Abstract
In cancer therapy novel concepts focus on phosphoinositide-3-kinase/protein kinase B/mammalian target of rapamycin (mTOR) inhibitors. In this context, phosphorylated S6 protein of the 40S ribosomal subunit (pS6) overexpression was previously shown to be associated with sensitivity to inhibitors of mTOR. The present study therefore evaluated pS6 expression in normal renal parenchyma (NRP), primary renal cell carcinomas (PRCC) and their metastases. pS6 and pmTOR expression was immunohistochemically analyzed in a tissue microarray (TMA) from localized primary renal cell carcinoma (lPRCC) (n = 35), metastasized primary renal cell carcinoma (mPRCC) (n = 45), their metastases (n = 45), and NRP (n = 45). pS6 expression was stronger in mPRCCs and metastases than in NRP and lPRCCs (p < 0.05). In mPRCCs high-grade and high-stage tumors showed higher pS6 levels. pS6 overexpression was more frequently found in metastases (40/45; 88.9%) than in mPRCC (24/45; 53.3%) (p < 0.05). Overexpression of pS6 in metastases without concomitant overexpression in their primary tumors was found in 16/45 (35.56%) cases. Patients with pS6 overexpression in mPRCCs but also in metastases showed a tendency to shorter overall survival. pS6 score and pmTOR score correlated positively in NRP and in tumorous tissue (mPRCC and metastases). In conclusion, the present study showed stronger pS6 expression and more frequent overexpression in metastases than in corresponding PRCCs. In approximately one-third of the cases pS6 overexpression was found exclusively in metastases, which is interesting with regard to the association between high pS6 expression and sensitivity to mTOR inhibitor therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The phosphorylated S6 protein of the 40S ribosomal subunit (pS6), causing protein synthesis [1, 2], is elevated in various malignancies (e.g. breast, ovary, renal, hepatocellular carcinoma, sarcoma, acute leukemia) [3–10] as a result of alterations in the phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT) pathway, and its overexpression has been reported to be associated with worse prognosis [8, 10]. In detail, the AKT pathway is activated by receptor tyrosine kinase growth factors (e.g. epidermal growth factor receptor, insulin-like growth factor receptor), which leads to generation of membrane-bound phosphoinositides and phosporylation of AKT (pAKT) by PI3K [11]. Consequently, pAKT activates mammalian target of rapamycin (mTOR), a serine/threonine kinase, effecting phosphorylation of S6 through kinases (e.g. p70S6 kinase) [1, 2, 12].
Since advanced and metastatic renal cell carcinomas are rather insensitive to radio- and chemotherapy, but also cytokine therapy rarely shows benefit (e.g. interleukin-2, interferon alpha), [13–15] novel, so far promising, therapeutic approaches focus on the use of mTOR inhibitors [4, 14]. Since associations between overexpression of pS6 and sensitivity to mTOR inhibitor therapy are described [4, 7] and few data are available on pS6 expression in metastases from renal cell carcinomas, the present study evaluated pS6 expression of primary renal cell carcinomas (PRCC) and their metastases.
Materials and Methods
A tissue microarray (TMA) was constructed from paraffin-embedded localized primary renal cell carcinoma (lPRCC) (n = 35), metastasized primary renal cell carcinoma (mPRCC) (n = 45) (Table 1), their metastases (n = 45), (Table 2) and adjacent tumor-negative renal parenchyma specimens (NRP) (n = 45). In detail, from representative regions of primary tumor, their metastases, and tumor-negative renal tissue four cores per case (diameter 0.6 mm) were arrayed on five recipient blocs. All primary renal cell carcinomas were graded [16] and TNM-staged [17]. Subtyping of renal cell carcinoma followed the WHO tumor classification [17] (Table 1). Follow-up was calculated from the date of nephrectomy and excision of metastases to the last recorded follow-up or death. Metastases occurred synchronously (n = 25) with the primary tumor and metachronously (n = 20). In eight cases the primary diagnosis of renal cell carcinoma was made on metastasectomy specimens prior to nephrectomy.
