Abstract
Hepatitis C virus (HCV) is a global health concern, notably in Southeast Asia, and in Laos the presentation of the HCV-induced liver disease is poorly known. Our objective was thus to describe a comprehensive HCV infection pattern in order to guide national health policies. A study on a group of 1765 patients formerly diagnosed by rapid test in health centres was conducted at the Centre of Infectiology Lao Christophe Merieux in Vientiane. The demographic information of patients, their infection status (viral load: VL), liver function (aminotransferases) and treatments were analysed. Results showed that gender distribution of infected people was balanced; with median ages of 53.8 for men and 51.6 years for women (13–86 years). The majority of patients (72%) were confirmed positive (VL > 50 IU/mL) and 28% of them had high VL (> 6log10). About 23% of patients had level of aminotransferases indicative of liver damage (> 40 IU/mL); but less than 20% of patients received treatment. Patients rarely received a second sampling or medical imaging. The survey also showed that cycloferon, pegylated interferon and ribavirin were the drugs prescribed preferentially by the medical staff, without following any international recommendations schemes. In conclusion, we recommend that a population screening policy and better management of patients should be urgently implemented in the country, respecting official guidelines. However, the cost of biological analysis and treatment are significant barriers that must be removed. Public health resolutions should be immediately enforced in the perspective of meeting the WHO HCV elimination deadline by 2030.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection with Hepatitis C virus (HCV) is one of the major causes of death and morbidity especially in low and middle income countries (Stanaway et al. 2016). According to Lim et al. (2017) the prevalence of Hepatitis C in the general population ranges from 0.1% to 4.7% in the Asia–Pacific region. In Laos, among Vientiane Capital blood donors, the prevalence of anti-HCV is 1.1% (Jutavijittum et al. 2007). Overall, the epidemiology of Hepatitis C remains poorly defined in Laos, despite its location in the centre of the HCV genotype-6 distribution area (Lee et al. 2017). Most notably, it is unknown in Laos whether HCV-infected patients correspond to an age-driven or are merely the result of on-going HCV transmission.
Chronic infection with HCV is a well-recognized risk factor for liver cirrhosis and hepatocellular carcinoma. Therefore it is important to (1) detect and (2) carefully follow infected subjects to propose adequate disease management. For this purpose, the Centre d’Infectiologie Lao-Christophe Mérieux (CILM) in Vientiane capital has been receiving patient samples since 2010. In the present study, we examined the course of HCV infection in 1765 patients who attended CILM between January 2010 and November 2016. Several parameters, such as gender, age, treatment allocation, HCV RNA viral load (VL) and liver damage [through aspartate (AST) and alanine aminotransferases (ALT) serum levels] were assessed. The ultimate aim of our work was to better characterize key epidemiologic features of HCV infections in Lao population and to identify unforeseen high-risk groups that should be primarily targeted by proactive screening campaigns.
Our results aim to help health authorities to apply cost-effective prevention and treatment measures to resolve the HCV infection burden as recommended by the World Health Organization in its 2030 agenda for sustainable WHO (2017).
Materials and Methods
Sample Collection
The study was conducted retrospectively by analysing the data of 1765 individuals positive for anti-HCV visiting CILM between January 2010 and November 2016. Anti-HCV antibody detection was performed using rapid point-of-care diagnostic tests according to manufacturer instructions in hospitals and private clinics before patients arrived at CILM. Patients never came to CILM on their own initiative; they were diagnosed in health care centres during routine health check-ups or during specialized consultations related to symptoms they experienced. Patients’ blood was collected on EDTA, plasma harvested and stored at − 20 °C prior further analysis. Age, gender, geographic origin, aspartate (AST) and alanine (ALT) aminotransferases serum levels, and treatments were registered.
Viral Load Determination
VL determination was performed through Multiplex Real-Time PCR by TaqMan® technology (Biorad, France) with a detection threshold of 50 IU/mL (see http://www.fast-trackdiagnostics.com/ for details).
