Abstract
Initiation of transcription by RNA polymerase II requires TATA-box-binding protein (TBP)-associated factors (TAFs). TAF1 is a major scaffold by which TBP and TAFs interact in the basal transcription factor. TAF1L is a TAF1 homologue with 95 % amino acid identity with TAF1. TAF1 is involved in apoptosis induction and cell cycle regulation, but roles of TAF1 and TAF1L in tumorigenesis remain unknown. The aim of this study was to explore whether TAF1 and TAF1L genes were mutated in gastric (GC) and colorectal cancers (CRC). In a public database, we found that TAF1 and TAF1L genes had mononucleotide repeats in the coding sequences that might be mutation targets in the cancers with microsatellite instability (MSI). We analyzed the mutations in 79 GC and 124 CRC by single-strand conformation polymorphism analysis and DNA sequencing. In the present study, we found TAF1 frameshift mutations (3.8 % of CRC with MSI-H) and TAF1L frameshift mutations (2.9 % of GC and 3.8 % of CRC with MSI-H). These mutations were not found in stable MSI/low MSI (MSS/MSI-L) (0/90). In addition, we analyzed intratumoral heterogeneity (ITH) of TAF1 and TAF1L frameshift mutations in 16 CRC and found that two and one CRC harbored regional ITH of TAF1 and TAF1L frameshift mutations, respectively. Our data indicate that TAF1 and TAF1L genes harbored not only somatic mutations but also mutational ITH, which together might play a role in tumorigenesis of GC and CRC with MSI-H. Our results also suggest that ultra-regional mutation analysis is required for a comprehensive evaluation of mutation status in these tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Initiation of transcription by RNA polymerase II that catalyzes synthesis of precursor mRNA requires activities of the basal transcription factor TFIID, which acts as a channel for regulatory signals [1–3]. TFIID is a multisubunit protein comprised of TATA-box-binding protein (TBP) and TBP-associated factors (TAFs). TAF1, the largest TAF, functions as a major scaffold by which TBP and TAFs interact in TFIID [1–3]. TAF1 possesses intrinsic protein kinase, histone acetyltransferase and ubiquitin-conjugating activities [4, 5]. Mutations in this gene result in Dystonia 3, torsion, X-linked, a dystonia-parkinsonism disorder, an adult onset, sex-linked, predominantly male, severe, progressive movement disorder with high penetrance [6]. Although TAF1 is known to play a critical role in the regulation of cell growth and cell cycle [1–3], its implication in cancer development is largely unknown. A genome-wide RNAi screen identifies TAF1 as an apoptosis regulator in response to genotoxic stress [7]. Silencing of TAF1 decreased expression of p27Kip1, allowing cells resistant from the apoptosis [7]. These data suggest a possibility that inactivation of TAF1 might be involved in tumorigenesis. Somatic mutations of TAF1 gene have been reported in several tumors, including uterine serous carcinoma [8]. Most of the TAF1 mutations in the COSMIC database (http://cancer.sanger.ac.uk/cancergenome/projects/cosmic/) are missense mutations. TAF1L, a TAF1 homologue, shows 95 % amino acid identity with TAF1 [9]. Biological and tumor-related functions of TAF1L are not known, either.
In a public genome database (http://genome.cse.ucsc.edu/), we found that human TAF1 and TAF1L had mononucleotide repeats in the coding sequences that could be targets for frameshift mutation in cancers with microsatellite instability (MSI). Frameshift mutation of genes containing mononucleotide repeats is a feature of gastric (GC) and colorectal cancers (CRC) with MSI [10]. To date, however, it is not known whether TAF1 and TAF1L genes are mutationally altered in GC and CRC with MSI. Cancer development initiates through a clonal expansion of a single cell. The resulting cell population usually becomes heterogeneous after branching sub-clonal expansions, which leads to intra-tumor heterogeneity (ITH). This ITH contributes to acquired tumor aggressiveness and may impede the accurate diagnosis/prognosis and the proper selection of tumor therapies [11]. In this study, we analyzed somatic mutations of TAF1 and TAF1L genes, and mutational ITH of these genes in GC and CRC.
