Abstract
The proportion of Ki-67 immunostained cells (Ki-67 labeling index, LI) is one of the most commonly used histology methods for estimating proliferation of breast carcinomas. Although the Ki-67 LI is used in treatment decision making, its reproducibility shows variation in different studies, and is generally less then optimal. The aim of the present study was to investigate how the use of a standardized, partially digitalized counting method could affect reproducibility of determining the Ki-67 LI. Thirty breast cancer core-biopsy samples were stained with B-56, SP-6 and MIB-1 monoclonal Ki-67 antibodies. Each sample was represented by a single digital photograph taken with a x20 objective. Four investigators determined the Ki-67 LI on these digital images by estimation, then by counting with the help of a grid overlaid on the same images. Altogether 720 evaluations were made by 4 independent pathologists. Good to excellent correlation was found between estimations and calculations of each observer. Kappa values >0.6 suggest substantial inter-observer agreement when classifying the cases into a 15 % and 30 % cut-off determined three-tiered or a 4-quarter-based four-tiered categorization, which is better than the fair reproducibility gained on the real slides in a previous study. The results also suggest that the type of the antibody may also impact on the consistency of both estimating and calculating the Ki-67 LIs. The results indicate that counting on digital images may significantly improve reproducibility of determining the KI-67 LI. Interestingly, estimation on the same images is not worse, but is obviously faster and more convenient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proliferation activity of different malignant tumors is an important prognostic factor which has a role in planning surgical and oncological treatment. This feature is also taken into account in breast carcinomas to distinguish between tumors with low and high proliferation [1–10]. This is especially true for ER positive, HER2 negative tumors which are more likely to respond to systemic chemotherapy when their proliferation is high, than when it is low [6, 11–14]. There are different ways to evaluate proliferation activity. Beresford et al. have shown many methods to be problematic and have recommended the use of Ki-67 as a standard proliferation marker [15]. This protein is expressed in all phases of cell cycle, except G0 [16, 17]. This feature makes it the best marker to be detected by immunohistochemistry in different malignant tumors including breast carcinoma. Proliferating tumor cells show positive nuclear reaction with anti-Ki-67 antibodies. Different studies have demonstrated that a high Ki-67 LI indicates an increased risk of recurrence, metastasis, and faster progression of the disease [6, 11, 12, 18–23]. The pathologic report generally refers to the percentage of positive tumor cells (Ki-67 labeling index, LI). The Ki-67 LI is determined either by estimation or by counting all over the world, but there are differences in the methods which are used [10, 24]. Because of its clinical role mentioned above, reproducibility is important, but is quite problematic in our opinion. Quality, type and size of the tissue, fixation time and human errors can all be causes of worse reproducibility. In 2009, a >30 % Ki-67 LI cut-off value was accepted at a St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer for high proliferation and for recommending adjuvant chemotherapy in endocrine responsive breast carcinomas [13, 25]. Two years later a >14 % cut-off was taken into account to delineate a surrogate approach for a part of luminal B carcinomas that could also be treated with systemic chemotherapy along with hormonal treatment [5, 14]. There are also different cut-off values of Ki-67 LI which have been proposed by others for the indication of chemotherapy for ER-positive patients [19, 22, 26–28]. Beside that, there are no standardized methods for the elimination of the different previously mentioned factors influencing the Ki-67 LI, although efforts have been made towards standardization [5].
In a previous study we highlighted the limitations and suboptimal reproducibility of counting Ki-67-positive tumor cells under the microscope [29]. We also underlined the differences in personal evaluating methods of the LI. In the present study, we aimed to investigate how the use of a standardized, partially digitalized counting method could affect reproducibility of determining the Ki-67 LI.
