Abstract
We explored environmental factors influencing soil pyrite formation within different wetland regions of Everglades National Park. Within the Shark River Slough (SRS) region, soils had higher organic matter (62.65 ± 1.88 %) and lower bulk density (0.19 ± 0.01 g cm−3) than soils within Taylor Slough (TS; 14.35 ± 0.82 % and 0.45 ± 0.01 g cm−3, respectively), Panhandle (Ph; 15.82 ± 1.37 % and 0.34 ± 0.009 g cm−3, respectively), and Florida Bay (FB; 5.63 ± 0.19 % and 0.73 ± 0.02 g cm−3, respectively) regions. Total reactive sulfide and extractable iron (Fe) generally were greatest in soils from the SRS region, and the degree of pyritization (DOP) was higher in soils from both SRS (0.62 ± 0.02) and FB (0.52 ± 0.03) regions relative to TS and Ph regions (0.30 ± 0.02 and 0.31 ± 0.02, respectively). Each region, however, had different potential limits to pyrite formation, with SRS being Fe and sulfide limited and FB being Fe and organic matter limited. Due to the calcium-rich soils of TS and Ph regions, DOP was relatively suppressed. Annual water flow volume was positively correlated with soil DOP. Soil DOP also varied in relation to distance from water management features and soil percent organic matter. We demonstrate the potential use of soil DOP as a proxy for soil oxidation state, thereby facilitating comparisons of wetland soils under different flooding regimes, e.g., spatially or between wet years versus dry years. Despite its low total abundance, Fe plays an important role in sulfur dynamics and other biogeochemical cycles that characterize wetland soils of the Florida coastal Everglades.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The formation of pyrite in soils/sediments is a major process controlled by both oxidation-reduction conditions, available reactive iron (Fe), and free sulfide (Berner 1984; Raiswell and Berner 1985). Reactive Fe is defined as the fraction of iron in soil that readily reacts with sulfide as a product of dissimilatory sulfate reduction (DSR) (Canfield 1989). Meanwhile, reactive sulfide can be defined as the fraction of sulfide that binds to metals including Fe and other toxic metals (Rozan et al. 2000; Bowles et al. 2002; Rozan et al. 2002). In anoxic environments, sulfide is produced by the coupling of organic matter oxidation with sulfate reduction. Reactive Fe can bind with sulfide to yield a metastable iron mono-sulfide that during early diagenesis is readily transformed to pyrite. Pyrite may also be formed in partially oxic to anoxic environments. In environments with low oxygen conditions, metastable polysulfides also may react with aqueous reduced iron (Fe2+) to produce pyrite (Berner 1984; Rickard 1997).
Soil pyrite forms where sulfate reduction and associated fermentation reactions occur during anaerobic decomposition of organic matter (Giblin 1988). Pyrite is a dynamic soil constituent and is sensitive to redox and oxygen conditions (Luther et al. 1988). For example, conversion of sedimentary pyrite to oxidized Fe (Fe3+) and sulfur (S) (sulfate) is possible when soils become oxidized during plant growing seasons and/or hydrologic fluctuation due to dry-out or drawdown conditions (Reddy and DeLaune 2008). When soil pores fill with water, dissolved oxygen is rapidly consumed by microbial respiration and the soil status transitions from oxidizing to reducing conditions (Corstanje and Reddy 2004). Temporal changes in the quantity and timing of surface water flow influence redox conditions within wetland soils and affect Fe and S interactions.
Hydrology (i.e., quantity, timing, and distribution) plays an integral role in the structure and function of wetland ecosystems (Todd et al. 2010; Kotun and Renshaw 2014). Within the Everglades ecosystem, intra-annual water flow varies with the pronounced wet and dry seasons typical of sub-tropical environments, and interannual variation in flooding and drought conditions are not uncommon. Further, approximately 26 % of the historic ridge and slough landscape has been drained for agriculture and urban development, with the remainder being compartmentalized by a series of levees, canals, and water control structures. Due to these system modifications, natural patterns of water flow have been disrupted. Hydrologic restoration is increasingly viewed as the key to revitalize and maintain ecosystem function within the Everglades ecosystem (McCormick et al. 2009; Watts et al. 2010). Hydrologic restoration related to the distribution, timing, and duration of water within the Everglades ecosystem is expected to improve, resulting in changes in current hydrologic characteristics which could result in a cascade effect of altered ecosystem processes (i.e., biogeochemistry, productivity, etc.).
Much of the research on Fe-S interactions has been completed in either marine/brackish waters (Giblin and Howarth 1984; Luther et al. 1988; Pallud and Van Cappellen 2006) or acidic lakes/acid mine tail ponds (White et al. 1989; Friese et al. 1998). Within the freshwater Everglades ecosystem, S has been and will continue to be a topic of intense research and interest owing to the influence of reduced sulfur on methylmercury formation (Orem et al. 2011). Very few studies, however, have been conducted to specifically study Fe dynamics and its interaction with S and other compounds within a large peat accreting wetland such as the Everglades ecosystem (Koch et al. 2001; Qualls et al. 2001; Chambers and Pederson 2006). Especially for carbonate-rich, biogenic soils of south Florida that are “anemic” relative to the iron-rich, terrigenous soils of most temperate wetland systems, iron availability for pyrite formation would be an important influence on soil biogeochemistry.