Materials and Methods
Tissue Samples and Microdissection
For the mutation analysis, cancer tissues of 79 sporadic GC and 124 sporadic CRC were used in this study. Of them, 54 CRC were frozen tissues and the other 149 tissues were methacarn-fixed tissues. All of the patients with the cancers were Koreans. The GC consisted of 34 GC with high MSI (MSI-H), 45 GC with stable MSI/low MSI (MSS/MSI-L), 79 CRC with MSI-H and 45 CRC with MSS/MSI-L. The MSI evaluation system used five mononucleotide repeats (BAT25, BAT26, NR-21, NR-24 and MONO-27), tumoral MSI status of which was characterized as: MSI-H, if two or more of these markers show instability, MSI-L, if one of the markers shows instability and MSS, if none of the markers shows instability [12–14]. For 54 CRC of the 124 CRC described above, we collected four to seven different tumor areas and one normal mucosal area from each fresh CRC specimen to analyze the mutational ITH. The tumor areas were 0.027–1 cm3 and at least 1.0 cm apart from each other. To confirm that these multi-regional biopsies were all areas of carcinoma (as opposed to areas of normal or dysplasia), they were frozen, stained with hematoxylin & eosin and examined under light microscope. The tumor cell purities of the ITH tissues were at least 70 %. Sixteen of the 54 CRC with ITH collection were identified as MSI-H. These 16 cases selected for ITH analysis were only chosen from the MSI-H group. These four to seven different tumor areas in the 16 CRC were used for detecting regional heterogeneity of TAF1 and TAF1L genes.
The pathologic features of the cancers are summarized in Table 1. The histologic features of CRC with MSI-H, including mucinous histology, tumor infiltrating lymphocytes, medullary pattern, and Crohn’s like inflammation, were evaluated in all blocks of all cases by a pathologist. Malignant cells and normal cells were selectively procured from hematoxylin and eosin-stained slides using a 30G1/2 hypodermic needle by microdissection as described previously [15, 16]. DNA extraction was performed by a modified single-step DNA extraction method by proteinase K treatment. Approval of this study was obtained from the Catholic University of Korea, College of Medicine’s institutional review board for this study.
Single Strand Conformation Polymorphism (SSCP) Analysis
TAF1 exon 12 (an A8 repeat) and TAF1L exon 1 (an A7 repeat) have mononucleotide repeats in their coding sequences. Also, another exon without repetitive sequences for TAF1 (exon 11) and another area in exon 1 without repetitive sequences for TAF1L were investigated as well. Genomic DNA from the microdissected cells was isolated, and was amplified by polymerase chain reaction (PCR) with specific primer pairs. Radioisotope ([32P]dCTP) was incorporated into the PCR products for detection by autoradiogram. After SSCP, mobility shifts on the SSCP gels (FMC Mutation Detection Enhancement system; Intermountain Scientific, Kaysville, UT, USA) were determined by visual inspection. Direct DNA sequencing reactions in both forward and reverse sequences were performed in the cancers with the mobility shifts in the SSCP using a capillary automatic sequencer (3730 DNA Analyzer, Applied Biosystem, Carlsbad, CA, USA). When mutations in the TAF1 and TAF1L genes were suspected by SSCP, analysis of an independently isolated DNA from another tissue section of the same patients was performed to exclude potential artifacts originated from PCR.
Results and Discussion
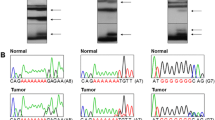
Genomic DNAs isolated from normal and tumor tissues of the 79 GC and 124 CRC were analyzed for the detection of mutation in TAF1 (exon 12 (A8)) and TAF1L (exon 1 (A7)) by PCR-SSCP analysis. On the SSCP, we observed aberrant bands in three cases for TAF1 and four cases for TAF1L (Fig. 1 and Table 2). DNA from normal tissues from the same patients showed no evidence of aberrant migration in SSCP, indicating the mutations had risen somatically (Fig. 1a and b). Direct DNA sequencing analyses of the cancer tissues with aberrantly migrating bands confirmed that the aberrant bands represented somatic mutations of TAF1 and TAF1L genes (Fig. 1b). Three of 79 CRC (3.8 %) with MSI-H harbored TAF1 frameshift mutations, while one of 34 GC (2.9 %) and three of 79 CRC (3.8 %) with MSI-H harbored TAF1L frameshift mutations. The three cases found to show ITH described below were from the seven cases detected with mutation of the TAF1 and TAF1L gene described above.
Representative SSCP and DNA sequencings of TAF1 A8 repeat in a colon carcinoma and TAF1L A7 in another colon carcinoma. SSCP (a) and DNA sequencing analyses (b) of TAF1 (left) and TAF1L (right) from tumor (Lane T) and normal tissues (Lane N). Direct DNA sequencing analyses (b) shows heterozygous A deletion within the TAF1 A8 (left) and the TAF1L A7 (right) in tumor tissue as compare to normal tissue
All of the TAF1 mutations were a heterozygous frameshift mutation (deletion of one base) in the A8 repeat (c.1937delA) that would result in a frame shifting change with lysine 646 as the first affected amino acid, changing to an arginine and creating a new reading frame ending in a stop at position 4 from the arginine (p. Lys646ArgfsX4). The TAF1L mutations were a heterozygous frameshift mutation (deletion of one base) in the A7 repeat (c.2553delA) that would result in a frameshift (p. Ala852ProfsX14) (Table 2). The mutations were detected in the cancers with MSI-H, but not in those with MSS/MSI-L (Table 2). In the cancers with MSI-H, however, there was no correlation between histological features of the tumors (histologic grade, subtypes, mucinous histology, medullary pattern and tumor-infiltrating lymphocytes) and presence of the mutations (p > 0.05). We could not find any mutations in other areas without repetitive sequences each for TAF1 and TAF1L.
