Abstract
Krüppel-like factor 8 (KLF8) plays a key role in cancer progression. However, its expression pattern and relationship with clinicopathological characteristics in non-small cell lung cancer (NSCLC) has not been completely elucidated. The study aimed to investigate the expression of KLF8 and its correlation with clinical pathologic features in NSCLC and to explore the potential mechanism. The expression of KLF8 in NSCLC was detected by RT-PCR, Western blot and immunohistochemistry. The expression of Vimentin and E-cadherin, epithelial-mesenchymal transition (EMT) markers, were detected by immunohistochemistry in the NSCLC. The relationship between KLF8 expression and various clinicopathological features or EMT markers was investigated. The results showed m-RNA and protein of KLF8 were overexpressed in NSCLC and the percent of KLF8 positive cells was positively correlated with TNM stage, lymph node metastasis and poor overall survive. Moreover, high expression of KLF8 correlated with E-cadherin low expression, and Vimentin overexpression. Additionally, COX multivariate regression analysis suggested that TNM stage, KLF8, E-cadherin and Vimentin were independent prognostic indicators for overall survival of patients with NSCLC. The data demonstrate that KLF8 is overexpressed in NSCLC. KLF8 overexpression promotes the malignancy of NSCLC, which mechanism may be involved in EMT. KLF8 maybe serve as a potential therapeutic target for NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related mortality in the majority of countries worldwide, with over 1 million deaths each year [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer cases [2]. Despite great advances of the therapies, the 5-year survival rate of lung cancer is still below 15% [3, 4]. Hence, investigating the genes involved in the process of tumor cell invasion and metastasis in NSCLC, as well as identifying their molecular biological mechanisms are essential steps for the treatment and prognosis prediction of NSCLC.
The Krüppel-like factor (KLF) family as transcription factors share homology in their three C2-H2 zinc finger DNA binding domains and play diverse roles, containing the regulation of cell cycle, proliferation, differentiation, apoptosis, development and tumorigenesis [5,6,7]. Like other members of the KLFs, KLF8 shares the well-conserved DNA-binding zinc finger domains on its C-terminus and a distinct sequence in the N-terminus domain which is thought to determine its functional specificity through recruiting other proteins [8]. KLF8 was initially identified as a transcription repressor in a vitro reporter system [9]. However, recent investigation identified KLF8 as a critical role in mesenchymal transition and invasion, which seemly plays a crucial role in metastatic progression of human carcinoma [10]. KLF8 was barely expressed in normal human epithelial cells but highly overexpressed in some certain human malignant tumors and played a significant role in cancer progression [10], such as ovarian [11], breast [12], renal [13], liver [14, 15], gastric cancer [16, 17]. Moreover, overexpression of KLF8 was strongly associated with early tumor recurrence and poor prognosis in patients with hepatocellular carcinoma (HCC) after surgery [15]. However, no data of KLF8 are available for NSCLC.
Epithelial-mesenchymal transition (EMT) is a cell biological program, characterized by the loss of epithelial features such as E-cadherin or gamma-catenin low expression and vimentin or periostin overexpression [18]. EMT is implicated in cancer progression and metastasis. Cancer cells undergoing EMT can acquire invasive properties and enter the surrounding stroma, creating a favorable microenvironment for cancer progression and metastasis [19, 20]. Recently, KLF8 has been linked to EMT, because KLF8 overexpression down-regulated the expression of E-cadherin and facilitated invasion in breast carcinoma cells [12]. In addition, KLF8 knockdown in gastric cancer cells significantly increases E-cadherin expression and reduces vimentin expression [14]. However, it is still unclear whether expression of KLF8 correlates with the EMT in NSCLC.
The present study aimed to investigate the expression of KLF8 and its correlation with clinicopathological features, as well as the relationship between KLF8 and indicators of EMT in NSCLC patient.
Materials and Methods
Patients and Tissue Samples
NSCLC tissues and the corresponding paracancer tissues were collected from surgical resection specimens of 134 patients who had not undergone radiotherapy and chemotherapy in the Affiliated Hospital of Nantong University between 2009 and 2011 and snap-frozen in liquid nitrogen. Use of tissue for this study was approved by our Local Research Ethics Committee. The TNM stage was accorded to the 7th Edition of TNM in Lung Cancer [21]. All patients provided written informed consent. Clinical data of these patients were summarized in Table 2.
