Abstract
Nek6 is a cell cycle regulatory gene, which can control cell proliferation and survival. Recent studies suggested that desregulation of Nek6 expression plays a key role in oncogenesis. This study was aimed to investigate the potential roles of Nek6 in hepatocellular carcinoma (HCC) development. Immunohistochemistry and Western blot analysis was performed for Nek6 in 80 hepatocellular carcinoma samples. The data were correlated with clinicopathological features. The univariate and multivariate survival analyses were performed to determine the prognostic significance of Nek6 in HCC. In addition, Nek6 expression vector was used to detect its role in cell cycle control. Nek6 was overexpressed in hepatocellular carcinoma as compared with the adjacent normal tissue. High expression of Nek6 was associated with histological grade and the level of alpha fetal protein, and Nek6 was positively correlated with proliferation marker Ki-67. Univariate analysis showed that Nek6 expression was associated with poor prognosis. Multivariate analysis indicated that Nek6 and Ki-67 protein expression was an independent prognostic marker for HCC. While in vitro, following release from serum starvation of HuH7 HCC cell, the expression of Nek6 was upregulated. Overexpression Nek6 in Huh7 cell could promote the cell cycle. In conclusion, Nek6 is involved in the pathogenesis of hepatocellular carcinoma. It may be a favorable independent poor prognostic parameter for hepatocellular carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC), which accounts for 80% to 90% of primary liver cancer, is the fifth most common cancer in the world, and the third most common cause of cancer-related death [1–3]. In worldwide, there are 500,000 to 1,000,000 new cases annually [4, 5]. Without specific treatment, the prognosis is still very poor, and the median survivals for patients with early and advanced tumors are 6–9 months and 1–2 months, respectively [5, 6]. Much effort has been spent to reveal the molecular pathogenesis of HCC, but the mechanisms underlying the pathogenesis are still far from clear. In these studies, cell cycle dysfunction plays an essential role in the process [7, 8], and the structure and expression of several tumor suppressor genes and oncogenes have been reported [9, 10].
Never in mitosis A (NIMA) was originally identified in Aspergillus nidulans as a serine/threonine kinase critical for cell cycle progression into mitosis. Dominant-negative version of NIMA can adversely affect the progression of human cells into mitosis [11]. Human NIMA-related kinases (NEKs) are a group of protein kinases that are homologous to NIMA, which have high homology to NIMA in their N-terminal catalytic domain sequences, but each diverges substantially from NIMA at its non-catalytic C-terminal [11, 12]. Eleven Neks have been identified in human genome. Evidence suggests that NEKs perform functions similar to those of NIMA [13]. Nek6 is a recently identified serine/threonine kinase that belongs to the Neks (NIMA-related kinases) family, which has been implicated in mitosis control [14]. Previous studies revealed that Nek6 plays an important role in mitotic cell cycle progression. It has been shown that the protein level and the activity of Nek6 are increased during mitosis [14, 15]. Moreover, the overexpression of a dominant negative mutant form of Nek6 or of an RNAi designed to knock down Nek6 induced spindle defects, abnormal chromosome segregation, mitotic arrest and eventually apoptosis, indicating that Nek6 is an essential mitotic kinase [15, 16]. Several lines of evidence suggested that the function of Nek6 is related to tumorigenesis. For example, it was recently shown that Nek6 mRNA levels were upregulated in several tumor cell lines [17]. And overexpression of inactive Nek6 decreases the growth rate and induces apoptosis in human breast cancer cells [17, 18].
In the present study, we compared the expression of Nek6 protein in normal human hepatic and HCC tissues using immunohistochemical and immunoblottingmethods. And we also investigated its associationswith clinical and pathologic factors, as well as the prognostic implications. At the same time, we have detected the function of Nek6 in cell cycle control by siRNA and overexpression in the HepG2 cell lines.
Materials and Methods
Patients and Tissue Specimens
HCC tissues were obtained from 80 patients. All underwent hepatic surgical resection without postoperative systemic chemotherapy at the Surgery Department, the Affiliated Hospital of Nantong University between January 2003 and December 2006. The diagnosis was confirmed histologically in all cases, based mainly on examination of sections stained with hematoxylin and eosin. After obtaining informed consent, patients were interviewed to obtain information on demographic characteristics, and clinical data were collected. The study population consisted of 58 males and 22 females, and their ages ranged from 32 to 75 years, with an average age of 54.8 years. Histological grades were classified according to the International Union Against Cancer TNM classification system, and assemble to well (grade I), moderately (grade II), and poorly differentiated (grade III). The main clinicopathological variables of the patients are shown in Table 1.