From 96 regional fragments of 16 CRC (4–7 fragments per case) with MSI-H were collected and analyzed with respect to their regional status of TAF1 and TAF1L frameshift mutations. Two of the 16 CRC (12.5 %) harbored ITH of TAF1 frameshift mutations, while one of the 16 CRC (6.3 %) harbored ITH of TAF1L frameshift mutations. A CRC case (#53) showed the TAF1 mutation in four of seven regional biopsies. Two CRC cases (#43 and 35, respectively) showed TAF1 and TAF1L mutation in one biopsy per CRC case (Table 3 and Fig. 2).
Intratumoral heterogeneity of TAF1 and TAF1L frameshift mutations in colon cancers. a Direct DNA sequencings show TAF1 c.1937delA mutation (MT) in four regional biopsies (53–1, 53–2, 53–6 and 53–7) and wild-type (WT) TAF1 in the other three regional biopsies (53–3, 53–4 and 53–5). b Direct DNA sequencings show TAF1L c.2553delA mutation (MT) in a regional biopsy (35–2) and wild-type (WT) TAF1L in the other three regional biopsies (35–1, 35–3 and 35–4)
Based on earlier reports that showed TAF1 had not only basal transcription-related functions but also an apoptosis activity, inactivation of which would be a cancer hallmark [17], we attempted to disclose whether somatic frameshift mutations of TAF1 and its homologue TAF1L genes were present in GC and CRC. Since mononucleotide repeats are common targets for frameshift mutations in cancers with MSI-H [10], we focused the analysis within the repeats in TAF1 and TAF1L genes. In the present study, we found TAF1 frameshift mutations (3.8 % of CRC with MSI-H) and TAF1L frameshift mutations (2.9 % of GC and 3.8 % of CRC with MSI-H). Despite the low incidences, these mutations appear to have interesting findings. First, the frameshift mutations detected in the present study would result in premature stops of amino acid synthesis in TAF1 and TAF1L proteins and hence resembles a typical loss-of-function mutation. At this stage, consequence of the mutations in tumorigenesis remains unknown. Given the p27-mediated anti-apoptotic function of TAF1, the TAF1 frameshift mutation would reduce the cell death and contribute to the survival of cancer cells.
Second, in the present study we found ITH of TAF1 and TAF1L mutations (12.5 % and 6.3 %, respectively). These results are in accordance with previous studies showing that genetic ITH for (non-coding) microsatellite markers, as well as repeat sequences within coding genes, may be encountered [18]. Presence of genetic ITH may have implications for predictive and prognostic biomarker strategies. For example, low frequency mutations with a potential to metastasize define clinical outcomes since the clones with ITH easily achieve clonal dominance during the progression and affect treatment efficacy [11, 19]. Practically, the data indicate that there could be under- or over-estimation of TAF1 and TAF1L mutations in the cancers. The data also suggest that when performing mutation analysis in cancers with MSI-H, multi-regional biopsies should be taken into account for a better evaluation. As for the clinicopathologic parameters, however, there was no definite difference between the CRCs with and without TAF1 and TAF1L mutation ITH. Therefore, we propose that the roles of ITH of TAF1 and TAF1L mutations remain to be clarified in conjunction with the identification of biological functions of TAF1 and TAF1L in cancers.
In summary, our study here reports inactivating mutations of TAF1 and TAF1L in GC and CRC with MSI-H, and their mutational ITH. The results may extend our insights that the basal transcription units themselves or their unknown functions might be involved in mechanisms of cancer development. Also, our study has added concerns about ITH of the mutations, which should be considered in the clinical application to GC and CRC with MSI-H. When performing mutation analyses in MSI-H GCs and CRCs, multi-regional biopsies should be considered for a better evaluation of their mutation status. The putative clinical implications of mutational ITH, especially for tumors with a MSI-H phenotype, warrant further investigation.