pS6 Staining
Deparaffinized TMA sections (4 μm) were treated in water bath at 95°C in pH9 Target Retrieval Solution (Dako, Denmark) for 40 min, followed by incubation in 3% H2O2 for 10 min to block endogenous peroxidase activity. The slides were incubated for 2 h at room temperature with a polyclonal rabbit antibody (1:100) specific for phospho-S6 ribosomal protein (S235/236) (pS6) (Cell Signaling, Danvers, MA, USA). After rinsing in wash buffer, primary antibody was detected using the EnVision™ + Dual Link Detection system (Dako, Denmark) and visualisation appeared with diaminobenzidine (DAB+) as the chromogen substrate (Dako, Denmark). The sections were counterstained with haematoxylin. Colon carcinoma specimens served as positive control. Negative controls were also included in the colon carcinoma control with absence of staining in normal vascular smooth muscle, normal muscularis propria, and resting fibroblasts, as previously reported [7]. In further negative controls colon cancer specimens were tested without the primary antibody.
pmTOR Staining
Deparaffinized TMA sections (4 μm) were incubated in a methanol solution containing 3% H2O2 for 30 min to block endogenous peroxidase activity. Following antigen retrieval with a 10 mM citrate buffer, the TMA sections were incubated overnight at 4°C with a monoclonal rabbit antibody (1:50) specific for pmTOR (Cell Signaling, Danvers, MA, USA) and subsequently with a secondary biotinylated goat anti-rabbit-antibody (1:500) (Dako, Glostrup, Denmark) for 30 min. Immunohistological staining was performed with a commercially available kit (ABC-Kit® Vectastain, Vector, Burlingame, CA, USA), and haematoxylin counterstaining was used. Breast cancer tissue sections served as positive control.
Scoring of pS6
For each core pS6 expression was separately scored for staining intensity (e.g. (0) negative; (1) low; (2) moderate; (3) strong) and percentage of stained tumor cells (e.g. (0) 0%; (1) 1% to 29%; (2) 30% to 69%; (3) 70% to 100%).
Scoring of pmTOR
For each core cytoplasmic pmTOR expression was separately scored for staining intensity (e.g. (0) negative, (1) low, (2) moderate, (3) strong) and percentage of stained cells (e.g. (0) 0%, (1) 1% to 10%, (2) 11% to 50%, (3) 51% to 75%, (4) 76% to 100%).
A total score was calculated from the staining intensity score and the score for the percentage of stained cells in tumor-positive and tumor-negative specimens. For cases with two or more evaluable cores the average score per case was evaluated. Subsequently for pS6, in mPRCCs and metastases overexpression was defined as a score higher than found in corresponding NRP.
Statistical Analysis
Data are given as mean ± SD. Descriptive statistics were used. Data were tested for normal distribution using the Kolmogorov-Smirnov Test. The T-test and the Mann–Whitney U Test were used for analysing comparisons. Correlations were calculated with the Spearman correlation coefficient. Kaplan-Meier survival curves were calculated and the log-rank test was used for univariate survival comparison. Statistical analysis employed the statistics software package SPSS 16.0.0 ® (SPSS, Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Results
Patients (n = 80) enrolled had a mean age of 61.2 ± 11.1 years (male (n = 43), female (n = 37)). Patients with metastasized disease experienced a mean time from diagnosis of primary renal cell carcinoma to that of metastases of 26 ± 39.9 months. Mean follow-up time for lPRCC, mPRCC, and metastases was 159.0 ± 9.2 months, 47.7 ± 49.0 months and 23.0 ± 23.1 months, respectively. Patients with lPRCC did not develop progressive or metastatic disease and were alive until end of follow-up.