The primary objective of this study was to characterize Fe-S interactions within the Everglades ecosystem by determining the degree of pyritization (DOP) in soils in recent depositional environments. The metric of DOP was developed originally to identify paleo-redox status within aquatic ecosystems and later to determine Fe or S limitation in pyrite formation (Berner 1984; Roychoudhury et al. 2003). We hypothesized that non-tidal, carbonate-rich wetland soils would have low DOP values relative to both organic, freshwater peat wetlands and sulfur-rich marine environments due to differences in limiting resources needed to form pyrite (or other Fe-S minerals) between the different systems. Due to the high natural production of organic matter driven in part by hydrology, the portion of the Everglades ecosystem dominated by freshwater peat wetlands provides an adequate substrate for microbial process and redox conditions (Aiken et al. 2011), whereas the marine environment has an abundance of sulfur sourced from marine waters. We addressed this hypothesis by comparing available iron and sulfide concentration and degree of pyrite formation among four hydrologic regions (i.e., Shark River Slough, Taylor Slough, Panhandle, and Florida Bay) and four ecosystem types (i.e., freshwater marsh, brackish ecotone, mangrove fringe, and marine) within Everglades National Park (ENP). The observed spatial variation across ENP and among wetland types led to a second hypothesis regarding the influence of hydrologic features on Fe-S dynamics, measured as the annual flow volume entering ENP and soil sampling distance from canals. We predicted that DOP values would be higher farther from canals due to reduced wetland drainage effects, as hydroperiods and frequency of flooding typically are shorter in areas with higher canal and water control structure densities (Julian 2013).
Methods
Study Area
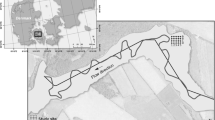
This study was conducted in ENP at the 17 original monitoring sites of the Florida Coastal Everglades Long-Term Ecological Research program (FCE LTER). Everglades National Park is located at the southern-most extent of the Everglades Protection Area and receives surface water from Water Conservation Area 3A and Big Cypress National Preserve (Osborne et al. 2011). Everglades National Park has two major drainages, Shark River Slough (SRS) and Taylor Slough (TS). Shark River Slough is the major water flow-pathway through ENP, and is approximately 32 km (km) wide along the northern border, narrowing to approximately 10 km near its discharge point at the Gulf of Mexico (GoM). This area has relatively low elevation and maintains a long hydroperiod facilitating the accretion of organic matter (Bazante et al. 2006; Osborne et al. 2011). TS is the second largest water flow path through the southeastern portion of the ENP. Upper reaches of TS are narrow, elevations are relatively high, the hydroperiod is relatively short, and soils are relatively low in organic matter. The Panhandle (Ph) portion of the ENP is located adjacent to TS, characterized as a marl prairie with shallow carbonate soils. The primary source of surface water to the Ph region is from the C-111 canal during the wet season, and thus this region experiences a very short hydroperiod (Ross et al. 2000).
Vegetation and ecosystem characteristics in ENP vary along a salinity gradient. The freshwater landscapes within ENP are dominated by a ridge and slough topography interspersed with sawgrass marshes and tree islands. Ecotone regions grade from freshwater to brackish environments and are characterized by oligohaline, mixed marsh/mangrove forests. Farther downstream in more saline water are fringing mangroves. Finally, Florida Bay receives seawater from the GoM and a small volume of freshwater runoff from ENP and is characterized as a seagrass-dominated marine environment (Chambers and Pederson 2006; Childers et al. 2006). For the purposes of this study, ENP has been divided into four hydrologic regions including Shark River Slough (SRS), Taylor Slough (TS), Panhandle (Ph), and Florida Bay (FB). The 17 monitoring sites were established to capture variation in ecosystem types among the four hydrologic regions.
Soil Collection and Analysis
Soil samples were collected annually during the wet season from the 17 monitoring sites within ENP between 2004 and 2014 (Fig. 1). From each of the sites, triplicate 60 mL syringe cores (internal diameter 2.6 cm) were pushed into the soil surface to a depth of 10 cm. The syringe barrels were capped with butyl rubber stoppers and stored on ice for transport to the laboratory. Cores were extruded, homogenized, and subsampled from each core.
Samples were dried at 80 °C for bulk density and ashed at 450 °C for 4 h for loss on ignition/percent organic matter. The ashed soils were then re-suspended in 1 N hydrochloric acid (HCl) and analyzed using the ferrozine method for extractable iron (FeHCl) (Stookey 1970). Acid volatile sulfide (AVS) was extracted from wet soil samples using a 1-N HCl solution then sequestered in a sodium hydroxide (NaOH) trap. Chromium reducible sulfur (CRS) was then extracted using a boiling solution of concentrated HCl and reduced chromium and sequestered in a NaOH trap (Chambers and Pederson 2006). Trapped AVS and CRS fractions were fixed using Cline’s reagent. Chromium reducible sulfur and AVS were analyzed colorimetrically according to methods outlined by (Cline 1969). Total reactive sulfide was determined by the sum of AVS and CRS. Reproducibility of extractable iron and sulfur species was typically within 5 %; anomalously high levels of extractable iron in 2008, however, could not be reproduced and those data were not used in the analysis. Analytical data has been archived by the Florida Coastal Everglades Long Term Ecological Research Program (Chambers and Russell 2016).
Flow data for the SRS, TS, and Ph transects were retrieved from the South Florida Water Management District online database (DBHYDRO; http://www.sfwmd.gov/dbhydro) for flow sites entering each region. Sites for Shark River Slough include water control structures along US-41 (Tamiami Trail) including S-12A, S-12B, S-12C, S-12D, S-333, and S-334. Total annual flow entering Everglades National Park (ENP) was computed as the sum of the S-12 structures and the difference between S-333 and S-334. Flow sites for Taylor Slough and Panhandle regions of ENP including the S-332D and S-18C, respectively (Fig. 1). Distance from the main flow path within each hydrologic region was determined by Euclidean distance using ArcGIS (Ver 10.2 ESRI, Redlands, CA, USA).
Data Analysis
Chromium reducible sulfur, AVS, and FeHCl data were converted from volume-based units (μmol cm−3) to mass-based units (μmol g−1) by dividing concentration data by soil bulk density. Mass-based units were used in all analyses. For purpose of analysis, the entire CRS fraction was assumed to be pyrite (FeS2). Degree of pyritization was calculated according to Eq. 1 using CRS and FeHCl concentrations. Based on DOP, site conditions were classified as DOP <0.46 indicating aerobic or oxidizing conditions, DOP between 0.46 and 0.75 indicating dysoxic conditions (low oxygen between anoxic and hypoxic), and DOP >0.75 indicating euxinic (anaerobic) or reducing conditions (Roychoudhury et al. 2003).