Real-Time PCR
The total RNA of cancer and paracancer samples from 7 NSCLC patients was isolated using UNIQ-10 Spin Column RNA Purified Kit (Sangon, Shanghai, China). The first strand cDNA was synthesized using RevertAidTM First Strand cDNA Synthesized Kit (Fermentas, Burlington, Canada). First Strand cDNA was subsequently subjected to Corbett RG-6000 PCR system (QIAGEN, Dusseldorf, German) using FastStart Universal SYBR Green Master Mix (Roche, Basel, Switzerland). The reactions were optimized by varying the annealing temperatures from 50 ~ 55 °C, the sense and antisense primers were synthesized as follows: GAPDH 5′-GCAAGTTCAACGGCACAG-3′, 5′-GCCAGTAGACTCCACGACAT-3′; KLF8 5′-GCTCACCGCAGAATCCATACA-3′, 5′- GTGCACCGAAAAGGCTTGAT-3′.
Western Blot Analysis
The tissues were lysed in RIPA buffer (50 mMTris-HCl, PH7.5; 150 mMNaCl; 1% NP-40; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate) containing complete protease inhibitor cocktail (Pirece Biotechnology, IL, USA) and placed on ice for 30 min. The supernatant was collected and concentrated by centrifugation at 15,000 g for 5 min at 4 °C. Protein concentrations were determined with the Bradfordassay (Bio-Rad, Hercules, California, USA). Denatured protein (200 μg) was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and sub-sequently transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was then blocked with 5% nonfat milk in TBST for 2 h and then incubated with polyclonal antibody to KLF8 (1:1000, Cell Signaling Technology, California, USA) or anti-GAPDH polyclonal antibody (1:500, Bioss, Beijing, China) at 4 °C for 24 h, and finally incubated with the second antibody at room temperature for 2 h. After three washes with TBST, the membrane was developed with enhanced chemiluminescence (ECL, Pierce Company, Kocktord, Illinois, USA). The optical densities were analyzed by using ImageMasterTM2D Platinum (Version 5.0, Amersham Biosciences, Piscataway, NJ).
Immunohistochemistry (IHC)
IHC was performed as described previously [22]. Polyclonal rabbit anti-human KLF8 (1:150; Aviva Systems Biology, California, USA), E-cadherin antibody (1:500; Abcam, San Francisco, USA) and Vimentin antibody (1:100; Santa Cruz, California, USA). Counterstaining with hematoxylin was performed and visualized on the DM IL LED microscope (Leica Microsystems GmbH).
The immunostaining results were interpreted independently by two expert pathologists who blinded to the clinical data. The staining intensity was scored using the following scale of four grades: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. At least 5 areas of each core were viewed and the proportion of cells in each core staining positively was assigned a score (0□100%). A semi-quantitative histopathology (H) score was obtained by multiplying the staining intensity score with the percentage score (0 ~ 300). An H-score higher than the median was considered positive. All samples were evaluated at 200 magnification.
Statistical Methods
Statistical analysis was performed using statistics package for social science 21.0 (SPSS 21.0). The expression of KLF8 mRNA and protein of cancer and paracancer samples was analyzed using independent t test. Associations between KLF8 expression and the clinicopathological characteristics or EMT indicators were analyzed using Person Chi-Square or Fisher’s exact test. Five-year overall survival (OS) was the primary outcome measure, estimated using the Kaplan-Meier method and differences in survival among groups were compared using the log-rank test. Multivariate analysis was performed using Cox regression model. A P-value less than 0.05 were considered statistically significant.
Results
KLF8 mRNA Expression Detected by Real-Time PCR
The KLF8 mRNA of cancer and paracancer samples from 7 NSCLC was detected by Real-time PCR. KLF8 gene level in cancer samples was ~12 folds higher than that in paracancer samples, the difference between the cancer and paracancer samples was statistically significant (Fig. 1).