Immunohistochemistry
For histological examination, tissue specimens were immediately processed after surgical removal. All tumorous and surrounding nontumorous tissue portions were processed into 10% buffered formalin-fixed, paraffin-embedded blocks. The sections were deparaffinized using a graded ethanol series, and endogenous peroxidase activity was blocked by soaking in 0.3% hydrogen peroxide. Thereafter, the sections were processed in 10 mmol/L citrate buffer (pH 6.0) to retrieve the antigen. After rinsing in PBS (pH 7.2), sections were blocked for 1 h at roomtemperature to remove any nonspecific reactions. The sections were then incubated overnight at 4°C with anti-Nek6 rabbit polyclonal antibody (diluted 1:100; Santa Cruze), and anti-Ki-67 mouse monoclonal antibody (diluted 1:100, Santa Cruze). Negative control slides were also processed in parallel using a nonspecific immunoglobulin IgG (rabbit and mouse, Santa Cruze) at the same concentration as the primary antibody. Immunostaining were performed using the avidin biotin peroxidase complex method and antigen-antibody reactions were visualized with diaminobenzidine tetrahydrochloride method. Two observers (X.C. and L.C.) independently evaluated the immunostaining results, similar results were obtained in these samples. For assessment of Nek6 and Ki-67, five highpower fields in each specimen were selected randomly, and nuclear (cytoplasma) staining was examined under high power magnification. More than 500 cells were counted to determine the labeling index, which represented the percentage of immunostained cells relative to the total number of cells.
Cell Culture and Cell Cycle Analysis
The human hepatocarcinoma cell line Huh7 was obtained from the Institute of Cell Biology, Academic Sinica and cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin in 5% CO2 at 37°C. The cell proliferation assay was performed with Brdu assay kit according to the kit’s protocol. Generally, cells were incubated with 100 μM Brdu labeling solution for 4 h at 37°C. After removing the culture media, the cells were fixed and the DNA was denatured by FixDenat solution. The anti-Brdu-POD working solution and substrate solution were then added, and the absorbances of the samples were measured by an ELISA plate reader at 370 nm wavelength.
Expression Plasmid and Transient Transfection
Full-length Nek6 cDNA was generated by polymerase chain reaction from a cDNA library of HCC. The polymerase chain reaction fragment was cloned into the pcDNA3.1-myc expression vector at the EcoRI/XhoI. The proper construction of the plasmid was confirmed by DNA sequencing and the plasmid for transfection was prepared using a Plasmid Extra Kit (Sigma). Transfections were performed using lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacture’s protocol. The cells were harvested 48 h after transfection and used for the experiment. The experiments were repeated at least 3 times.
Immunoblot Analysis
For immunoblot examination, all tumor and surrounding nontumor tissue specimens were immediately processed after surgical removal, and stored at −80°C. Tissue and cell protein were promptly homogenized in SDS loading buffer, centrifuged at 10,000 g for 30 min to collect the supernatant. Protein concentrations were determined with a Bio-Rad protein assay (Bio-Rad). Proteins were separated with SDS polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidine difluoride filter (PVDF) membranes (Millipore). The membranes were blocked with 5% dried skim milk in TBST for 2 h at room temperature. The membranes were then incubated overnight with polyclonal antibody against using the primary antibodies. And then, horseradish peroxidase-linked IgG was used as the secondary antibody. Immunoreactive bands were visualized by chemiluminescence (Cellsignal). After the chemiluminescence was exposed to X-ray films, scanned using a Molecular Dynamics densitometer (Imaging Technology, Ontario, Canada). Values are responsible for at least three independent reactions.
Statistical Analysis
Statistical analysis was performed using the Stat View 5.0 software package. The association between Ki-67 and Nek6 expression and clinico-pathological features was analyzed using χ2 test. Ki-67 and Nek6 expression in human hepatocellular carcinoma (HCC) was studied using the Spearman rank correlation test because the data were not normally distributed. Survival curves were calculated using the Kaplan–Meier method, and the log-rank test was used for analysis. Multivariate analysis was performed using Cox’s proportional hazards model. One-way-ANOVA followed by the Tukey’s post-hocmultiple comparison tests was used for statistical analysis of the data of immunoblot. The results of the HCC cells are expressed as the mean ± SE. P < 0.05 was considered statistically significant.