Aminotransferases Quantification
ALT and AST concentrations were measured on HumaStar 600 machine (CoreLab, Germany), according to manufacturer’s instructions. De Ritis ratio (De Ritis et al. 2006) or AST/ALT ratio was also calculated as in chronic liver disease, and especially in case of infection with HCV, as when the ratio is even slightly above 1.0 (e.g. 1.09) it often correlates with the presence of fibrosis.
Statistics
Data were inputted using Microsoft Excel software (14.4.7) and analysed with Minitab software (17.3.1). Comparisons between groups (numerical data) or proportions (categorical data) were performed using Student’s t-test, Fisher’s exact test, Chi squared test, ANOVA or non-parametric tests (e.g. Mann–Whitney, Kruskal–Wallis), as appropriate. Two-sided P-values lower than 0.05 were considered as statistically significant.
Results
Samples
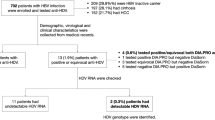
Our laboratory has received a total of 3102 blood samples, corresponding to 1765 different individuals seropositive for anti-HCV between January 2010 and October 2016 previously diagnosed through rapid tests in health care structures (see Tables 1 and 2 for details). A large majority of patients (n = 1233; 70%) had a single viral load measurement, while 220 (12%) had two, 129 (7.3%) had three VL tested and the others more than 3 (one patient had been tested 18 times). At the beginning of the activity of CILM in 2010, only 44 patients were received annually (Fig. 1). Then the number of patients increased to 378 in 2015 and the number of collected samples was about 2 times greater. Since 2013 the number of patients and samples have been almost stable.
Whole Group and Sub-Groups Configuration
The 1765 patients who presented at the CILM for VL measurement were sub-divided into 3 sub-groups: true positive (the largest group with 1278 people), true negative treated and not treated (487) and true negative not treated (407). The sex-ratio of the whole group of patients (and also of true positive and true negative non-treated) was 1.2. The age ranged from 13 to 86 years, with median ages of 53.8 for men and 51.6 years for women (P = 0.006). Patients with an on-going infection (true positive) were displaying higher median values for aminotransferases (P < 0.0001) than negative patients. A subset of 407 naive patients (28.4%) were negative for HCV RNA (called “true negative and non-treated” in Table 1). These naïve patients could reflect the natural course of HCV infection in Lao populations with spontaneous cleared infection (if any). However, among this sub-group, 42 benefited from a second VL measurement and 7 (16.7%) of them turned out to have VL > 50 IU/mL suggesting that HCV loads were possibly oscillating around detection threshold in a sizable proportion of patients. Individuals with apparent spontaneous recovery at initial sampling were significantly younger than RNA(+) patients (48.4 ± 12.7 vs. 52.4 ± 11.3; P < 0.0001). No gender difference was noticed in this sub-group. Remarkably, despite the absence of circulating HCV genomes, 41.0% (n = 52) of HCV RNA(−) subjects tested for aminotransferases displayed levels above the upper limit of normal.
Treated Patients
Treatment prescription reflected the availability of drugs in Laos and economic status of the patients; as a result, international guidelines were seldom followed by the prescribing physicians.
Ten drugs were used in mono to quadruple therapy (Table 3). In total, 329 patients (18.7% of the whole group) received an antiviral treatment including a subset of 80 (24.4%) subjects negative for HCV RNA(−) who were receiving or had already received antiviral treatment at the time of initial VL determination (Tables 1 and 2). We then compared treated individuals (those with confirmed presence of the virus), who had been cured of the virus with those patients who remained chronically infected. Gender distribution was somewhat skewed towards men in patients who displayed a sustained viral response to treatment, albeit without reaching the level of significance (P = 0.0906, ns). As for untreated patients, disappearance of HCV RNA in treated patients was the characteristic of younger age (49.8 ± 11.1 vs. 52.6 ± 9.3, P = 0.031).