Soil bulk density and percent organic matter were analyzed using log-log regression. DOP was compared among hydrologic regions using the Kruskal-Wallis/Mann-Whitney multiple comparisons in the “pgirmess” package in R (Giraudoux 2016). Analysis of variance (ANOVA) was used to compare annual median DOP for each flow path and total flow. To ensure the data fit the assumptions of the statistical test, data were tested for normality using the Shapiro-Wilk’s and Anderson-Darling test. Chi-square (RxC) analysis compared soil DOP site condition definitions both by hydrologic region and by ecosystem type. Percent organic matter, total flow to each flow path, and distance from canal were compared to square root transformed DOP using multiple regression analysis. Degree of pyritization values were transformed so that the data fit the assumptions of linear models. Regression model assumptions were tested for all regression models using the “gvlma” global linear model validation package in R (Peña and Slate 2006). All statistical operations were performed using R (Ver 3.1.2, R Foundation for Statistical Computing, Vienna Austria) and the critical level of significance was set at α = 0.05.
Results and Discussion
General Soil/Sediment Characteristics
Among the 17 monitoring sites, soil bulk density values ranged from 0.03 to 1.94 g cm−3 with soils from the mangrove fringe exhibiting the largest degree of variation, ranging from 0.08 to 1.94 g cm−3. Within the freshwater and brackish ecotone systems of the study area, bulk density values ranged from 0.03 to 1.08 g cm−3 (Table 1). Generally, higher bulk density values were associated with soils with a higher proportion of mineral content (marl-soils, sand, etc.). Percent organic matter varied substantially along the gradient from freshwater to marine ecosystems, ranging from 1.4 to 85.3 % in soils from the mangrove fringe, 3.2 to 91.5 % from the brackish ecotone, and 2.7 to 95.0 % from freshwater areas (Table 1). The relationship between bulk density and organic matter followed a log-log relationship (Fig. 2; R 2 = 0.73, F(1558) = 1534, ρ < 0.001). High percent organic matter, low bulk density soils are indicative of organic, peat type soils whereas low percent organic matter, high bulk density soils are indicative of mineral, marl-based soils. This relationship is consistent with data collected in the region (Osborne et al. 2011; Osborne et al. 2014) and elsewhere (Harrison and Bocock 1981; Morris et al. 2016).
Iron and Sulfur Dynamics
Generally, surface water iron concentrations are relatively low within freshwater environments of the Everglades ecosystem, ranging between 0.81 and 1.78 μM (Julian et al. 2015). Atmospheric deposition and groundwater inputs are the primary iron sources for the Everglades (Shotyk 1988; Blodau et al. 2002). Wet deposition of iron dust has been reported to range from ~50 to 225 μg cm−2 year−1 throughout the greater Everglades ecosystem (Landing et al. 1995; Prospero et al. 2010). These deposition values correspond to an atmospheric loading of approximately 5.5–25.1 kmol year−1 (3111 to 14,000 metric tons year−1) of iron to Everglades National Park (assuming an area of 6222 km2) including both terrestrial and aquatic portions. Soil FeHCl concentrations within ENP are generally low (Chambers and Pederson 2006) with FeHCl pools varying between regions and ecosystems ranging in concentration from 0.02 to 127.08 μmol g−1. Mean FeHCl concentrations were greatest along Ph (35.73 ± 2.39 μmol g−1), followed by SRS and TS (25.22 ± 2.32 and 23.98 ± 1.31 μmol g−1, respectively) with the lowest FeHCl concentrations measured in FB soils (11.15 ± 0.95 μmol g−1) (Fig. 3). With the exception of SRS, FeHCl concentrations were largest in mangrove fringe environments compared to the brackish ecotone or freshwater environments (Table 1). Iron directly and indirectly effects that influence the growth of plants and algae; therefore, the distribution and availability of Fe are important variables that biogoechemically drives ecosystem structure (Chambers et al. 2001). Directly, Fe is needed by organisms and is integrated into many biochemical interactions. Phototrophs require minerals to synthesize cell contents and to manufacture structural and reproductive cells. More specifically, Fe is used to biosynthesize cytochromes, chlorophyll, and other iron-containing proteins (Marschner 2011). Indirectly, Fe buffers the phytotoxic and growth-limiting effects of sulfide on plants by precipitating sulfide as iron-sulfur minerals (i.e., pyrite, FeS, etc.) (Koch et al. 1990; Carlson et al. 1994; Alongi 2010). Elevated Fe concentrations within the mangrove fringe areas of ENP could provide an optimal soil condition to facilitate recruitment and establishment of mangrove species both by supplementing Fe for growth (Alongi 2010) and diminishing sulfide toxicity affects (Wilhelmina et al. 2006).
Iron cycling is driven by both abiotic and biotic reactions coupled with other biogeochemical cycles. Cycling of Fe and S is a significant process in the biogeochemistry of estuarine and marine sediments (Canfield 1989; Canfield et al. 1992; Keene et al. 2010) and could play a significant role in freshwater sediments. In aerobic and circumneutral pH conditions, ferrous Fe (Fe2+) is rapidly oxidized to ferric Fe (Fe3+) and precipitates as iron oxide. Conversely, chemosynthetic microbes can oxidize Fe2+ to Fe3+ under a variety of pH and oxygen conditions (Fortin and Langley 2005). In environments with abundant reactive Fe or limited reduced sulfur (S2−), aqueous Fe species are important biogeochemically, with Fe2+ having the potential to form a series of weak ion pairs with most anions including carbonate, chloride, and sulfate (Rickard and Morse 2005). Complexation of Fe with carbonate greatly reduces pyrite formation; furthermore, depending on pH and redox conditions, calcium can complex with available sulfate to form CaSO4 (Berner 1984; Hammes and Verstraete 2002). Thus, if sediment is dominated by CaCO3 (calcite), pyrite formation is low (Berner 1984). Taylor Slough and Panhandle regions, for example, are in the eastern marl prairies of ENP, which are calcium-dominated systems due to the growth and decomposition of calcareous periphyton and the dissolution of the underlying limestone and Pleistocene reef platform (Osborne et al. 2011).