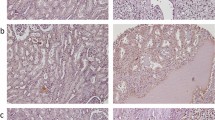
pS6 showed a cytoplasmic staining pattern and its expression was observed in 34/45 (75.56%) NRP, 15/35 (42.86%) lPRCC, 35/45 (77.78%) mPRCC, and 44/45 (97.78%) metastases. pS6 score was higher in mPRCCs and metastases (Fig. 1b) than in NRP (mean score 1.22 ± 1.04) (Fig. 1c) and lPRCCs (p < 0.05) (Tables 1, 2 and 3). Metastases showed a higher pS6 score and more frequent pS6 overexpression than did their PRCCs (p < 0.05) (Tables 1 and 2). In mPRCC tumor grade correlated positively with pS6 score, and pS6 overexpression was more frequently observed in higher-stage tumors (Table 1). pS6 score and pS6 overexpression in mPRCCs correlated positively with its expression and overexpression in metastases (p < 0.05, R = 0.45 and p < 0.05, R = 0.43). mPRCC with synchronous metastases showed higher tumor size (8.2 ± 3.6 cm) than did those with metachronous metastases (5.9 ± 2.3 cm) (p < 0.05). No correlations were found between tumor size and pS6 expression.
Cytoplasmic pmTOR staining was shown in 43/45 (95.6%) NRP, 7/35 (20.0%) lPRCC, 24/45 (53.3%) mPRCC, and 27/45 (60.0%) metastases. pS6 score and pmTOR score correlated positively in NRP and in tumorous tissue (mPRCC and metastases) (p < 0.05, R = 0.53 and R = 0.37).
Concordance in pS6 over- and non-overexpression in mPRCCs and metastases was demonstrated in 29/45 (64.44%) cases, with 24/45 (53.33%) showing pS6 over- and 5/45 (11.11%) showing pS6 non-overexpression (Table 4). In 16/45 (35.56%) cases pS6 overexpression was found only in metastases and not in their primary tumors. The opposite could not be demonstrated (Table 4).
Patients with pS6 overexpression in mPRCCs and in metastases showed a trend to shorter overall survival than did those with pS6 non-overexpression (p = 0.15 and p = 0.16) (Fig. 2a, b).
Discussion
pS6 expression and overexpression were higher and more frequently found in metastases than in primary renal cell carcinoma. Moreover, pS6 overexpression in metastases without concomitant overexpression in primary renal cell carcinoma was found in approx. one-third of the specimens. Furthermore, patients with pS6 overexpression in mPRCCs but also in metastases showed a tendency to shorter overall survival.
pS6, causing protein synthesis [1, 2], is elevated in various malignancies (e.g. breast, ovary, renal, hepatocellular carcinoma, sarcoma, acute leukemia) [3–10] as a result of alterations in the PI3K/AKT pathway, and its overexpression has been reported to be associated with worse prognosis [8, 10]. pS6 is phosphorylated by kinases (e.g. p70S6 kinase), activated by mammalian target of rapamycin (mTOR) [1, 2, 12]. Especially in advanced and metastatic renal cell carcinoma, which are rather insensitive to radio- and chemotherapy and in which cytokine therapy rarely shows a benefit (e.g. interleukin-2, interferon alpha) [13–15], novel, so far promising, therapeutic approaches focus on the use of mTOR inhibitors [4, 14]. Following reports of an association between sensitivity to therapy with mTOR inhibitors and increased pS6 expression [4, 7], the present study evaluated pS6 expression of both localized and metastasized PRCCs and their metastases.
In previous studies pS6 expression was reported to vary from 69.4% to 85% with higher pS6 levels in primary renal cell carcinomas than in NRP [8, 9] and overexpression in 55.56% [9] of PRCC. The present study found pS6 expression in 42.86% of lPRCC and 77.8% of mPRCC with higher levels in mPRCC than in NRP and overexpression in 53.3% of mPRCC. As confirmed by us in the presence of metastatic disease, pS6 was reported to be more strongly expressed in PRCC [8] without knowledge of pS6 expression in corresponding metastases [8]. Our results demonstrate a positive correlation between pS6 score in mPRCC and tumor grade and more frequent overexpression in higher-stage tumors, similar to the findings of Campbell et al. for grade [18] and Pantuck et al. [8]. The current investigation showed metastases with higher and more frequently overexpressed (88.89%) pS6 than mPRCCs in comparison to a former study that found no differences in pS6 expression between metastatic versus primary tumor samples, but that did not evaluate primary tumor and metastatic lesion of the same patient [4]. These findings suggest more severe pS6 activation in more advanced tumorous disease.