KLF8 Protein Expression Detected by Western Blot
KLF8 protein of cancer and paracancer samples from 7 NSCLC was detected by Western Blot. Semi-quantitative assay showed KLF8 protein level in cancer samples was higher than that in paracancer samples, the difference between the cancer and paracancer samples was statistically significant (Fig. 2).
IHC Assessment of KLF8, E-Cadherin and Vimentin Expression
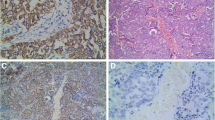
KLF8 protein was mainly located in the nuclear, and some in the cytoplasm. KLF8 strong staining was found in cancer samples from patients with NSCLC, whereas there was no or low KLF8 expression in paracancer tissues (Fig. 3a, b). The positive expression of KLF8 was more frequent in 62 of 134 (46.27%) cases of NSCLC than that in paracancer samples (10 of 134; 7.46%) (Fig. 3g).
IHC assessment of KLF8, E-cadherin and Vimentin expression. KLF8 protein was mainly located in the nuclear, and some in the cytoplasm. KLF8 strong staining was found in cancer samples from patients with NSCLC (a, ×200), whereas there was no or low KLF8 expression in paracancer tissues (b, ×200). The positive expression of KLF8 in cancer samples was more than that in paracancer samples (g). Expression of E-cadherin was mainly located in the cell membrane. Weak staining was found in cancer samples (c, ×200), whereas there was strong or moderate E-cadherin expression in paracancer tissues (d, ×200). The positive expression of E-cadherin in cancer samples was low than that in paracancer samples (g). Vimentin expression was mainly observed in the cytoplasm. Vimentin strong staining was found in cancer samples from patients with NSCLC (e, ×200), whereas there was no or low Vimentin expression in paracancer tissues (f, ×200). The positive expression of Vimentin in cancer samples was more than that in paracancer samples (g). * vs. cancer samples P < 0.05
Expression of E-cadherin was mainly located in the cell membrane. Weak staining was found in cancer samples, whereas there was strong or moderate E-cadherin expression in paracancer tissues (Fig. 3c, d). The positive expression of E-cadherin was low frequent in 62 of 134 (46.27%) cases of NSCLC than that in paracancer samples (10 of 134; 7.46%) (Fig. 3g).
Vimentin expression was mainly observed in the cytoplasm. Vimentin strong staining was found in cancer samples from patients with NSCLC, whereas there was no or low Vimentin expression in paracancer tissues (Fig. 3e, f). The positive expression of Vimentin was more frequent in 76 of 134 (56.72%) cases of NSCLC than that in paracancer samples (15 of 134; 11.19%) (Fig. 3g).
Correlation between Expression of KLF8 and the EMT Indicator Proteins
The relationship between expression of KLF8 and the two EMT indicators were analyzed and were outlined in Table 1. The result showed that high expression of KLF8 was positively correlated with anomalous positivity of Vimentin (P = 0.006), and inversely with a loss of E-cadherin expression (P = 0.019) in NSCLC samples (Table 1).
The Relationships between KLF8 Expression and Clinicopathological Characteristics
An overview of KLF8 expression and clinicopathological parameters was shown in Table 2. KLF8 overexpression was significantly associated with TNM stage (P < 0.001), and lymph node metastases (P < 0.001), respectively. And there was no significant relationship between KLF8 protein level and variables like tumor histology, age, gender, smoking history, tumor grade (P > 0.05) (Table 2).
The Five-Year OS Rate
The mean five-year OS of patients with KLF8 positive was 35 months (95% CI: 30.0 to 40.3 months). The mean five-year OS of patients with KLF8 negative was 43 months (95% CI: 38.1 to 48.2 months). The five-year OS rate was significantly shorter for KLF8 positive patients than for KLF8 negative patients, the difference was statistically significant (Fig. 4a). A high level of KLF8 expression was associated with decreased survival time.