Chromium reducible sulfur concentrations were much greater than AVS concentrations for all flow paths and ecosystems, indicating that the majority of sulfide in soils resides as CRS (Table 1). Acid volatile sulfide is predominately a measure of iron monosulfides (FeS) such as mackinawite in Fe-rich environments. In Fe-poor systems such as the carbonate systems of TS and Ph, free sulfide and other sulfur compounds, such as thiosulfate and dithionite, are dominant (Rees et al. 2010). The abundance of CRS in the Everglades systems suggests that it is a pyrite-preferred geochemical system as opposed to other minerals such as mackinawite or greigite which are considered metastable and precursors to the most thermodynamically favored pyrite (Schoonen and Barnes 1991a; Schoonen and Barnes 1991b; Burton et al. 2011). Acid volatile sulfide concentrations ranged from 0.02 to 85.72 μmol g−1. Chromium reducible sulfur concentrations ranged from 0.02 to 365.45 μmol g−1 with the greatest CRS concentration observed within SRS (54.89 ± 3.52 μmol g−1), followed by Ph (40.68 ± 6.24 μmol g−1), TS (30.03 ± 4.11 μmol g−1), and FB (24.39 ± 2.38 μmol g−1) during the period of this study.
The interaction of Fe and S could also potentially influence or explain variability expressed in the hypothetical S-mercury (Hg) relationship. Mercury methylation is facilitated by microbial methylation via sulfate-reducing bacteria (SRB), Fe-reducing bacteria, and methanogenic bacteria with the necessary genes required to complete the methylation process (Gilmour et al. 2013; Parks et al. 2013; Bae et al. 2014). Elevated sulfate concentrations have been linked to regions of elevated methyl-Hg concentrations (i.e., hotspots) through potential generation of methyl-Hg during sulfate reduction via Hg-methylating SRB (Compeau and Bartha 1985; Gilmour and Henry 1991; Gilmour et al. 1992). Therefore, processes that interact with S cycling could have a potential to influence Hg methylation. In the water conservation areas north of ENP, porewater Fe and porewater sulfide concentrations significantly differed between hotspots and non-hotspots, suggesting that the interaction between Fe and S could play a role in Hg methylation or cycling (Julian et al. 2016).
Pyrite Formation
This study contrasts three drastically different regions along an ecosystem gradient with respect to soil characteristics. Shark River Slough is a high organic matter, peat-based system; TS and Ph are low organic matter, high calcium marl-based systems (Osborne et al. 2011), and FB is a marine system with characteristic biogenic carbonate mud (Bosence 1989). Pyrite formation and burial is dependent upon three factors: the rate at which sulfide is produced via sulfate reduction, the availability of iron, and the rate that reduced sulfur compounds are subsequently oxidized.
Sulfate reduction rates depend upon the amount and quality of organic matter present as well as the availability of sulfate. In marine sediments, due to the abundance of sulfate, sulfate reduction rates are limited by the supply of organic carbon. In contrast, sulfate concentrations in freshwater systems are at least an order of magnitude less than marine systems and thus sulfate reduction is limited by the availability of sulfate (Giblin 1988). The availability of iron is tied to sources including atmospheric deposition (discussed above), complexation/precipitation (Canfield 1989), redox chemistry (Qualls et al. 2001), and sedimentation (i.e., terrigenous versus calcareous) (Berner 1984). Once deposited, detrital iron minerals undergo redox reactions which make them susceptible to other biogeochemical reactions during diagenesis. Oxidation of reduced sulfur—including pyrite—frequently occurs within the freshwater portions of the Everglades ecosystem because of frequent dry-out and rewetting cycles that result in large spikes in sulfate surface water concentrations (Dierberg et al. 2014; Julian et al. 2014) and presumably rapid changes in water column pH.
Pyrite formation, as expressed by DOP, varied significantly among flow paths (Fig. 4; χ 2 = 107.10, df = 3, ρ < 0.001). As expected, due to regional characteristics, TS and Ph DOP values throughout the course of the study were similar, while SRS and FB were different from one another (Fig. 4). Differences in DOP values between TS and Ph to that of SRS could be explained by the differences in soil organic matter pools within each respective region. The role of organic matter in pyrite formation is one of the essential pieces to the pyrite puzzle since organic carbon is necessary for sulfate-reducing bacteria to reduce sulfate to sulfide by using organic carbon as an oxidizing agent. Based on the interquartile range (IQR) of each flow path, SRS has the greatest percent organic matter (Q1, 40.7 %; Q3, 86.1 %; IQR, 45.4) followed by Ph (Q1, 7.4 %; Q3, 23.2 %; IQR, 15.8), TS (Q1, 8.0 %; Q3, 17.0 %; IQR, 9.0), and FB (Q1, 4.7 %; Q3, 6.5 %; IQR, 1.8). Even though FB had relatively less organic matter (by volume), DOP was relatively higher than Ph and TS and similar to that of the SRS flow path due to the differences in limiting conditions. Shark River Slough is a sulfate-limited system, and FB is Fe limited and potentially to some degree organic matter/carbon limited with respect to pyrite formation. Although seagrass beds can have higher organic matter content (7.2–18.8 % organic matter) (Borum et al. 2005) and sulfide concentrations (32–2143 μM porewater sulfide) (Carlson et al. 1994), high DOP values observed in FB is the result of Fe limitation due to the low reactive Fe (high bound Fe) of FB sediment relative to SRS soils. Furthermore, Chambers et al. (2001) observed a pronounced north-to-south gradient of CRS-Fe in FB. The relatively depressed DOP values observed in the TS and Ph flow paths could be explained by the abundant supply of CaCO3 within the upper portions of these flow paths.