The De Ritis ratio (AST/ALT ratio) was similar in RNA(+) and RNA(−) groups with 38% of the latter displaying a value above 1.1, indicative of significant liver fibrosis.
When we considered treatments received, we observed that patients who had recovered from viral infection received Pegylated interferon significantly more often than other drugs.
Among infected patients, VLs were significantly higher in treated than in naive patients (6.4 ± 1.7 vs. 5.4 ± 1.3, P < 0.0001). Furthermore, an important subset (64.3%) of treated patients had VL > 6 log10, a threshold considered by some Asian authors as a predictor for liver disease progression (Noh et al. 2016). This rate was significantly lower in treated naive patients (31.8%, P < 0.0001). Concerning liver damage, treated patients displayed decreased enzyme values when compared with untreated ones.
Impact of Treatment as Seen on VL and Transaminases
Among the 249 HCV RNA(+) treated patients (detailed in Table 2), 108 have AST and 104 have ALT measurements; 65 patients were treated before aminotransferases determination and 39 were treated subsequently. AST and ALT medians were significantly lower (respectively P = 0.005 and P = 0.026) when the treatment was received before transaminases evaluation. Among the 39 patients treated after the first transaminase sampling, 17 had a second determination after treatment. Only the AST median was significantly lower after treatment (P = 0.014). However, time intervals between samplings were extremely variable and this group rather small. A group of 197 infected treated patients had 2 serial VL determinations (data not shown). We observed in them a significant decrease of the mean and median VL (P < 0.0001), but there was no difference between therapeutic schemes regarding the magnitude of VL decrease.
58% of this group received monotherapy with acridone-acetic acid (Cycloferon) predominantly employed. Therefore we compared Cycloferon impact on VL in patients who received it in mono or dual therapies (Table 4). We noticed that the viral response to Cycloferon was stronger when monotherapy was used (P = 0.0001).
We also analysed the impact of dual therapy on true positive patients with 2 samplings. A subset of 88 patients received dual therapy: 31 ribavirin + Cycloferon (group 1), 30 ribavirin + Pegasys (group 2) and 27 “others” (group 3). We found that VL were more strongly impacted in group 2, while groups 1 and 3 were similar (same decrease of the VL) P < 0.0001).
The global impact of monotherapy versus dual therapy on VL was also assessed. We observed that there was no difference between these therapeutic schemes regarding viral response.
Evolution of Medications Administered to Patients
As shown in Fig. 2, 10 different medicines were used between January 2010 and November 2016. The drug classes included adenosine analogues (adefovir and tenofovir), guanosine analogue (ribavirin), cytidine analogue (lamivudine), immunostimulant (cycloferon), and Interferon-like (Interferon, Pegasys), NS5A replication complex inhibitors (ledipasvir, daclatasvir) and nucleoside NS5B polymerase inhibitors (sofosbuvir). Ribavirin, cycloferon and pegasys were the most prescribed drugs; they peaked in 2012, 2014 and 2015 respectively. The least used drug was sofosbuvir.
Discussion
In recent years, worldwide, almost half of the patients with HCC have been diagnosed positive for HCV (Chassagne et al. 2016; El-Serag and Mason 1999). This has been confirmed in a study we conducted at the Calmette hospital in Cambodia on around 500 patients, where HCV was associated with half the cases of HCC, with higher prevalence in women (Chassagne et al. 2016). In Laos, comprehensive epidemiological data are lacking but those obtained by Jutavijittum et al. (2007), aggregated to ours, are a source of concern. Between 2010 and 2016, nearly 2000 patients diagnosed in Lao public or private health care structures came to CILM for the determination of their VL. Of course, this figure does not reflect the prevalence of the disease, as there is no systematic screening for HCV in the Lao population. Patients usually come to be diagnosed at CILM following the advice of a physician. The cost of HCV viral load measurement is around 45 USD in Vientiane, which is a substantial amount of money for most people in the country (minimum salary is 111 USD per month). It is probable that such expense may deter many Lao people to visit diagnostic laboratories. Overall, the fact that HCV infection is commonly associated with low income status both in developed and developing countries implies that the problem in Lao PDR is much more serious than the situation we can describe with the evidence at our disposal (Mahajan et al. 2017; Alter et al. 1994).