Materials and Methods
Pretreatment diagnostic core biopsy samples of patients scheduled for neoadjuvant chemotherapy for breast cancer were analyzed in the study. The biopsies were taken from patients with operable T2 ≥ 3 cm or T3-4 and/or N1-2 and M0 breast cancer. For better comparability, the cases were identical with the cases of a previous study assessing interobserver and intraobeserver reproducibility of assessing the Ki-67 LI by routine work microscopy [29]. The tumor samples were fixed in buffered formalin and embedded in paraffin. Samples have been routinely stained with hematoxylin and eosin (HE) and routinely immunostained for estrogen receptor, progesterone receptor, HER-2 and topoisomerase II-alpha. Samples were also immunostained for Ki-67 with the following 3 antibodies: SP6 (monoclonal rabbit antibody, Hisztopatologia Kft., Pécs, Hungary), B56 (monoclonal mouse antibody, Hisztopatologia Kft., Pécs, Hungary) and MIB-1 (monoclonal mouse antibody, Dako, Glostrup, Denmark) for the purpose of a study. Wet antigen retrieval consisted of pretreatment of all samples in microwave oven in a citrate buffer with pH6 for 30, 30 and 50 min in case of MIB-1, B56 and SP6, respectively. All antibodies were diluted at 1:100. Expression of Ki-67 was determined using Dako EnVision FLEX/HRP, DAB + Chromogen (Dako, Glostrup, Denmark).
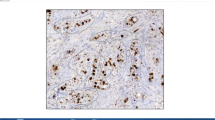
Microphotographs of each immunostained core-biopsy sample were taken with a x20 objective. A hot-spot area was photographed in all cases where such a hot spot could be identified. Pictures were entered in a Microsoft PowerPoint file. One photograph was analyzed in each case. Four different investigators first determined the Ki-67 LI by estimating the proportion of stained cells with 5 % precision in the same areas (i.e. the same digital image displayed on a screen). No counting was involved in this assessment. Time needed for the evaluation was recorded in series of cases for all investigators. In a second round, a uniform grid composed of equidistant parallel horizontal lines was laid on all digital images (Fig. 1), previously used for estimation. The observers were asked to count the tumor cells crossed by the lines or touching the lines. The lines of the grid can be followed and the touching or crossed cells can be recorded (counted) continuously without the doubt of double counting or omitting single cells. Both immunohistochemically negative and positive nuclei were counted. Non-cancerous cells (stromal elements, lymphocytes etc.) were ignored as much as possible. The ratio of positive cells was derived from these values. In further analyses, rounded values (to the next integer) were used. Evaluation time was also recorded for this method. In all cases, the participating pathologists were asked to consider positive any cell with a brown (stained) rather than blue (unstained) hue.
Comparisons were performed between the estimated and counted values of each investigator. Different investigators’ values were also compared with each other.
Kappa statistics were used to evaluate the interobserver reproducibility regarding estimation and counting. The following cut-off values were used (taking the values mentioned by the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer in 2009): 0–15 %, 16–30 % and >30 %. Beside this categorization, Ki-67 values were divided into four quarters (0–25 %, 26–50 %, 51–75 % and 76–100 % Ki–67 LI). Kappa values were calculated according to Fleiss [30] and were interpreted as reflecting slight (0–0.2), fair (0.21–0.4), moderate (0.41–0.6), substantial (0.61–0.8) and almost perfect (>0.8) agreement between observations according to Landis and Koch [31].
Spearman and Pearson correlation analyses were also used in order to compare the intra- and interobserver estimated and calculated values. Coefficients were categorized as follows: values between 0.9 and 1 show excellent, 0.75–0.9 good, 0.5–0.75 moderate, 0.25–0.5 week correlation, whereas values between 0.0 and 0.25 reflect lack of correlation. Comparisons were made for each antibody alone (30 values per observer) and for the three different antibodies combined (90 values per observer) for each pair of investigators. The analyses were performed both for the estimated and the calculated values, using software package SPSS 15.0 for Windows (SPSS Inc., Chicago, Illinois).