In wetlands, pyrite formation and the underlying biogeochemical reactions are dependent upon redox state which in turn is influenced by hydrologic conditions. Thus, pyrite formation should be significantly influenced by the amount of flow entering a specific region. Flow significantly influenced annual median DOP (F(1,24) = 11.94, ρ < 0.01). During high flow years, hydrologic loading is higher, resulting in a larger surface area covered with water for a longer duration (i.e., longer hydroperiod) therefore driving anaerobic/reducing biogeochemical reactions increasing the potential for formation of pyrite. Alternatively, in drier years (i.e., periods of less flow), the duration of inundation and intensity of reducing biogeochemical conditions is less, resulting in a greater oxidizing environment and depressed pyrite production. Degree of pyritization was significantly explained by percent organic matter, total flow, and distance from canal (Table 2; F(3434) = 83.41, ρ < 0.001). Percent organic matter, total flow, and distance from canal are very important in the overall formation of pyrite within both freshwater systems as well as marine systems within the Everglades ecosystem (Table 2).
Total flow and distance from canal are hydrologic factors that most directly influence redox conditions in the upper portions of the flow paths (i.e., freshwater system) by inundating areas. Areas closer to the canals during high flow years would experience longer hydroperiods, which in combination with organic matter results in largely reducing conditions. However, as distance from canal increases, the potential for longer hydroperiods decreases until the flow path reaches its terminus (i.e., FB) making redox conditions slightly more variable and driven by different factors (e.g., tidal supply of water and electron acceptors, less organic matter). Therefore, factors driving pyrite formation may differ along the flow path but each factor is important in its own right.
Each region and ecosystem differed significantly with respect to DOP site condition definitions. Throughout the course of the study, site conditions differed significantly by region (Fig. 5; χ 2 = 124.79, df = 6, ρ < 0.001). Based on DOP site condition analysis, overall TS and Ph flow paths were considered more aerobic than dysoxic or euxinic with a higher proportion of DOP values less than 0.46. These results correspond to results of an intensive soil survey of TS and Ph regions presented by Osborne and Ellis (2015). This study determined the underlying geology combined with the region’s hydrology resulted in a narrow channelized path of organic matter with areas outside of the main channel experiencing significant dry periods that favored organic matter oxidation and a net loss of organic carbon. Shark River Slough and FB exhibited a variety of conditions with FB experiencing less euxinic conditions (i.e., DOP >0.76) and relative equal aerobic and dysoxic conditions while SRS experienced a greater proportion of aerobic and euxinic conditions. Florida Bay flow and circulation is extremely dynamic with restricted circulation and higher summertime salinity values (Zieman et al. 1989; Wang et al. 1994). Meanwhile, SRS experiences a high degree of variability with respect to flow volume and timing along the length of the slough, especially hydroperiod duration in the northern portions of the slough (close to inflows) (Todd et al. 2010; Cook 2012).
Sites-specific conditions were significantly different between ecosystems (Fig. 5; χ 2 = 40.47, df = 6, ρ < 0.001) with freshwater and ecotone systems being more aerobic and estuary systems being dominantly aerobic or dysoxic with respect to DOP values. However, these conditions could reflect overall hydrologic biogeochemical drivers (i.e., drier, oxidizing conditions versus wetter, reducing conditions), interaction with the Ca-rich substrate in the case of TS and Ph, the lack or abundance of organic matter to effectively cycle sulfur, and the overall availability of sulfur. Degree of pyritization, however, does provide insight into the integrated redox condition of the region or flow path with respect to Fe and S cycling, and may be used as a proxy for soil oxidation status in wetlands that experience seasonal and interannual variation in flooding. Furthermore, this analysis highlights the drastic ecosystem biochemical differences across ENP. These site condition definitions provide an integrated viewpoint regarding pyrite forming conditions at a given site or flow path. Therefore, high DOP conditions or euxinic conditions can be translated to favorable pyrite forming conditions, dysoxic as intermediate pyrite forming conditions, and aerobic as unfavorable pyrite forming conditions. More research is needed to determine whether DOP is a reliable indicator of modern pyrite formation and diagenesis and if DOP can be used as a metric to further evaluate biogeochemical processes related to water quality conditions.
Conclusions and Further Research
This study suggests that Fe plays an important role in biogeochemical cycling in the Everglades system, especially in the context of sulfur biogeochemistry and the formation of minerals such as pyrite. These dynamics are exemplified across an ecosystem gradient from freshwater to marine systems, each with unique characteristics, driving forces, and biological conditions. Iron-S dynamics are vitally important to the biological integrity of the Everglades ecosystem due to the hypothesized role of Fe availability in the recruitment and establishment of mangroves (Alongi 2010), the ability of pyrite to regulate sulfide exposure to plants and affect seagrass growth (Chambers et al. 2001), and the potential of Fe-S interactions to influence mercury biogeochemistry (Julian et al. 2016). Additional study is needed to further characterize Fe-S interaction, Fe interaction with other biogeochemical cycles, and pyrite formation within the Everglades system.
References
Aiken, George R., Cynthia C. Gilmour, David P. Krabbenhoft, and William Orem. 2011. Dissolved organic matter in the Florida Everglades: implications for ecosystem restoration. Critical Reviews in Environmental Science and Technology 41: 217–248. doi:10.1080/10643389.2010.530934.
Alongi, Daniel M. 2010. Dissolved iron supply limits early growth of estuarine mangroves. Ecology 91: 3229–3241.
Bae, H., F.E. Dierberg, and A. Ogram. 2014. Syntrophs dominate sequences associated with the mercury-methylating gene hgcA in the water conservation areas of the Florida Everglades. Applied and Environmental Microbiology: AEM.01666–14. doi:10.1128/AEM.01666-14.
Bazante, Jose, Gary Jacobi, Helena M. Solo-Gabriele, David Reed, Sherry Mitchell-Bruker, Daniel L. Childers, Lynn Leonard, and Michael Ross. 2006. Hydrologic measurements and implications for tree island formation within Everglades National Park. Journal of Hydrology 329: 606–619. doi:10.1016/j.jhydrol.2006.03.011.
