 inhibitor 17-N-allylamino-17-demethoxy geldanamycin (17-AAG) synergizes with cisplatin and induces apoptosis in cisplatin-resistant esophageal squamous cell carcinoma cell lines via the Akt/XIAP pathway. Oncol Rep 31(2):619–624. https://doi.org/10.3892/or.2013.2899
Jin Z, Yan W, Jin H, Ge C, Xu Y (2016) Psoralidin inhibits proliferation and enhances apoptosis of human esophageal carcinoma cells via NF-kappaB and PI3K/Akt signaling pathways. Oncol Lett 12(2):971–976. https://doi.org/10.3892/ol.2016.4716
Wang X, Li X, Li C, He C, Ren B, Deng Q, Gao W, Wang B (2016) Aurora-A modulates MMP-2 expression via AKT/NF-kappaB pathway in esophageal squamous cell carcinoma cells. Acta Biochim Biophys Sin 48(6):520–527. https://doi.org/10.1093/abbs/gmw030
Xue L, Yang L, Jin ZA, Gao F, Kang JQ, Xu GH, Liu B, Li H, Wang XJ, Liu LJ, Wang BL, Liang SH, Ding J (2014) Increased expression of HSP27 inhibits invasion and metastasis in human esophageal squamous cell carcinoma. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 35(7):6999–7007. https://doi.org/10.1007/s13277-014-1946-5
Chen JH, Chen LM, Xu LY, Wu MY, Shen ZY (2006) Expression and significance of heat shock proteins in esophageal squamous cell carcinoma. Zhonghua zhong liu za zhi [Chinese journal of oncology] 28(10):758–761
Farkas R, Pozsgai E, Bellyei S, Cseke L, Szigeti A, Vereczkei A, Marton S, Mangel L, Horvath OP, Papp A (2011) Correlation between tumor-associated proteins and response to neoadjuvant treatment in patients with advanced squamous-cell esophageal cancer. Anticancer Res 31(5):1769–1775
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92 (3):205–216
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 73(11):2680–2686
Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M, Bedenne L (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 24(28):4620–4625. https://doi.org/10.1200/JCO.2006.06.7629
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17):1731–1740. https://doi.org/10.1056/NEJMoa040694
Bellyei S, Szigeti A, Boronkai A, Pozsgai E, Gomori E, Melegh B, Janaky T, Bognar Z, Hocsak E, Sumegi B, Gallyas F, Jr. (2007) Inhibition of cell death by a novel 16.2 kD heat shock protein predominantly via Hsp90 mediated lipid rafts stabilization and Akt activation pathway. Apoptosis 12 (1):97–112. doi:https://doi.org/10.1007/s10495-006-0486-x
Bellyei S, Szigeti A, Pozsgai E, Boronkai A, Gomori E, Hocsak E, Farkas R, Sumegi B, Gallyas F, Jr. (2007) Preventing apoptotic cell death by a novel small heat shock protein. Eur J Cell Biol 86 (3):161–171. doi:https://doi.org/10.1016/j.ejcb.2006.12.004
Szigeti A, Bellyei S, Gasz B, Boronkai A, Hocsak E, Minik O, Bognar Z, Varbiro G, Sumegi B, Gallyas F, Jr. (2006) Induction of necrotic cell death and mitochondrial permeabilization by heme binding protein 2/SOUL. FEBS Lett 580 (27):6447–6454. doi:https://doi.org/10.1016/j.febslet.2006.10.067
Somji S, Sens MA, Lamm DL, Garrett SH, Sens DA (2001) Metallothionein isoform 1 and 2 gene expression in the human bladder: evidence for upregulation of MT-1X mRNA in bladder cancer. Cancer Detect Prev 25(1):62–75
de Manzoni G, Pedrazzani C, Laterza E, Pasini F, Grandinetti A, Bernini M, Ruzzenente A, Zerman G, Tomezzoli A, Cordiano C (2005) Induction chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus: impact of increased dosage on long-term results. Ann Thorac Surg 80(4):1176–1183. https://doi.org/10.1016/j.athoracsur.2005.02.048