Results
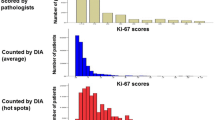
Digital images of 30 core-biopsy samples were analyzed. The mean ± SE age of the patients represented was 46 ± 2 (range: 26–70) years. Samples included 28 invasive ductal carcinomas of no special type, and 2 invasive lobular carcinomas. Altogether 720 evaluations were made by 4 independent pathologists with special interest in breast pathology (GC, AV, EC, BK). Mean ± SE estimated and counted Ki-67 LI values provided by the 4 pathologists are shown in Table 1. The calculated Ki-67 LI was based on the assessment of an average of 75–91 cells. There were no major differences in the cells counted from the same set of digital images by different investigators. The range of cells counted on the grid marked images was 9 to 194, as a single image with the same grid was used for each tumor and core biopsy.
Ki-67 values for individual cases and individual observers are presented in Fig. 2. The graphs demonstrate a very good overlap between investigators, both for the estimated and the counted Ki-67 values. Furthermore, the similar shapes of the graphs for the estimated and calculated Ki-67 values suggest a good overlap between the two methods of assessment. These impressions were also substantiated by the statistical analyses.
Good to excellent correlation was observed both with the Pearson’s and the Spearman’s methods when comparing the estimated and the calculated Ki-67 values of each investigator when analyzing all antibodies (90 cases per observer) (Table 2). The 90 assessments (all antibodies included) of each pair of investigators were also compared both for the estimated and the calculated Ki-67 LIs. The inter-observer correlation coefficients demonstrate an excellent correlation (Table 3).
Similar correlation analyses were repeated in case of each antibody, one by one (i.e. only 30 cases with the same antibody per observer), in order to see whether different antibodies were associated with different correlations. To compare the effect of the antibodies on the intra-observer correlation of estimated and calculated Ki-67 LIs, as a basic approach, the 4 correlation coefficients by observers were averaged. In case of SP6 the mean ± SE values of the Person’s and Spearman’s correlation coefficients for the estimated Ki-67 LIs were 0.855 ± 0.044 and 0.857 ± 0.035, respectively. These values were 0.922 ± 0.004 and 0.926 ± 0.008 in case of B56, and 0.904 ± 0.012 and 0.879 ± 0.026 in case of MIB-1, respectively. These results suggest that SP6 might have at least a trend for a slightly weaker intra-observer correlation, than the others. With inter-observer analyses by antibody type, comparing the calculated values (6 pairs of investigators) in case of SP6 the mean ± SE values of correlation coefficients derived from Pearson and Spearmen tests were 0.871 ± 0.044 and 0.881 ± 0.039, respectively. In case of B56 these values were 0.967 ± 0.005 and 0.947 ± 0.005, respectively, while for MIB-1 they were 0.957 ± 0.006 and 0.960 ± 0.006, respectively. For the estimated values, the following results were found using Pearson and Spearman tests (mean ± SE): 0.942 ± 0.008 and 0.943 ± 0.009 for SP6, 0.947 ± 0.008 and 0.962 ± 0.006 for B56, while 0.926 ± 0.012 and 0.896 ± 0.013 for MIB-1, respectively. These results also suggest that SP6 might be associated with less concordance between observers, but only in case of the calculated Ki-67 LIs while in case of MIB-1, the estimation of Ki-67 LIs showed lower correlations.
When all Ki-67 values (30 cases stained with 3 antibodies, i.e. 90 values per observer) estimated by eyeballing were considered, reproducibility of the proliferative activity was substantial both for the classification into 4 equal quarters (kappa: 0.68) and the classification into three categories (kappa: 0.65). The kappas were 0.67 and 0.73, respectively in case of the calculated Ki-67 values, all corresponding to substantial agreement.
Examining the antibodies one by one, using four categories and estimated Ki-67 values, the kappas were 0.65, 0.69 and 0.64 for the MIB1, B56 and SP6 antibodies, respectively. For the three-tiered estimated Ki-67 categories, kappa values were 0.59, 0.69 and 0.67, respectively. Analyzing the calculated Ki-67 LIs, kappas were 0.66, 0.71 and 0.65, respectively for the four categories and 0.90, 0.60 and 0.69, respectively for the three categories. The agreement of the Ki-67 LIs gained by different antibodies was therefore almost always substantial, with two instances suggesting moderate reproducibility but falling just short of the substantial agreement category, and one instance with an almost perfect agreement.