Nevertheless, the number of patients attending CILM to control their HCV RNA VL increased constantly from 2010 to 2016. This increase could reflect increased economic status of the patients, the effectiveness of public health prevention campaigns for hepatitis, the high repute of the CILM (meaning that less patients cross the border to be diagnosed in Thailand), or last but not least because the hepatitis epidemic is spreading in the country.
All patients presenting at CILM were diagnosed anti-HCV(+) using rapid point-of-care tests with questionable efficacy according to our experience (Paboriboune et al. 2018). This diagnosis was established during routine health check-ups or specialized gastroenterology consultations. Patients never come to CILM on their own initiative.
Among them 18.6% had undetectable virus. In the small subset of initially HCV RNA(−) patients tested twice, a substantial proportion (16.7%) turned out to be actually infected with HCV. Thus, a sizeable subset of anti-HCV(+)/RNA(−) was, in fact, positive. This observation questions the sensitivity of the initial analysis that was performed in the presence of undetectable VL according to our standards but could also reflect the fluctuation of the viral load. Moreover, despite the absence of circulating HCV genomes, a large section among the untreated anti-HCV(+)/RNA(−) subjects displayed increased levels of AST and ALT > 40 with a De Ritis ratio value above 1.1 considered as a predictor of liver fibrosis. This suggests that additional liver injury such as chronic high alcohol intake, occult B infection or infection by liver flukes commonly found in Laos were actually on-going in these patients (Sripa et al. 2012).
The significance of the VL is source of debate as oscillation in HCV RNA levels has been reported as a “stamp” of the viral dynamics in the early stages of HCV infection, and was described by some investigators as a “yo–yo” pattern (Aberle et al. 2006; Smith et al. 2010). We also observed this phenomenon in patients who had multi samplings. Noh et al. (2016) showed that the cumulative risk of HCC development was higher in patients with high titer of HCV RNA than patients with low titer. These authors also stated that VL is “high” when > 6log10. In our hands 27% of patients have VL > 6 log10 and among them 105 patients have VL > 6 log10 associated with liver injury evidenced by AST and ALT > 40 IU/mL (6% of the whole group of patients). A subset of 38 patients among the 105, received treatments (36% of this sub-group i.e. only 3% of the RNA(+) group). According to Botros and Sikari (2013) the De Ritis ratio reflects the time course of acute viral hepatitis and is generally a vital clue to the patient’s prognosis. We found that 218 patients who had one sampling had high fibrosis risk (AST/ALT = 1.09–2.0), but only 46 received treatments. We also observed that 39 patients had acute viral hepatitis flares (AST/ALT > 2.0) but only 10 received treatments (with means AST around 200 and ALT > 50 IU/mL). Only 74 on 615 patients had a second sampling and AST/ALT measured; 50% of them scored > 1.09, and less than 1/3 were treated.
Some authors have demonstrated that HCV genotype 1b was more often related to the development of HCC than non-1b genotypes (Ishiguro et al. 2011). Unfortunately, we did not have the opportunity to perform genotyping on the sample-set described herein. Nevertheless, it could be considered that genotype determination could soon become obsolete since drugs such as Sofosbuvir (and many other direct-acting antivirals) are effective on all known genotypes (Linas et al. 2015). The cost of these therapies is, however, shamefully prohibitive for the vast majority of infected people in the world. Fortunately, affordable alternatives exist (although less effective than Sofosbuvir, used in only 2.1% of patients in this study).
Ten different medicines and four therapeutic schemes (mono, dual, triple, and quadruple therapy) were used. Curiously, we observed that anti-hepatitis B antivirals were prescribed to a small subset of HCV patients (n = 37). This may be due to the fact that hepatitis B is much more frequent in Laos than HCV and that medical staff prescribe drugs in a probabilistic manner when they consider the treatment necessary.