In contrast to an investigation of localized clear cell renal cell carcinoma [19], the literature reports pS6 overexpression in primary tumors (e.g. kidney, ovary) [8, 10] and worse prognosis [8, 18]. Our findings demonstrate a trend to shorter overall survival in patients with overexpressed pS6 in metastasized primary renal cell carcinoma and their metastases.
Various studies have focused on the clinical use of inhibitors of the PI3K/AKT/mTOR pathway [13, 14, 20–22], which block transcription factors and thereby cause cell cycle arrest [23]. mTOR inhibitors (e.g. rapamycin and its analogs (e.g. temsirolimus)), which are already approved as immunosuppressive agents in transplant patients [24], were previously shown to significantly prolong survival as compared to interferon alpha therapy in a subgroup of patients suffering from advanced, metastatic renal cell carcinoma [13]. Now temsirolimus and everolimus are approved for systemic treatment of metastasized renal cell carcinoma [25]. Consequently, identifying potential responders to an antitumor mTOR inhibitor therapy could significantly improve survival in these patients. Most interestingly, in various malignancies (e.g. sarcoma, renal cell carcinoma) an increased expression of pS6 in tumorous tissue was reported to be useful for assessing sensitivity to mTOR inhibitor therapy [4, 7].
Thus, with regard to renal cell carcinoma three questions arise. Firstly, is the primary tumor’s pS6 overexpression pattern comparable to that of its metastases and what is the pS6 overexpression concordance rate? Secondly, does pS6 expression reflect mTOR activity? Thirdly, does the respective pS6 overexpression pattern identify potential responders to an antitumor mTOR inhibitor therapy? The third question clearly awaits further experimental and clinical investigation, which definitely goes beyond the scope of the present study.
With regard to pS6 overexpression in the mPRCC and simultaneously in the metastases the present study demonstrates a concordance rate of 53.33%. Since pS6 overexpression is reported to be associated with sensitivity to mTOR inhibitor therapy, it is noteworthy that despite the correlation between pS6 overexpression in mPRCC and metastases, as has been reported for molecules of the PI3K/AKT/mTOR pathway “upstream” from pS6 [26], in 35.56% of cases pS6 overexpression was found only in metastsases and not in the corresponding PRCC and that the opposite was not observed (Table 4). In this respect an additional evaluation of pS6 expression in metastases of patients without pS6 overexpression in the primary tumors would be of value in order to not withhold mTOR inhibitor therapy from patients who would profit from it. This standpoint is further reinforced by the results of a previous study, finding greater clinical benefit and higher response rates to temsirolimus in patients with RCC, whose tumors showed stronger pS6 expression, and no response in patients, whose tumors demonstrated no pS6 expression [4].
Regarding the second question the current study demonstrated a correlation between pS6 and pmTOR expression in tumor-negative and tumor-positive specimens (mPRCC and metastases), showing that pS6 expression reflects activity of mTOR.
A limitation, which has to be acknowledged, is the small number of patients in the study. Ideally, our findings must be verified by a further multicenter study with a larger number of patients
In conclusion, the present study showed higher pS6 expression and more frequent overexpression in metastases than in PRCCs. In approximately one-third of the cases pS6 overexpression was exclusively found in metastases, which is interesting with regard to the association between high pS6 expression and sensitivity to mTOR inhibitor therapy.