The five-year OS rate was estimated using the Kaplan-Meier method and differences in survival among groups were compared using the log-rank test. a: The five-year OS rate was significantly shorter for KLF8 positive patients than for KLF8 negative patients, the difference was statistically significant. A high level of KLF8 expression was associated with decreased survival time. b: The five-year OS rate was significantly shorter for E-cadherin negative patients than for E-cadherin positive patients, the difference was statistically significant. A low level of E-cadherin expression was associated with decreased survival time. c: The five-year OS rate was significantly shorter for Vimentin positive patients than for Vimentin negative patients, the difference was statistically significant. A high level Vimentin expression was associated with decreased survival time
The mean five-year OS of patients with E-cadherin positive was 45 months (95% CI: 40.0 to 50.9 months). The mean five-year OS of patients with E-cadherin negative was 36 months (95% CI: 31.1 to 40.6 months). The five-year OS rate was significantly shorter for E-cadherin negative patients than for E-cadherin positive patients, the difference was statistically significant (Fig. 4b). A low level of E-cadherin expression was associated with decreased survival time.
The mean five-year OS of patients with Vimentin positive was 28 months (95% CI: 23.6 to 32.4 months). The mean five-year OS of patients with Vimentin negative was 55 months (95% CI: 51.2 to 58.3 months). The five-year OS rate was significantly shorter for Vimentin positive patients than for Vimentin negative patients, the difference was statistically significant (Fig. 4c). A high level Vimentin expression was associated with decreased survival time.
The Result of COX Multivariate Regression Analysis
The Cox’s proportional hazards regression model proved that KLF8, E-cadherin, Vimentin and TNM stage were independent prognostic indicators for overall survival of patients with NSCLC (P<0.05, Table 3).
Discussion
In this study, we emphasized the prognostic value of KLF8 expression in NSCLC. KLF8 overexpression was found in tumor samples of NSCLC and was significantly correlated with TNM stage and lymph node metastasis. KLF8 overexpression was correlated with decreased overall survival. Overexpression of the KLF8 gene was also observed in ovarian [11], breast [12], renal [13], liver [14, 15], gastric [16, 17] cancers. Previous study confirmed that KLF8 was a valuable prognostic indicator for poor survival in HCC [15]. The latest research showed that KLF8 was closely associated with gastric tumor progression, angiogenesis and poor prognosis [17]. Our present results are consistent with previous studies.
However, the exact mechanisms of KLF8 tumorigenic effects in NSCLC have not been elucidated. KLF8 has been identified to enhance the cell cycle progression in ovarian cancer cells by transducing FAK to PI3K to AKT to SP1 signaling to elevate cyclin D1 expression [11]. It is reported that KLF8 promoted HCC cell proliferation through mediating Wnt to β-catenin signaling [14].
EMT is implicated in cancer progression and metastasis. Cancer cells undergoing EMT can acquire invasive properties and enter the surrounding stroma, creating a favorable microenvironment for cancer progression and metastasis [19, 20]. EMT is characterized by the loss of epithelial features such as E-cadherin or gamma-catenin low expression and vimentin or periostin overexpression [18]. Overexpression of KLF8 protein suppressed the expression of E-cadherin and promoted invasion in breast carcinoma cells [12]. Down-regulated expression of KLF8 in gastric cancer cells significantly increased E-cadherin expression while reduced vimentin expression [14]. To identify the relation between KLF8 and EMT in NSCLC, two EMT markers were investigated in NSCLC using IHC. The results showed that high levels of expression of the KLF8 protein correlated with low expression of E-cadherin and overexpression of Vimentin. It indicated a potential role of KLF8 in EMT of NSCLC. It is confirmed that EMT phenomenon is correlated with poor survival in NSCLC [19, 20]. It is reported that E-cadherin low expression was significantly linked with the poor overall survival of lung cancer [23]. However, whether Vimentin expression is associated with survival in lung cancer is still controversial. Some reports showed that vimentin overexpression was associated with a poor outcome of the patients [24, 25]. By contrast, other researchers reported that the expression of Vimentin in primary lung adenocarcinomas was not associated with the overall survival, postoperative recurrence and progression-free survival [26, 27]. In this study, we showed that both E-cadherin and vimentin expression were associated with NSCLC outcome.