The mean time to evaluate the Ki-67 LI on a single digital image was calculated on the basis of the time used for the investigation of 30 biopsy samples stained by a given antibody. By eyeballing this time ranged between 18 and 50 s per investigator, and this range was between 90 and 180 s when the cells were counted, and the Ki-67 LI was derived from the calculated proportion of stained and all tumor cells.
Discussion
Proliferation assessed by Ki-67 immunostaining is a recognized prognosticator of breast carcinomas [2–4, 11]. Determining the proliferation activity of breast cancer is an important task for the pathologist because it is a factor considered in therapeutic decision making, especially when chemotherapy is needed, since most chemotherapeutic agents act on proliferating cells. Higher proliferation may result in better chemosensitivity and may also reflect better response to specific hormonal agents (e.g. letrozole versus tamoxifen) [11]. Immunostaining with Ki-67 monoclonal antibodies is the most widely used assessment of proliferative activity today. Pathologists have to try to determine this value as accurately as possible. There are several technical issues relating to the processing of the tissues, areas of the tumor considered, intensity of staining considered positive… etc. that may affect the final LI, but even without these, reproducibility has been found less than optimal in several studies, including our previous work assessing the proliferation on the same set of needle core biopsies [29, 32]. In our previous article, we found that KI-67 LI values were significantly influenced by the investigator even if unified rules were used during the evaluation. Not only the inter-observer, but also the intra-observer agreement was found to be poor to moderate [29]. Although an international consensus recommends the examination of at least 500, but optimally 1,000 cells for deriving the Ki-67 LI [5], this practice is rarely followed, and counting about 100 cells or estimating the overall stained proportion of tumor cells are common methods of assessing proliferation. The lack of time is one of the most important factors deviating from the counting of high number of cells.
The presented results support that by choosing a limited area of the tumor as represented by a digital image, and by helping to choose which cells to count with a grid, improves reproducibility of determining the Ki-67 LI. Indeed, the inter-observer agreement on the Ki-67 LI reached on the real slides of the same cases and derived on the basis of about 100 cells from the area with the highest staining proportion was only fair on the basis of the overall kappa values <0.4 [29], but changed to substantial (overall kappa >0.6) for the digital images. Such an improvement in reproducibility was achieved by counting somewhat less cells on average than the 100 cells in the previous investigation, but the present study did not assess how many cells needed to be evaluated to reflect the proliferative activity of a tumor on the basis of a needle core biopsy, it only concentrated on reproducibility issues. It may well be, that several digital images would be required to reflect tumor proliferation. We expect similarly acceptable reproducibility with 2, 3 or more images. On the dark side of such an improvement, we must accept a loss in time. Making digital images of given areas of a tumor histology slide and adding a standard grid to the image may be fast in some settings, but may also take too much time to be affordable. The evaluation itself is also somewhat time-taking, requiring 2 to 3 min per digital image, depending on the cell density. Therefore, the finding that a rough estimate of the stained proportion of tumor cells may be as reproducible as the calculated LI is of interest. A similar estimation based method is generally used for establishing the percentage of estrogen or progesterone receptor positive cells.
Varga et al. in a very carefully designed study showed, that better reproducibility could be achieved by estimation rather than accurate counting [32]. Our results are in keeping with this observation, as good to excellent correlation was found between the estimated and the counted Ki-67 LI values and the overall kappa values also suggested substantial reproducibility for the eyeballing based estimation of the Ki-67 LI. Eyeballing obviously require less time, as supported by our data.