Ribavirin, Cycloferon and Pegasys were the most prescribed drugs of all therapies. Nonetheless only 30 patients received the WHO-validated therapeutic scheme (Ribavirin + Pegasys) with 2 samplings to ascertain the efficacy of the therapy (WHO 2014). As expected, we showed in these cases that the combination was effective on the VL.
Ribavirin is part of the World Health Organization’s list of essential medicines (WHO 2016). It has been extensively used in dual therapy (51.6%) but for unknown reason we found that its use was declining since 2012. Cycloferon is considered as an early inductor of types 1 and 2 Interferons. This particularly cheap medicine (10 US$/month) is imported from Russia into Laos and was the most used drug in monotherapy scheme. It was used extensively when Russian clinicians were present in the public health system. Pegasys (Pegylated interferon alfa-2a) has been used in a stable and continuous manner since 2011, and was almost as used as Cycloferon in monotherapy schemes. The other drugs were used in less than 5% of all therapeutic schemes (direct acting antivirals, Sofosbuvir and Ledipasvir were used in only 11 patients).
A meta-analysis of controlled trials has shown that antiviral therapy reduced the rate of HCC development in patients with liver cirrhosis (Craxì and Cammà 2005). Moreover, it has been shown that in patients with chronic hepatitis C (with normal aminotransferases level) interferon therapy improves liver histology (Voronkova et al. 2002). It is then of utmost importance to make “curative” drugs affordable to infected patients and to encourage physicians to follow international guidelines as soon as possible in Laos.
Nevertheless, we showed that among true positive patients who were treated and sampled twice, those receiving monotherapy had a greater VL decrease than those treated with dual therapy. This is in contradiction with the therapeutic schemes recommended by the World Health Organization who advocate for a combination of Interferon and Ribavirin, effective against all the genotypes of HCV (WHO 2014).
Our results also suggest that the use of Cycloferon should be examined in combination. In other studies it was shown to increase other antiviral drugs’ efficacy, providing earlier normalization of clinical and biochemical parameters; it reduces the incidence and degree of adverse reactions and increases the quality of life in treated patients (Romantsov et al. 2008, 2010; Sologub et al. 2010; Malyĭ et al. 2013). Last but not least it is economically sustainable in a country like Laos. Therefore, clinical trials should be conducted in Laos to re-define the most effective and affordable drugs to be recommended by health authorities, including Cycloferon.
The biological follow-up of patients should be also updated. As a matter of fact, meta-analysis regression demonstrated that advanced stages of fibrosis were significantly correlated with platelet count, AST/ALT ratio and AFP levels but there was no correlation with viral load. Fanning et al. (1999) observed correlation between VL and the degree of inflammation while they did not detect any correlation between VL and the degree of fibrosis nor between serum viral load and ALT, although there was a correlation between ALT and the degree of inflammation. Su et al. (2009) showed that 42% of patients achieving sustained virological response following peg-interferon alpha and ribavirin therapy showed delayed biochemical normalization (persistently abnormal transaminase levels). Delayed biochemical response was associated with the baseline hepatic fibrosis stage and liver cirrhosis. All these data suggest that we should consider other alternatives to VL for the follow-up of HCV infected patients. According to Durante-Mangoni et al. (2013), hepatitis C virus core antigen (HCVcoreAg) levels are significantly correlated with HCV-RNA, ALT levels and liver necroinflammatory activity. Although these findings must be confirmed by additional studies, the determination of HCVcoreAg could be useful for the monitoring of chronic hepatitis C patients in the future.
In conclusion, it is urgent to challenge on-going diagnostic and therapeutic strategies in Lao PDR, with better follow-up of patients, in order to optimize the reduction in the burden of HCV-related diseases. Field studies should be conducted to determine the routes of infection in the Lao population, which population groups are the most vulnerable and why preventive campaigns have failed in some areas of the country.