As far as we know, this is the first report of the significance of KLF8 expression in NSCLC. In conclusion, our data demonstrate that KLF8 is overexpressed in NSCLC. KLF8 overexpression promotes the malignancy of NSCLC, which mechanism may be involved in EMT. KLF8 maybe serve as a potential therapeutic target for NSCLC.
References
Jemal A et al (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics. CA Cancer J Clin 62(1):10–29
Gao W et al (2011) Circulating microRNAs: possible prediction biomarkers for personalized therapy of non-small-cell lung carcinoma. Clin Lung Cancer 12(1):14–17
Smith CB, Kelley AS, Meier DE (2010) Evidence for new standard of care in non-small cell lung cancer patients. Semin Thorac Cardiovasc Surg 22(3):193–194
Pearson R et al (2008) Kruppel-like transcription factors: a functional family. Int J Biochem Cell Biol 40(10):1996–2001
Zhao J et al (2003) Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell 11(6):1503–1515
Wang X, Zhao J (2007) KLF8 transcription factor participates in oncogenic transformation. Oncogene 26(3):456–461
Quadrini KJ, Bieker JJ (2002) Kruppel-like zinc fingers bind to nuclear import proteins and are required for efficient nuclear localization of erythroid Kruppel-like factor. J Biol Chem 277(35):32243–32252
van Vliet J, Turner J, Crossley M (2000) Human Kruppel-like factor 8: a CACCC-box binding protein that associates with CtBP and represses transcription. Nucleic Acids Res 28(9):1955–1962
Wang X et al (2007) Kruppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res 67(15):7184–7193
Wang X et al (2008) Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem 283(20):13934–13942
Wang X et al (2011) KLF8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of MMP9. Oncogene 30(16):1901–1911
Fu WJ et al (2010) Small interference RNA targeting Kruppel-like factor 8 inhibits the renal carcinoma 786-0 cells growth in vitro and in vivo. J Cancer Res Clin Oncol 136(8):1255–1265
Yang T et al (2012) Kruppel-like factor 8 is a new Wnt/beta-catenin signaling target gene and regulator in hepatocellular carcinoma. PLoS One 7(6):e39668
Li JC et al (2010) Up-regulation of Kruppel-like factor 8 promotes tumor invasion and indicates poor prognosis for hepatocellular carcinoma. Gastroenterology 139(6):2146–2157 e12
Liu L et al (2012) Lentivirus-delivered Kruppel-like factor 8 small interfering RNA inhibits gastric cancer cell growth in vitro and in vivo. Tumour Biol 33(1):53–61
Wang WF et al (2013) Kruppel-like factor 8 overexpression is correlated with angiogenesis and poor prognosis in gastric cancer. World J Gastroenterol 19(27):4309–4315
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454
Iwatsuki M et al (2010) Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci 101(2):293–299
Thiery JP et al (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Goldstraw P (2009) The 7th Edition of TNM in Lung Cancer: what now? J Thorac Oncol 4(6):671–673
Tischler V et al (2011) L1CAM protein expression is associated with poor prognosis in non-small cell lung cancer. Mol Cancer 10:127
Tamura M et al (2005) Prognostic significance of dysadherin expression in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 130(3):740–745
Maeda J et al (2008) Proteomic analysis of stage I primary lung adenocarcinoma aimed at individualisation of postoperative therapy. Br J Cancer 98(3):596–603
Richardson F et al (2012) The evaluation of E-Cadherin and vimentin as biomarkers of clinical outcomes among patients with non-small cell lung cancer treated with erlotinib as second- or third-line therapy. Anticancer Res 32(2):537–552
Chikaishi Y, Uramoto H, Tanaka F (2011) The EMT status in the primary tumor does not predict postoperative recurrence or disease-free survival in lung adenocarcinoma. Anticancer Res 31(12):4451–4456
Soltermann A et al (2008) Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res 14(22):7430–7437
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
None.
Rights and permissions
About this article
Cite this article
Li, J., Liu, Y., Xue, J. et al. Krüppel-Like Factor 8 Overexpression Correlates with Poor Prognosis in Non-Small Cell Lung Cancer. Pathol. Oncol. Res. 25, 115–121 (2019). https://doi.org/10.1007/s12253-017-0321-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-017-0321-4