Most of the studies investigating reproducibility of Ki-67 based proliferation have been performed on surgical samples. We stress on the fact that core-biopsy samples were used in our studies. This factor theoretically decreases tumor sample heterogeneity and different investigators examine the same area with higher chance, due to the small size of the sample. Therefore, in theory, Ki-67 assessment on core biopsy samples could result in better reproducibility, but this was rebutted in our previous work documenting only fair to poor inter-observer agreement [29]. As concerns the use of different antibodies, MIB-1 is the most widely used and generally recommended one [5], but some other antibodies are also used for the estimation of proliferation. Our results suggest that the type of the antibody may also impact on the consistency of both estimating and calculating the Ki-67 LIs.
Conclusion
The use of a simple digital technology, taking microphotographs of proliferating areas of breast cancers and adding a grid to the pictures to better delineate which cells to consider in the count makes possible for different investigators to examine the same area and the same cells when determining the Ki-67 LI. This can significantly improve reproducibility. We found that calculating the LI on the basis of such grid labeled digital images results in better reproducibility than the frustratingly low one found on the basis of counting stained cells on the histology slides of the same core biopsy specimens. However, we also found that estimating the proportion of Ki-67 stained cells on the same digital images is not only faster than counting the stained and unstained cells, but also results in acceptable and substantial reproducibility and the estimated and counted values correlate strongly. Therefore estimation should not be considered an inadequate method of establishing the Ki-67 LI, simply because it is not based on objective numbers.
References
Urruticoechea A, Smith IE, Dowsett M (2005) Proliferation marker Ki-67 in early breast cancer. J Clin Oncol 23:7212–20
Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ (2005) Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol 16:1723–39
Jalava P, Kuopio T, Juntti-Patinen L, Kotkansalo T, Kronqvist P, Collan Y (2006) Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology 48:674–82
de Azambuja E, Cardoso F, de Castro G, Jr CM, Mano DV et al (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96:1504–13
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst 103:1–9
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA (2010) Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 11:174–83
Billgren AM, Tani E, Liedberg A, Skoog L, Rutqvist LE (2002) Prognostic significance of tumor cell proliferation analyzed in fine needle aspirates from primary breast cancer. Breast Cancer Res Treat 71:161–70
Liu S, Edgerton SM, Moore DH 2nd, Thor AD (2001) Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res 7:1716–23
Seshadri R, Leong AS, McCaul K, Firgaira FA, Setlur V, Horsfall DJ (1996) Relationship between p53 gene abnormalities and other tumour characteristics in breast-cancer prognosis. Int J Cancer 69:135–41
Luporsi E, André F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A, Egele C, Poulet B, Clough KB, Crouet H, Fourquet A, Lefranc JP, Mathelin C, Rouyer N, Serin D, Spielmann M, Haugh M, Chenard MP, Brain E, de Cremoux P, Bellocq JP (2012) Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat 132:895–915
Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell’Orto P et al (2008) Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1–98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol 26:5569–75
Viale G, Regan MM, Mastropasqua MG, Maffini F, Maiorano E, Colleoni M, Price KN, Golouh R, Perin T, Brown RW, Kovács A, Pillay K, Ohlschlegel C, Gusterson BA, Castiglione-Gertsch M, Gelber RD, Goldhirsch A, Coates AS, International Breast Cancer Study Group (2008) Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst 100:207–12
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20:1319–29
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22:1736–47