References
Aberle JH, Formann E, Steindl-Munda P, Weseslindtner L, Gurguta C, Perstinger G, Grilnberger E, Laferl H, Dienes HP, Popow-Kraupp T, Ferenci P, Holzmann H (2006) Prospective study of viral clearance and CD4(+) T-cell response in acute hepatitis C primary infection and reinfection. J Clin Virol 36:24–31
Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS (1994) The prevalence of hepatitis C virus infection in the United States, 1988 through. N Engl J Med 341:556–562
Botros M, Sikaris KA (2013) The De Ritis ratio: the test of time. Clin Biochem Rev 34:117–130
Chassagne F, Rojas Rojas T, Bertani S, Bourdy G, Eav S, Ruiz E, Pineau P, Deharo E (2016) A 13-year retrospective study on primary liver cancer in cambodia: a strikingly high hepatitis C occurrence among hepatocellular carcinoma cases. Oncology 91:106–116
Craxì A, Cammà C (2005) Prevention of hepatocellular carcinoma. Clin Liver Dis 9:329–346
De Ritis F, Coltorti M, Giusti G (2006) An enzymic test for the diagnosis of viral hepatitis: the transaminase serum activities. Clin Chim Acta 369:148–152
Durante-Mangoni E, Vallefuoco L, Sorrentino R, Iossa D, Perna E, Molaro R, Braschi U, Zampino R, Sodano G, Adinolfi LE, Utili R, Portella G (2013) Clinico-pathological significance of hepatitis C virus core antigen levels in chronic infection. J Med Virol 85:1913–1918
El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340:745–750
Fanning L, Kenny E, Sheehan M, Cannon B, Whelton M, O’Connell J, Collins JK, Shanahan F (1999) Viral load and clinicopathological features of chronic hepatitis C (1b) in a homogeneous patient population. Hepatol Baltim Md 29:904–907
Ishiguro S, Inoue M, Tanaka Y, Mizokami M, Iwasaki M, Tsugane S (2011) JPHC Study Group. Impact of viral load of hepatitis C on the incidence of hepatocellular carcinoma: a population-based cohort study (JPHC Study). Cancer Lett 300:173–179
Jutavijittum P, Yousukh A, Samountry B, Samountry K, Ounavong A, Thammavong T, Keokhamphue J, Toriyama K (2007) Seroprevalence of hepatitis B and C virus infections among Lao blood donors. Southeast Asian J Trop Med Public Health 38:674–679
Lee MH, Hsiao TI, Subramaniam SR, Le AK, Vu VD, Trinh HN, Zhang J, Jin M, Wong VWS, Wong GL-H, Nguyen MH (2017) HCV genotype 6 increased the risk for hepatocellular carcinoma among Asian patients with liver cirrhosis. Am J Gastroenterol 112:1111–1119
Lim SG, Aghemo A, Chen P-J, Dan YY, Gane E, Gani R, Gish RG, Guan R, Jia JD, Lim K et al (2017) Management of hepatitis C virus infection in the Asia-Pacific region: an update. Lancet Gastroenterol Hepatol 2:52–62
Linas BP, Barter DM, Morgan JR, Pho MT, Leff JA, Schackman BR, Horsburgh CR, Assoumou SA, Salomon JA, Weinstein MC, Freedberg KA, Kim AY (2015) The cost-effectiveness of sofosbuvir-based regimens for treatment of hepatitis C virus genotype 2 or 3 infection. Ann Intern Med 162:619–629
Mahajan R, Midha V, Goyal O, Mehta V, Narang V, Kaur K, Singh A, Singh D, Bhanot R, Sood A (2017) Clinical profile of hepatitis C virus infection in a developing country-India. J Gastroenterol Hepatol 33:926–933
Malyĭ VP, Pen’kov DV, Chiriukina OI (2013) Cycloferon therapy of acute and chronic virus hepatitis C. Antibiot Khimioter Antibiot Chemoter Sic 58:34–40 (in Russian)
Noh R, Lee DH, Kwon BW, Kim YH, Kim SB, Song IH (2016) Clinical impact of viral load on the development of hepatocellular carcinoma and liver-related mortality in patients with hepatitis c virus infection. Gastroenterol Res Pract 2016:7476231