Pasini F, de Manzoni G, Pedrazzani C, Grandinetti A, Durante E, Gabbani M, Tomezzoli A, Griso C, Guglielmi A, Pelosi G, Maluta S, Cetto GL, Cordiano C (2005) High pathological response rate in locally advanced esophageal cancer after neoadjuvant combined modality therapy: dose finding of a weekly chemotherapy schedule with protracted venous infusion of 5-fluorouracil and dose escalation of cisplatin, docetaxel and concurrent radiotherapy. Annals of oncology : official journal of the European Society for Medical Oncology 16(7):1133–1139. https://doi.org/10.1093/annonc/mdi207
Hanna A, Birla R, Iosif C, Boeriu M, Tomsa R, Puscasu A, Constantinoiu S (2015) Evaluation of Neoadjuvant Radiochemotherapy Response (RCT) in Squamous Esophageal Cancer (ESC) and Implications in Therapeutic Conduct. Chirurg 110(3):214–223
Parcellier A, Schmitt E, Brunet M, Hammann A, Solary E, Garrido C (2005) Small heat shock proteins HSP27 and alphaB-crystallin: cytoprotective and oncogenic functions. Antioxid Redox Signal 7(3–4):404–413. https://doi.org/10.1089/ars.2005.7.404
Xu Y, Chen Z, Zhang G, Xi Y, Sun R, Wang X, Wang W, Chai F, Li X (2016) HSP90B1 overexpression predicts poor prognosis in NSCLC patients. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine 37(10):14321–14328. https://doi.org/10.1007/s13277-016-5304-7
Chang X, Zhao J, Tian F, Jiang Y, Lu J, Ma J, Zhang X, Jin G, Huang Y, Dong Z, Liu K, Dong Z (2016) Aloe-emodin suppresses esophageal cancer cell TE1 proliferation by inhibiting AKT and ERK phosphorylation. Oncol Lett 12(3):2232–2238. https://doi.org/10.3892/ol.2016.4910
Sha K, Yeh S, Chang C, Nastiuk KL, Krolewski JJ (2015) TNF signaling mediates an enzalutamide-induced metastatic phenotype of prostate cancer and microenvironment cell co-cultures. Oncotarget 6(28):25726–25740. 10.18632/oncotarget.4535
Li X, Wang S, Ren H, Ma J, Sun X, Li N, Liu C, Huang K, Xu M, Ming L (2016) Molecular correlates and prognostic value of tmTNF-alpha expression in colorectal cancer of 5-Fluorouracil-Based Adjuvant Therapy. Cancer biology & therapy 17(6):684–692. https://doi.org/10.1080/15384047.2016.1187551
Plonowski A, Schally AV, Letsch M, Krupa M, Hebert F, Busto R, Groot K, Varga JL (2002) Inhibition of proliferation of PC-3 human prostate cancer by antagonists of growth hormone-releasing hormone: lack of correlation with the levels of serum IGF-I and expression of tumoral IGF-II and vascular endothelial growth factor. Prostate 52(3):173–182. https://doi.org/10.1002/pros.10105
Kahan Z, Arencibia JM, Csernus VJ, Groot K, Kineman RD, Robinson WR, Schally AV (1999) Expression of growth hormone-releasing hormone (GHRH) messenger ribonucleic acid and the presence of biologically active GHRH in human breast, endometrial, and ovarian cancers. J Clin Endocrinol Metab 84(2):582–589. https://doi.org/10.1210/jcem.84.2.5487
Garcia-Fernandez MO, Schally AV, Varga JL, Groot K, Busto R (2003) The expression of growth hormone-releasing hormone (GHRH) and its receptor splice variants in human breast cancer lines; the evaluation of signaling mechanisms in the stimulation of cell proliferation. Breast Cancer Res Treat 77(1):15–26
Rekasi Z, Czompoly T, Schally AV, Halmos G (2000) Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci U S A 97(19):10561–10566. https://doi.org/10.1073/pnas.180313297
Halmos G, Schally AV, Varga JL, Plonowski A, Rekasi Z, Czompoly T (2000) Human renal cell carcinoma expresses distinct binding sites for growth hormone-releasing hormone. Proc Natl Acad Sci U S A 97(19):10555–10560. https://doi.org/10.1073/pnas.180313097