Berner, Robert A. 1984. Sedimentary pyrite formation: an update. Geochimica et Cosmochimica Acta 48: 605–615. doi:10.1016/0016-7037(84)90089-9.
Blodau, C., C.L. Roehim, and T.R. Moore. 2002. Iron, sulfur, and dissolved carbon dynamics in a northern peatland. Archiv für Hydrobiologie 154: 561–583.
Borum, J., O. Pedersen, T.M. Greve, T.A. Frankovich, J.C. Zieman, J.W. Fourqurean, and C.J. Madden. 2005. The potential role of plant oxygen and sulphide dynamics in die-off events of the tropical seagrass, Thalassia testudinum. Journal of Ecology 93: 148–158. doi:10.1111/j.1365-2745.2004.00943.x.
Bosence, Daniel. 1989. Biogenic carbonate production in Florida Bay. Bulletin of Marine Science 44: 419–433.
Bowles, Karl C., Russell A. Bell, Michael J. Ernste, James R. Kramer, Helen Manolopoulos, and Nancy Ogden. 2002. Synthesis and characterization of metal sulfide clusters for toxicological studies. Environmental Toxicology and Chemistry 21: 693–699
Burton, Edward D., Richard T. Bush, Scott G. Johnston, Leigh A. Sullivan, and Annabelle F. Keene. 2011. Sulfur biogeochemical cycling and novel Fe–S mineralization pathways in a tidally re-flooded wetland. Geochimica et Cosmochimica Acta 75: 3434–3451. doi:10.1016/j.gca.2011.03.020.
Canfield, Donald E. 1989. Reactive iron in marine sediments. Geochimica et Cosmochimica Acta 53: 619–632. doi:10.1016/0016-7037(89)90005-7.
Canfield, Donald E., Robert Raiswell, and Simon H. Bottrell. 1992. The reactivity of sedimentary iron minerals toward sulfide. American Journal of Science 292: 659–683. doi:10.2475/ajs.292.9.659.
Carlson, Jr, R. Paul, Laura A. Yarbro, and Timothy R. Barber. 1994. Relationship of sediment sulfide to mortality of Thalassia testudinum in Florida Bay. Bulletin of Marine Science 54: 733–746.
Chambers, R.M., and K.A. Pederson. 2006. Variation in soil phosphorus, sulfur, and iron pools among South Florida wetlands. Hydrobiologia 569: 63–70. doi:10.1007/s10750-006-0122-3.
Chambers, R.M., and T.M. Russell. 2016. Physical and chemical characteristics of soil sediments from the Shark River slough and Taylor slough, Everglades National Park (FCE) from August 2004 to present, Grant No. DEB-1237517, DBI-0620409, and Grant No. DEB-9910514. Miami, FL: Florida Coastal Everglades Long-Term Ecological Research Program.
Chambers, R.M., J.W. Fourqurean, S.A. Macko, and et al. 2001. Biogeochemical effects of iron availability on primary producers in a shallow marine carbonate environment. Limnology and Oceanography 46:1278–1286
Childers, Daniel L., Joseph N. Boyer, Stephen E. Davis, Christopher J. Madden, David T. Rudnick, and Fred H. Sklar. 2006. Relating precipitation and water management to nutrient concentrations in the oligotrophic“ upside-down” estuaries of the Florida Everglades. Limnology and Oceanography 51: 602–616.
Cline, J.D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnology and Oceanography 14: 454–458.
Compeau, G.C., and R. Bartha. 1985. Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Applied and Environmental Microbiology 50: 498–502.
Cook, A. 2012. Development of an integrated surface and subsurface model of Everglades National Park.
Corstanje, R., and K.R. Reddy. 2004. Response of biogeochemical indicators to a drawdown and subsequent reflood. Journal of Environmental Quality 33: 2357–2366.
Dierberg, F.E., T.A. DeBusk, M Jerauld, and B Gu. 2014. Appendix 3B-1: Evaluation of factors influencing methylmercury accumulation in South Florida marshes. In 2014 South Florida Environmental Report. West Palm Beach, FL: South Florida Water Management District.
Fortin, Danielle, and Sean Langley. 2005. Formation and occurrence of biogenic iron-rich minerals. Earth-Science Reviews 72: 1–19. doi:10.1016/j.earscirev.2005.03.002.
Friese, K., K. Wendt-Potthoff, D.W. Zachmann, A. Fauville, B. Mayer, and J. Veizer. 1998. Biogeochemistry of iron and sulfur in sediments of an acidic mining Lake in Lusatia, Germany. Water, Air, and Soil Pollution 108: 231–247. doi:10.1023/A:1005195617195.
Giblin, Anne E. 1988. Pyrite formation in marshes during early diagenesis. Geomicrobiology Journal 6: 77–97. doi:10.1080/01490458809377827.
Giblin, Anne E., and Robert W. Howarth. 1984. Porewater evidence for a dynamic sedimentary iron cycle in salt marshes. Limnology and Oceanography 29: 47–63. doi:10.4319/lo.1984.29.1.0047.
Gilmour, C.C., and E.A. Henry. 1991. Mercury methylation in aquatic systems affected by acid deposition. Environmental Pollution 71: 131–169.
Gilmour, C.C., E.A. Henry, and R. Mitchell. 1992. Sulfate stimulation of mercury methylation in freshwater sediments. Environmental Science & Technology 26: 2281–2287.
Gilmour, C.C., M. Podar, A.L. Bullock, A.M. Graham, S.D. Brown, A.C. Somenahally, A. Johs, R.A. Hurt, K.L. Bailey, and D.A. Elias. 2013. Mercury methylation by novel microorganisms from new environments. Environmental Science & Technology 47: 11810–11820. doi:10.1021/es403075t.
Giraudoux, P. 2016. pgirmess: data analysis in ecology. R (version 1.6.4).