Paboriboune P, Vial T, Chassagne F, Sitbounlang P, Soundala S, Bertani S, Sengmanothong D, Babin F-X, Steenkeste N, Dény P, Pineau P, Deharo E (2018) A seven-year retrospective study on the surveillance of hepatitis B in Laos. Int J Hepatol. https://doi.org/10.1155/2018/9462475
Romantsov MG, Kovalenko SN, Sologub TV, Anikina OV (2008) Immunomodulators in the ‘gold standard’ of the chronic viral hepatitis C therapy. Antibiot Khimioter 53:18–22 (in Russian)
Romantsov MG, Kremen’ NV, Sologub TV (2010) Use of immunomodulators in the therapy of chronic hepatitis C: improving standard approach. Eksp Klin Farmakol 73:14–17 (in Russian)
Smith J, Aberle JH, Fleming VM, Ferenci P, Thomson EC, Karayiannis P, McLean AR, Holzmann H, Klenerman P (2010) Dynamic coinfection with multiple viral subtypes in acute hepatitis C. J Infect Dis 202:1770–1779
Sologub TV, Romantsev MG, Shul’diakov AA, Lin’kova IN, Radchenko VG, Kovalenko AL (2010) Efficiency of using cycloferon as part of combined therapy for chronic hepatitis C (a review of multicenter clinical trials). Ter Arkh 82:78–81 (in Russian)
Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, Bethony JM, Loukas A (2012) The tumorigenic liver fluke Opisthorchis viverrini—multiple pathways to cancer. Trends Parasitol 28:395–407
Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B et al (2016) The global burden of viral hepatitis from 1990 to 2013: findings from the global burden of disease study 2013. Lancet 388:1081–1088
Su WP, Peng CY, Lai HC, Liao KF, Huang WH, Chuang PH, Chen CB, Jeng LB (2009) Persistent transaminase elevations in chronic hepatitis C patients with virological response during peginterferon and ribavirin therapy. Hepatogastroenterology 56:798–801
Voronkova NV, Blokhina NP, Kelli EI, Mikhaĭlov MI, Malyshev NA (2002) Prospects for interferon therapy in chronic hepatitis C with normal transaminase levels. Ter Arkh 74:12–15 (in Russian)
WHO (2014) Hepatitis C. http://www.who.int/mediacentre/factsheets/fs164_apr2014/en/. Accessed 20 April 2018
WHO (2016) Model lists of essential medicines. http://www.who.int/medicines/publications/essentialmedicines/en/. Accessed 20 April 2018
WHO (2017) Towards access 2030. http://www.who.int/medicines/publications/towards_access2030/en/. Accessed 20 April 2018
Acknowledgements
We would like to express our sincere thanks to medical staff and patients. Phimpha Paboriboune was supported by CILM, Institut de Recherche pour le Développement, Campus France and the Fondation Mérieux. We are grateful to Elizabeth Elliott for editing assistance.
Author information
Authors and Affiliations
Contributions
P. Paboriboune, TV, PS, ED made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. SB, CT, PD, FXB, NS, P. Pineau were involved in drafting the manuscript or revising it critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
The study was approved by the Ethic Committee of the National Authority. All participants provided written informed consent. Written consents were obtained from all children’s parents involved in the study.
Rights and permissions
About this article
Cite this article
Paboriboune, P., Vial, T., Sitbounlang, P. et al. Hepatitis C in Laos: A 7-Year Retrospective Study on 1765 Patients. Virol. Sin. 33, 295–303 (2018). https://doi.org/10.1007/s12250-018-0039-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-018-0039-9