Szereday Z, Schally AV, Szepeshazi K, Bajo AM, Hebert F, Halmos G, Nagy A (2003) Effective treatment of H838 human non-small cell lung carcinoma with a targeted cytotoxic somatostatin analog, AN-238. Int J Oncol 22(5):1141–1146
Hohla F, Moder A, Mayrhauser U, Hauser-Kronberger C, Schally AV, Varga JL, Zarandi M, Buchholz S, Huber R, Aigner E, Ritter M, Datz C (2008) Differential expression of GHRH receptor and its splice variant 1 in human normal and malignant mucosa of the oesophagus and colon. Int J Oncol 33(1):137–143
Wu CC, Chang CM, Hsu TW, Lee CH, Chen JH, Huang CY, Lee CC (2016) The effect of individual and neighborhood socioeconomic status on esophageal cancer survival in working-age patients in Taiwan. Medicine 95(27):e4140. https://doi.org/10.1097/MD.0000000000004140
Koppert LB, Lemmens VE, Coebergh JW, Steyerberg EW, Wijnhoven BP, Tilanus HW, Janssen-Heijnen ML (2012) Impact of age and co-morbidity on surgical resection rate and survival in patients with oesophageal and gastric cancer. Br J Surg 99(12):1693–1700. https://doi.org/10.1002/bjs.8952
Kandaz M, Ertekin MV, Bilici M (2012) Retrospective analysis of patients with esophageal cancer treated with radiotherapy and/or chemoradiotherapy. Tumori 98(4):445–450. https://doi.org/10.1700/1146.12638
Neuhof D, Neumayer F, Einbeck W, Haschemian K, Mai SK, Hochhaus A, Willeke F, Rudi J, Debus J, Wenz F (2005) Retrospective evaluation of combined modality treatment and prognostic factors in patients with esophageal cancer. Acta Oncol 44(2):168–173. https://doi.org/10.1080/02841860510029563
Di Fiore F, Lecleire S, Pop D, Rigal O, Hamidou H, Paillot B, Ducrotte P, Lerebours E, Michel P (2007) Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol 102(11):2557–2563. https://doi.org/10.1111/j.1572-0241.2007.01437.x
Zemanova M, Novak F, Vitek P, Pazdro A, Smejkal M, Pazdrova G, Petruzelka L (2012) Outcomes of patients with oesophageal cancer treated with preoperative chemoradiotherapy, followed by tumor resection: influence of nutritional factors. Journal of BUON : official journal of the Balkan Union of Oncology 17(2):310–316
Papp A, Cseke L, Farkas R, Pavlovics G, Horvath G, Varga G, Szigeti A, Bellyei S, Marton S, Poto L, Kalmar K, Vereczkei A, Pozsgai E, Horvath OP (2010) Chemo-radiotherapy in locally advanced squamous cell oesophageal cancer--are upper third tumours more responsive? Pathol Oncol Res 16(2):193–200. https://doi.org/10.1007/s12253-009-9206-5
Papp A, Cseke L, Pavlovics G, Farkas R, Varga G, Marton S, Poto L, Esik O, Horvath OP (2007) The effect of preoperative chemo-radiotherapy in the treatment of locally advanced squamous cell carcinoma in the upper- and middle-thirds of the esophagus. Magy Seb 60(3):123–129. https://doi.org/10.1556/MaSeb.60.2007.3.1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that he/she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies animals performed by any of the authors.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study. This was approved by the Local Ethics Committee.
Rights and permissions
About this article
Cite this article
Zoltan, L., Farkas, R., Schally, A.V. et al. Possible Predictive Markers of Response to Therapy in Esophageal Squamous Cell Cancer. Pathol. Oncol. Res. 25, 279–288 (2019). https://doi.org/10.1007/s12253-017-0342-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-017-0342-z