References
Wassarman DA, Sauer F (2001) TAF(II)250: a transcription toolbox. J Cell Sci 114:2895–2902
Lee DH, Gershenzon N, Gupta M, Ioshikhes IP, Reinberg D, Lewis BA (2005) Functional characterization of core promoter elements: the downstream core element is recognized by TAF1. Mol Cell Biol 25:9674–9686
Juven-Gershon T, Kadonaga JT (2010) Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev Biol 339:225–229
Hilton TL, Li Y, Dunphy EL, Wang EH (2005) TAF1 histone acetyltransferase activity in Sp1 activation of the cyclin D1 promoter. Mol Cell Biol 25:4321–4332
Tavassoli P, Wafa LA, Cheng H, Zoubeidi A, Fazli L, Gleave M, Snoek R, Rennie PS (2010) TAF1 differentially enhances androgen receptor transcriptional activity via its N-terminal kinase and ubiquitin-activating and -conjugating domains. Mol Endocrinol 24:696–708
Makino S, Kaji R, Ando S, Tomizawa M, Yasuno K, Goto S, Matsumoto S, Tabuena MD, Maranon E, Dantes M, Lee LV, Ogasawara K, Tooyama I, Akatsu H, Nishimura M, Tamiya G (2007) Reduced neuron-specific expression of the TAF1 gene is associated with X-linked dystonia-parkinsonism. Am J Hum Genet 80:393–406
Kimura J, Nguyen ST, Liu H, Taira N, Miki Y, Yoshida K (2008) A functional genome-wide RNAi screen identifies TAF1 as a regulator for apoptosis in response to genotoxic stress. Nucleic Acids Res 36:5250–5259
Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, Guzzo F, English DP, Varughese J, Gasparrini S, Bortolomai I, Buza N, Hui P, Abu-Khalaf M, Ravaggi A, Bignotti E, Bandiera E, Romani C, Todeschini P, Tassi R, Zanotti L, Carrara L, Pecorelli S, Silasi DA, Ratner E, Azodi M, Schwartz PE, Rutherford TJ, Stiegler AL, Mane S, Boggon TJ, Schlessinger J, Lifton RP, Santin AD (2013) Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A 110:2916–2921
Wang PJ, Page DC (2002) Functional substitution for TAF(II)250 by a retroposed homolog that is expressed in human spermatogenesis. Hum Mol Genet 11:2341–2346
Imai K, Yamamoto H (2008) Carcinogenesis and microsatellite instability: the interrelationship between genetics and epigenetics. Carcinogenesis 29:673–680
Marusyk A, Almendro V, Polyak K (2012) Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 12:323–334
Murphy K, Zhang S, Geiger T, Hafez MJ, Bacher J, Berg KD, Eshleman JR (2006) Comparison of the microsatellite instability analysis system and the Bethesda panel for the determination of microsatellite instability in colorectal cancers. J Mol Diagn 8:305–311
Geiersbach KB, Samowitz WS (2011) Microsatellite instability and colorectal cancer. Arch Pathol Lab Med 135:1269–1277
Ogino S, Goel A (2008) Molecular classification and correlates in colorectal cancer. J Mol Diagn 10:13–27
Yoo NJ, Kim HR, Kim YR, An CH, Lee SH (2012) Somatic mutations of the KEAP1 gene in common solid cancers. Histopathology 60:943–952
Je EM, Kim MR, Min KO, Yoo NJ, Lee SH (2012) Mutational analysis of MED12 exon 2 in uterine leiomyoma and other common tumors. Int J Cancer 131:E1044–E1047
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Calin GA, Gafà R, Tibiletti MG, Herlea V, Becheanu G, Cavazzini L, Barbanti-Brodano G, Nenci I, Negrini M, Lanza G (2000) Genetic progression in microsatellite instability high (MSI-H) colon cancers correlates with clinico-pathological parameters: a study of the TGRbetaRII, BAX, hMSH3, hMSH6, IGFIIR and BLM genes. Int J Cancer 89:230–235
Almendro V, Cheng YK, Randles A, Itzkovitz S, Marusyk A, Ametller E, Gonzalez-Farre X, Muñoz M, Russnes HG, Helland A, Rye IH, Borresen-Dale AL, Maruyama R, van Oudenaarden A, Dowsett M, Jones RL, Reis-Filho J, Gascon P, Gönen M, Michor F, Polyak K (2014) Inference of tumor evolution during chemotherapy by computational modeling and in situ analysis of genetic and phenotypic cellular diversity. Cell Rep 6:514–527
Acknowledgments
This work was supported grants from National Reserach Foundation of Korea (2012R1A5A2047939 and 2015R1D1A1A01057355).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Hye Rim Oh and Chang Hyeok An contributed equally to this work.
Rights and permissions
About this article
Cite this article
Oh, H.R., An, C.H., Yoo, N.J. et al. Frameshift Mutations in the Mononucleotide Repeats of TAF1 and TAF1L Genes in Gastric and Colorectal Cancers with Regional Heterogeneity. Pathol. Oncol. Res. 23, 125–130 (2017). https://doi.org/10.1007/s12253-016-0107-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-016-0107-0