Results
The Expression of Nek6 Protein in HCC and Adjacent Normal Tissue
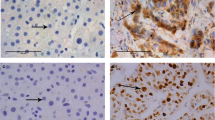
Of the 80 HCC, Nek6 was predominantly found in the nucleus, although weak cytoplasmic immunoreaction was also observed (Fig. 1). Representative examples of reactivity for Nek6 and Ki-67 are shown in Fig. 1. There was no or low Nek6 and Ki-67 expression in adjacent normal tissue (Fig. 1a,c). The percentage of Nek6 positive tumor cells ranged from 5% to 80% with a mean of 53 ± 23.23%. Ki-67 expression in HCC was scored as positive when strong nuclei was stained (Fig. 1b,d). Its LI ranged from 0.85% to 84.38%. with a mean percentage of 23 ± 12.53% (Fig. 2).
Immunohistochemical staining of Nek6 and Ki-67 in adjacent normal tissues and HCC tissues. Paraffin-embedded tissue sections were stained with antibodies for Nek6 and Ki-67 and counterstained with hematoxylin. a Nek6 negative staining was shown in adjacent normal tissues. b Nek6 immunoreactivity was detected in HCC, with staining predominant in the nucleus. c Ki-67 negative staining was shown in normal human hepatic tissue. d Ki-67 positive nuclear staining was shown in HCC (×400)
Correlation of Nek6 Expression with Clinicopathological Parameters in HCC
The clinico-pathological data of the patients are summarized in Table 1. As shown in Table 1, we evaluated the association of Ki-67 and Nek6 expression with clinical variables. For statistical analysis of the expression of Nek6 and Ki-67, the carcinoma specimens were divided into two groups: high expression and low expression, according to the percentage of Nek6 and Ki-67 positive cells, using a cut off level of 53% and 23% representing the mean value of Nek6 and Ki-67 expression. Nek6 expression did not correlate significantly with gender, age, metastasis, tumor size, HBsAg, cirrhosis, but its expression was significantly correlated with histological grade (P = 0.009) and the level of AFP (P = 0.003) (Table 1). Furthermore, in most specimens, the proportion of Nek6 positive tumor cells was similar to the proportion of Ki-67 positive tumor cells. A positive correlation between Nek6 expression and Ki-67 based proliferate activity was found (P < 0.01; Fig. 3).
Survival Analysis
At the end of clinical follow-up, survival information was available in 80 cases of 80 patients (100%). Of these 80 patients, only 16 of 47 (33%) patients in the Nek6 high-expression group were alive versus 24 of 33 (72%) in the Nek6 low-expresser group (Table 2). When all variables were compared separately to survival status, only Nek6 (P = 0.004), Ki-67(P = 0.000), and histological grade (P = 0.006) significantly influenced survival (Table 2). In univariate analysis, the Kaplan–Meier survival curves showed that high Nek6 expression related to a poor survival with statistical significance (Fig. 3). The Cox’s proportional hazards regression model proved that Nek6, Ki67 expression and histological grade were independent prognostic factors in patients with HCC (Table 3).
Nek6 Promotes Cell Proliferation in HCC cells
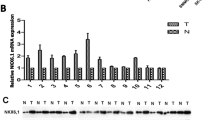
Based on our current study, we further detected the role of Nek6 in the proliferation of HCC cells. As previous reported, HuH7 cells were arrested in G1 phase by serum deprivation for 72 h. Upon serum addition, the cells were reentered S phase. Western blot displayed that the expression of Nek6 was increased as early as 12 h after serum stimulation (Fig. 4a). To further detect the role of Nek6 in cell proliferation, myc-peptide target Nek6 expression vector were transfected to Huh7 cell line (Fig. 4b). Cell proliferation were enhanced in the Nek6 overexpression cells (Table 4).