Hammes, F., and W. Verstraete. 2002. Key roles of pH and calcium metabolism in microbial carbonate precipitation. Reviews in Environmental Science and Biotechnology 1: 3–7. doi:10.1023/A:1015135629155.
Harrison, A.F., and K.L. Bocock. 1981. Estimation of soil bulk-density from loss-on-ignition values. The Journal of Applied Ecology 18: 919. doi:10.2307/2402382.
Julian, P. 2013. Mercury hotspot identification in water conservation area 3, Florida, USA. Annals of GIS 19: 79–88. doi:10.1080/19475683.2013.782469.
Julian, P, G.G. Payne, and S.K. Xue. 2014. Chapter 3A: water quality in the Everglades Protection Areas. In 2014 South Florida environmental report. West Palm Beach, FL: South Florida Water Management District.
Julian, P, G.G. Payne, and S.K. Xue. 2015. Chapter 3A: water quality in the Everglades Protection Areas. In 2015 South Florida environmental report. West Palm Beach, FL: South Florida Water Management District.
Julian, P., A.L. Wright, and T.Z. Osborne. 2016. Iron and sulfur porewater and surface water biogeochemical interactions in a subtropical peatland. Soil Science Society of America Journal 80: 794–802. doi:10.2136/sssaj2015.11.0418.
Keene, Annabelle F., Scott G. Johnston, Richard T. Bush, Leigh A. Sullivan, Edward D. Burton, Angus E. McElnea, Colin R. Ahern, and Bernard Powell. 2010. Effects of hyper-enriched reactive Fe on sulfidisation in a tidally inundated acid sulfate soil wetland. Biogeochemistry 103: 263–279. doi:10.1007/s10533-010-9461-2.
Koch, M.S., I.A. Mendelssohn, and K.L. Mckee. 1990. Mechanism for the hydrogen sulfide-induced growth limitation in wetland macrophytes. Limnology and Oceanography 35: 399–408. doi:10.4319/lo.1990.35.2.0399.
Koch, M.S., R.E. Benz, and D.T. Rudnick. 2001. Solid-phase phosphorus pools in highly organic carbonate sediments of northeastern Florida Bay. Estuarine, Coastal and Shelf Science 52: 279–291. doi:10.1006/ecss.2000.0751.
Kotun, Kevin, and Amy Renshaw. 2014. Taylor slough hydrology: fifty years of water management 1961-2010. Wetlands 34: 9–22. doi:10.1007/s13157-013-0441-x.
Landing, W.M., J.J. Perry Jr., J.L. Guentzel, G.A. Gill, and C.D. Pollman. 1995. Relationships between the atmospheric deposition of trace elements, major ions, and mercury in Florida: the FAMS project (1992–1993). Water, Air, and Soil Pollution 80: 343–352. doi:10.1007/BF01189684.
Luther, W. George, and Thomas M. Church. 1988. Seasonal cycling of sulfur and iron in porewaters of a Delaware salt marsh. Marine Chemistry 23: 295–309. doi:10.1016/0304-4203(88)90100-4.
Marschner, Horst. 2011. Mineral nutrition of higher plants. New York: Academic Press.
McCormick, Paul, Susan Newman, and Les Vilchek. 2009. Landscape responses to wetland eutrophication: loss of slough habitat in the Florida Everglades, USA. Hydrobiologia 621: 105–114. doi:10.1007/s10750-008-9635-2.
Morris, James T., Donald C. Barber, John C. Callaway, Randy Chambers, Scott C. Hagen, Charles S. Hopkinson, Beverly J. Johnson, et al. 2016. Contributions of organic and inorganic matter to sediment volume and accretion in tidal wetlands at steady state: sediment bulk density and ignition loss. Earth’s Future 4: 110–121. doi:10.1002/2015EF000334.
Orem, William, Cynthia Gilmour, Donald Axelrad, David Krabbenhoft, Daniel Scheidt, Peter Kalla, Paul McCormick, Mark Gabriel, and George Aiken. 2011. Sulfur in the South Florida ecosystem: distribution, sources, biogeochemistry, impacts, and management for restoration. Critical Reviews in Environmental Science and Technology 41: 249–288. doi:10.1080/10643389.2010.531201.
Osborne, T.Z., and L.R. Ellis. 2015. Monitoring of phosphorus storage in Park Marsh Land Sediments: an assessment of the C-111 Spreader Canal Project. Report to National Park Service, Everglades National Park
Osborne, T.Z., G.L. Bruland, S. Newman, K.R. Reddy, and S. Grunwald. 2011. Spatial distributions and eco-partitioning of soil biogeochemical properties in the Everglades National Park. Environmental Monitoring and Assessment 183: 395–408. doi:10.1007/s10661-011-1928-7.
Osborne, T.Z., K.R. Reddy, L.R. Ellis, N.G. Aumen, D.D. Surratt, M.S. Zimmerman, and J. Sadle. 2014. Evidence of recent phosphorus enrichment in surface soils of Taylor Slough and Northeast Everglades National Park. Wetlands 34: 37–45. doi:10.1007/s13157-013-0381-5.
Pallud, Céline, and Philippe Van Cappellen. 2006. Kinetics of microbial sulfate reduction in estuarine sediments. Geochimica et Cosmochimica Acta 70: 1148–1162. doi:10.1016/j.gca.2005.11.002.
Parks, Jerry M., Alexander Johs, Mircea Podar, Romain Bridou, Richard A. Hurt, Steven D. Smith, Stephen J. Tomanicek, et al. 2013. The genetic basis for bacterial mercury methylation. Science 339: 1332–1335. doi:10.1126/science.1230667.
Peña, Edsel A., and Elizabeth H. Slate. 2006. Global validation of linear model assumptions. Journal of the American Statistical Association 101: 341–354. doi:10.1198/016214505000000637.
Prospero, Joseph M., William M. Landing, and Michael Schulz. 2010. African dust deposition to Florida: temporal and spatial variability and comparisons to models. Journal of Geophysical Research: Atmospheres 115: D13304. doi:10.1029/2009JD012773.