Abbreviations
- PI3K:
-
Phosphoinositide-3-kinase
- AKT:
-
Protein kinase B
- pAKT:
-
Phosphorylated protein kinase B
- mTOR:
-
Mammalian target of rapamycin
- pS6:
-
S6 protein of the 40S ribosomal subunit
- NRP:
-
Normal renal parenchyma
- PRCC:
-
Primary renal cell carcinoma
- mPRCC:
-
Metastasized primary renal cell carcinoma
- lPRCC:
-
Localized primary renal cell carcinoma
- TMA:
-
Tissue microarray
References
Dufner A, Thomas G (1999) Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 253:100–109
Ferrari S, Bandi HR, Hofsteenge J et al (1991) Mitogen-activated 70K S6 kinase. Identification of in vitro 40 S ribosomal S6 phosphorylation sites. J Biol Chem 266:22770–22775
Bose S, Chandran S, Mirocha JM et al (2006) The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol 19:238–245
Cho D, Signoretti S, Dabora S et al (2007) Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin Genitourin Canc 5:379–385
Chow S, Minden MD, Hedley DW (2006) Constitutive phosphorylation of the S6 ribosomal protein via mTOR and ERK signaling in the peripheral blasts of acute leukemia patients. Exp Hematol 34:1183–1191
Cotler SJ, Hay N, Xie H et al (2008) Immunohistochemical expression of components of the Akt-mTORC1 pathway is associated with hepatocellular carcinoma in patients with chronic liver disease. Dig Dis Sci 53:844–849
Iwenofu OH, Lackman RD, Staddon AP et al (2008) Phospho-S6 ribosomal protein: a potential new predictive sarcoma marker for targeted mTOR therapy. Mod Pathol 21:231–237
Pantuck AJ, Seligson DB, Klatte T et al (2007) Prognostic relevance of the mTOR pathway in renal cell carcinoma: implications for molecular patient selection for targeted therapy. Cancer 109:2257–2267
Robb VA, Karbowniczek M, Klein-Szanto AJ et al (2007) Activation of the mTOR signaling pathway in renal clear cell carcinoma. J Urol 177:346–352
Trinh XB, Tjalma WA, Vermeulen PB et al (2009) The VEGF pathway and the AKT/mTOR/p70S6K1 signalling pathway in human epithelial ovarian cancer. Br J Canc 100:971–978
Fresno Vara JA, Casado E, De Castro J et al (2004) PI3K/Akt signalling pathway and cancer. Canc Treat Rev 30:193–204
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Gene Dev 18:1926–1945
Hudes G, Carducci M, Tomczak P et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. New Engl J Med 356:2271–2281
Kapoor A, Figlin RA (2009) Targeted inhibition of mammalian target of rapamycin for the treatment of advanced renal cell carcinoma. Cancer 115:3618–3630
Merseburger AS, Kuczyk MA (2008) Value of targeted therapies for renal cell cancer. Urologe A 47:1303–1310
Fuhrman SA, Lasky LC, Limas C (1982) Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 6:655–663
Eble JN, Sauter G, Epstein JI et al (2004) WHO classification of tumours. Tumors of the urinary system and male genital organs. IARC, Lyon, pp 23–34
Campbell L, Jasani B, Edwards K et al (2008) Combined expression of caveolin-1 and an activated AKT/mTOR pathway predicts reduced disease-free survival in clinically confined renal cell carcinoma. Br J Canc 98:931–940
Klatte T, Seligson DB, La Rochelle J et al (2009) Molecular signatures of localized clear cell renal cell carcinoma to predict disease-free survival after nephrectomy. Canc Epidemiol Biomarkers Prev 18:894–900
Amato RJ, Jac J, Giessinger S et al (2009) A phase 2 study with a daily regimen of the oral mTOR inhibitor RAD001 (everolimus) in patients with metastatic clear cell renal cell cancer. Cancer 115:2438–2446
Atkins MB, Hidalgo M, Stadler WM et al (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22:909–918
Motzer RJ, Escudier B, Oudard S et al (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372:449–456
Patel PH, Chadalavada RS, Chaganti RS et al (2006) Targeting von Hippel-Lindau pathway in renal cell carcinoma. Clin Canc Res 12:7215–7220
Akcakanat A, Sahin A, Shaye AN et al (2008) Comparison of Akt/mTOR signaling in primary breast tumors and matched distant metastases. Cancer 112:2352–2358
Ljungberg B, Cowan NC, Hanbury DC et al (2010) EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 58:398–406
Youssif TA, Fahmy MA, Koumakpayi IH et al (2011) The mammalian target of rapamycin pathway is widely activated without PTEN deletion in renal cell carcinoma metastases. Cancer 117:290–300
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hager, M., Haufe, H., Alinger, B. et al. pS6 Expression in Normal Renal Parenchyma, Primary Renal Cell Carcinomas and their Metastases. Pathol. Oncol. Res. 18, 277–283 (2012). https://doi.org/10.1007/s12253-011-9439-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-011-9439-y