The function of Nek6 in HCC cells proliferating regulation. a HuH7 cells were serum starved for 72 h and upon serum releasing, cell lysates were prepared and analyzed by Western blot using antibodies directed against Nek6. β-actin was used as a control for protein load and integrity. b HuH7 cells were transfected with myc-NLK vector and then serum starved for 72 h and upon serum releasing, MTT assay shown cell proliferation rate. The data are means ± SEM (n = 3, *P < 0.01, compared with controls)
Discussion
HCC is one of the most common cancers worldwide and is a major cause of death in many countries, especially in Asia [2, 4]. Although morbidity and mortality rates have decreased in recent years in patients with surgically treated HCC, the prognosis of HCC remains unsatisfactory and the 5-year survival rate is limited to 25–49% after surgery [19]. A deeper understanding of the molecular events associated with the HCC is necessary [20, 21].
Recently studies have demonstrated that the Aurora kinase family, polo-like kinases family and NIMA kinase family could control cell cycle in prokaryotic and eucaryotic cells [22, 23]. NIMA is a serine/threonine kinase and could control cell cycle progression into mitosis. NEKs are a group of protein kinases that homologous to NIMA, and have high homology sequence in their N-terminal catalytic domain sequences. Neks have been identified in 11 isotype according its non-catalytic C-terminal [11, 22]. Nek6 is a recently identified serine/threonine kinase belongs to the Neks family, which has been implicated in mitosis control. Previous studies revealed that Nek6 plays an important role in mitotic cell cycle progression, and could arrest cell in G2/M phase if the expression was blocked. Over expression of a dominant negative mutant form of Nek6 or of an RNAi was designed to knock down Nek6 induced spindle defects, abnormal chromosome segregation, mitotic arrest and eventually apoptosis, indicating that Nek6 is an essential mitotic kinase [14–16]. Several lines of evidence suggest that the function of Nek6 is related to tumorigenesis, and it was recently shown that Nek6 mRNA levels were upregulated in several tumor cell lines [22, 23]. Overexpression of inactive Nek6 decreases the growth rate and induces apoptosis in human breast cancer cells [17, 18]. In HCC, previous studies have indicated its expression was up-regulated in more HCC cell lines [24]. In our studies, we have also found the expression of Nek6 was up-regulated in the HCC tissues compared with the benign normal tissue, which showed no or low Nek6 expression, and that Nek6 was mainly located in the nuclei of tumor cells. In further, we have also found the expression of Nek6 was increased accompanying with the increasing of the HCC tissue grade. Our study suggested that increased Nek6 levels may be closely associated with the pathogenesis of HCC. We also investigated the correlation between Nek6 expression and Ki-67 immunoreactivity, which has been reported to be a useful marker of tumor proliferative activity [25–27]. A positive correlation between Nek6 and Ki-67 was observed in HCC.
The malignant conversion of a tumor is a complex process. Overexpression of Nek6 could promote cell proliferation in Huh7 cell line. So Nek6 may represent an important mechanism of development of HCC. In addition, we evaluated the correlation between Nek6 and clinicopathological parameters, as well as the prognosis of patients. We found that Nek6 expression was significantly associated with histological grade and the level of AFP. Results of the survival analysis showed that Nek6 high expression was significantly associated with poor prognosis. These findings suggested that Nek6 might be a reliable indicator of prognosis in HCC patients. Previous studies has reported Nek6 could regulated Pin 1 expression and sate-1 activity in HCC, in which the two proteins played an essential role of HCC development [24, 28].
In conclusion, we have showed that Nek6 expression was increased in HCC, and positively correlated with HCC cell proliferation. These results indicate that Nek6 may play an important role in HCC and its evaluation provides important prognostic information on HCC. Further studies are necessary to elucidate the molecular mechanisms of Nek6 in HCC pathogenesis.