Qualls, R.G., C.J. Richardson, and L.J. Sherwood. 2001. Soil reduction-oxidation potential along a nutrient-enrichment gradient in the Everglades. Wetlands 21: 403–411. doi:10.1672/0277-5212(2001)021[0403:SROPAA]2.0.CO;2.
Raiswell, Robert, and Robert A. Berner. 1985. Pyrite formation in euxinic and semi-euxinic sediments. American Journal of Science 285: 710–724. doi:10.2475/ajs.285.8.710.
Reddy, K.R., and R.D. DeLaune. 2008. Biogeochemistry of wetlands: science and applications. Boca Raton: CRC Press.
Rees, Gavin N., Darren S. Baldwin, Garth O. Watson, and Karina C. Hall. 2010. Sulfide formation in freshwater sediments, by sulfate-reducing microorganisms with diverse tolerance to salt. Science of the Total Environment 409: 134–139. doi:10.1016/j.scitotenv.2010.08.062.
Rickard, David. 1997. Kinetics of pyrite formation by the H2S oxidation of iron (II) monosulfide in aqueous solutions between 25 and 125 °C: the rate equation. Geochimica et Cosmochimica Acta 61: 115–134. doi:10.1016/S0016-7037(96)00321-3.
Rickard, David, and John W. Morse. 2005. Acid volatile sulfide (AVS). Marine Chemistry 97: 141–197. doi:10.1016/j.marchem.2005.08.004.
Ross, M.S., J.F. Meeder, J.P. Sah, P.I. Ruiz, and G.J. Telesnicki. 2000. The Southeast Saline Everglades revisited: 50 years of coastal vegetation change. Journal of Vegetation Science 11: 101–112. doi:10.2307/3236781.
Roychoudhury, Alakendra N., Joel E. Kostka, and Philippe Van Cappellen. 2003. Pyritization: a palaeoenvironmental and redox proxy reevaluated. Estuarine, Coastal and Shelf Science 57: 1183–1193. doi:10.1016/S0272-7714(03)00058-1.
Rozan, Tim F., Michael E. Lassman, Douglas P. Ridge, and George W. Luther. 2000. Evidence for iron, copper and zinc complexation as multinuclear sulphide clusters in oxic rivers. Nature 406: 879–882
Rozan, Tim F., Martial Taillefert, Robert E. Trouwborst, Brian T. Glazer, Shufen Ma, Julian Herszage, Lexia M. Valdes, Kent S. Price, and George W. Luther III. 2002. Iron-sulfur-phosphorus cycling in the sediments of a shallow coastal bay: implications for sediment nutrient release and benthic macroalgal blooms. Limnology and Oceanography 47: 1346–1354
Schoonen, M.A.A., and H.L. Barnes. 1991a. Reactions forming pyrite and marcasite from solution: I. Nucleation of FeS2 below 100 °C. Geochimica et Cosmochimica Acta 55: 1495–1504. doi:10.1016/0016-7037(91)90122-L.
Schoonen, M.A.A., and H.L. Barnes. 1991b. Reactions forming pyrite and marcasite from solution: II. Via FeS precursors below 100 °C. Geochimica et Cosmochimica Acta 55: 1505–1514. doi:10.1016/0016-7037(91)90123-M.
Shotyk, William. 1988. Review of the inorganic geochemistry of peats and peatland waters. Earth-Science Reviews 25: 95–176. doi:10.1016/0012-8252(88)90067-0.
Stookey, Lawrence L. 1970. Ferrozine—a new spectrophotometric reagent for iron. Analytical Chemistry 42: 779–78
Todd, M. Jason, R. Muneepeerakul, D. Pumo, S. Azaele, F. Miralles-Wilhelm, A. Rinaldo, and I. Rodriguez-Iturbe. 2010. Hydrological drivers of wetland vegetation community distribution within Everglades National Park, Florida. Advances in Water Resources 33 . doi:10.1016/j.advwatres.2010.04.003.Special Issue on Novel Insights in Hydrological ModellingRome-2009: 1279–1289
Wang, John D., Jacobus van de Kreeke, N. Krishnan, and DeWitt Smith. 1994. Wind and tide response in Florida Bay. Bulletin of Marine Science 54: 579–601.
Watts, Danielle L., Matthew J. Cohen, James B. Heffernan, and Todd Z. Osborne. 2010. Hydrologic modification and the loss of self-organized patterning in the ridge–slough mosaic of the Everglades. Ecosystems 13: 813–827. doi:10.1007/s10021-010-9356-z.
White, Jeffrey R., Chad P. Gubala, Brian Fry, Jeffrey Owen, and Myron J. Mitchell. 1989. Sediment biogeochemistry of iron and sulfur in an acidic lake. Geochimica et Cosmochimica Acta 53: 2547–2559. doi:10.1016/0016-7037(89)90127-0.
Wilhelmina, M.E., W. Van Der Welle, M. Cuppens, L.P.M. Lamers, and J.G.M. Roelofs. 2006. Detoxifying toxicants: interactions between sulfide and iron toxicity in freshwater wetlands. Environmental Toxicology and Chemistry 25.
Zieman, Joseph, James W. Fourqurean, and Richard L. Iverson. 1989. Distribution, Abundance and Productivity of Seagrasses and Macroalgae in Florida Bay. Bulletin of Marine Science 44: 292–311
Acknowledgments
We would like to thank the FCE LTER crew for field support and the anonymous peer reviewer(s) and editor(s) for their efforts and constructive review of this manuscript. This material was developed in collaboration with the FCE LTER program which is funded by National Science Foundation Grant No. DEB-9910514, Grant No. DBI-0620409, and Grant No. DEB-1237517.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marianne Holmer
Rights and permissions
About this article
Cite this article
Julian, P., Chambers, R. & Russell, T. Iron and Pyritization in Wetland Soils of the Florida Coastal Everglades. Estuaries and Coasts 40, 822–831 (2017). https://doi.org/10.1007/s12237-016-0180-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0180-3