Beresford MJ, Wilson GD, Makris A (2006) Measuring proliferation in breast cancer: practicalities and applications. Breast Cancer Res 8:216
Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C et al (1991) Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol 138:867–73
Gerdes J, Schwab U, Lemke H, Stein H (1983) Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 31:13–20
Goodson WH 3rd, Moore DH 2nd, Ljung BM, Chew K, Mayall B, Smith HS, Waldman FM (2000) The prognostic value of proliferation indices: a study with in vivo bromodeoxyuridine and Ki-67. Breast Cancer Res Treat 59:113–23
Fasching PA, Heusinger K, Haeberle L, Niklos M, Hein A, Bayer CM, Rauh C, Schulz-Wendtland R, Bani MR, Schrauder M, Kahmann L, Lux MP, Strehl JD, Hartmann A, Dimmler A, Beckmann MW, Wachter DL (2011) Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 11:486
Jung SY, Han W, Lee JW, Ko E, Kim E, Yu JH, Moon HG, Park IA, Oh DY, Im SA, Kim TY, Hwang KT, Kim SW, Noh DY (2009) Ki-67 expression gives additional prognostic information on St. Gallen 2007 and adjuvant! Online risk categories in early breast cancer. Ann Surg Oncol 16:1112–21
Mohammed ZM, McMillan DC, Elsberger B, Going JJ, Orange C, Mallon E, Doughty JC, Edwards J (2012) Comparison of visual and automated assessment of Ki-67 proliferative activity and their impact on outcome in primary operable invasive ductal breast cancer. Br J Cancer 106:383–8
Kashiwagi S, Yashiro M, Takashima T, Aomatsu N, Ikeda K, Ogawa Y, Ishikawa T, Hirakawa K (2011) Advantages of adjuvant chemotherapy for patients with triple-negative breast cancer at Stage II: usefulness of prognostic markers E-cadherin and Ki67. Breast Cancer Res 13:R122
Murri AM, Hilmy M, Bell J, Wilson C, McNicol AM, Lannigan A, Doughty JC, McMillan DC (2008) The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br J Cancer 99:1013–9
Fasanella S, Leonardi E, Cantaloni C, Eccher C, Bazzanella I, Aldovini D, Bragantini E, Morelli L, Cuorvo LV, Ferro A, Gasperetti F, Berlanda G, Dalla Palma P, Barbareschi M (2011) Proliferative activity in human breast cancer: Ki-67 automated evaluation and the influence of different Ki-67 equivalent antibodies. Diagn Pathol. doi:10.1186/1746-1596-6-S1-S7
Gnant M, Harbeck N, Thomssen C (2011) St. Gallen 2011: summary of the consensus discussion. Breast Care (Basel) 6:136–141
Spyratos F, Ferrero-Poüs M, Trassard M, Hacène K, Phillips E, Tubiana-Hulin M, Le Doussal V (2002) Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer 94:2151–9
Jones RL, Salter J, A’Hern R, Nerurkar A, Parton M, Reis-Filho JS, Smith IE, Dowsett M (2010) Relationship between oestrogen receptor status and proliferation in predicting response and long-term outcome to neoadjuvant chemotherapy for breast cancer. Breast Cancer Res Treat 119:315–23
Dowsett M, A’Hern R, Salter J, Zabaglo L, Smith IE (2009) Who would have thought a single Ki67 measurement would predict long-term outcome? Breast Cancer Res. doi:10.1186/bcr2434
Vörös A, Csörgő E, Nyári T, Cserni G (2012) An intra- and interobserver reproducibility analysis of the Ki-67 proliferation marker assessment on core biopsies of breast cancer patients and its potential clinical implications. Pathobiology 80:111–118
Fleiss JL (1980) The measurement of interrater agreement. In: Fleiss JL (ed) Statistical methods for rates and proportions, 2nd edn. John Wiley and Sons Inc, New York, pp 212–236
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Zs V, Diebold J, Dommann-Scherrer C, Frick H, Kaup D, Noske A, Obermann E, Ohlschlegel C, Padberg B, Rakozy C, Sancho Oliver S, Schobinger-Clement S, Schreiber-Facklam H, Singer G, Tapia C, Wagner U, Mastropasqua M, Viale G, Lehr H-A (2012) How reliable is Ki-67 immunohistochemistry in grade 2 breast carcinomas? A QA study of the Swiss Working Group of Breast- and Gynecopathologists. Plos One 7:e37379
Disclosures
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vörös, A., Csörgő, E., Kővári, B. et al. The Use of Digital Images Improves Reproducibility of the Ki-67 Labeling Index as a Proliferation Marker in Breast Cancer. Pathol. Oncol. Res. 20, 391–397 (2014). https://doi.org/10.1007/s12253-013-9708-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-013-9708-z