References
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362:1907–1917
Di Bisceglie AM (2004) Issues in screening and surveillance for hepatocellular carcinoma. Gastroenterology 127:S104–S107
Parkin DM, Bray F, Ferlay J et al (2001) Estimating the world cancer burden: GLOBOCAN 2000. Int J Cancer 94:153–156
Curado MP, Edwards B, Shin HR et al (2007) Cancer incidence in five continents (IARC, Lyon, France) IARC Scientific Publications, No. 160, Vol. IX
Sean FA, Katherine AM, Marsha ER (2009) Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. JCO 27(9):1485–1491
Bosch FX, Ribes J, Diaz M, Cleries R (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127(5):S6–S16
Lai PBS, Tianyi C, George GC (2007) Different levels of p53 induced either apoptosis or cell cycle arrest in a doxycycline-regulated hepatocellular carcinoma cell line in vitro. Apoptosis 12(2):387–393
Siddhartha K, Meifang W, Brian IC (2008) 2-Methoxyestradiol inhibits hepatocellular carcinoma cell growth by inhibiting Cdc25 and inducing cell cycle arrest and apoptosis. Cancer Chemother Pharmacol 62(5):831–840
Wang H, Pan K, Zhang H et al (2008) Increased polycomb-group oncogene Bmi-1 expression correlates with poor prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol 134(5):535–541
Laura G, Manuela F, Francesca F et al (2007) Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res 67(13):6092–6099
O’Connell MJ, Krien MJ, Hunter T (2003) Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol 13(5):221–228
Belham C, Roig J, Caldwell JA et al (2003) A mitotic cascade of NIMA family kinases Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J Biol Chem 278(37):34897–34909
Manning G, Whyte DB, Martinez R et al (2002) The protein kinase complement of the human genome. Science 298(5600):1912–1934
Min Jean Y, Lihua S, David V et al (2003) The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J Biol Chem 278(52):52454–52460
O’Regan L, Fry AM (2009) The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis. Mol Cell Biol 29(14):3975–3990
Joseph R, Marta N, Aaron G et al (2008) The NIMA-family kinase Nek6 phosphorylates the kinesin Eg5 at a novel site necessary for mitotic spindle formation. Journal of Cell Science 121(pt23):3912–3921
Rounak N, Lihua S, Peter F et al (2010) Nek6 mediates human cancer cell transformation and is a potential cancer therapeutic target. Mol Cancer Res 8(5):717–728
Ahmed S, Thomas G, Ghoussaini M et al (2009) Newly discovered breast cancer susceptibility loci on 3p24 and 17q23. 2. Nat Genet 41(5):585–590
Ke H, John ML, Lee NPY et al (2009) Predicting prognosis in hepatocellular carcinoma after curative surgery with common clinicopathologic parameters. BMC Cancer 9:389
Thorgeirsson SS, Grisham JW (2002) Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 31:339–346
Di Bisceglie AM (2009) Hepatitis B and hepatocellular carcinoma. Hepatology 49(S5):S56–S60
Guillermo C, Ignacio PC, Marcos M (2007) Targeting cell cycle kinases for cancer therapy. Curr Med Chem 14(9):969–985
Marcos M, Mariano B (2007) Cell cycle kinases in cancer. Curr Opin Genet Dev 17:60–65
Chen J, Li L, Zhang Y et al (2006) Interaction of Pin1 with Nek6 and characterization of their expression correlation in Chinese hepatocellular carcinoma patients. Biochem Biophys Res Commun 341(4):1059–1065
Grigioni WF, D’errico A, Bacci F et al (1989) Primary liver neoplasms: evaluation of proliferative index using MoAb Ki-67. J Pathol 158(1):23–29
Ng IO, Na J, Lai EC et al (1995) Ki-67 antigen expression in hepatocellular carcinoma using monoclonal antibody MIB1: a comparison with proliferating cell nuclear antigen. Am J Clin Pathol 104(3):313–318
Scholzen T, Gerde J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182(3):311–322
Pang R, Yuen J, Yuen MF et al (2004) PIN1 overexpression and β-catenin gene mutations are distinct oncogenic events in human hepatocellular carcinoma. Oncogene 23(23):4182–4186
Acknowledgements
The authors wish to thank Qun E for helpful in technology support. This work was supported by the Natural Science Foundation of Jiangsu Colleges and Universities Grant (09KJB320010); “Top Six Types of Talents” Financial Assistance of Jiangsu Province Grant (No. 6);Social Development Mentoring Programs of Nantong City (S2010039).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiaolei Cao and Yunfei Xia contributed equally in the work.
Rights and permissions
About this article
Cite this article
Cao, X., Xia, Y., Yang, J. et al. Clinical and Biological Significance of Never in Mitosis Gene A-Related Kinase 6 (NEK6) Expression in Hepatic Cell Cancer. Pathol. Oncol. Res. 18, 201–207 (2012). https://doi.org/10.1007/s12253-011-9429-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-011-9429-0